Abstract
In this study, we analyzed the length-weight relationships (LWRs), total length (TL)—standard length (SL) relationships (LLRs), and Fulton’s condition factor (K) of male and female black rockfish Sebastes schlegelii Hilgendorf, 1880 in Lidao Bay, Yellow Sea, China. Among the 729 S. schlegelii specimens sampled seasonally in February, May, August and November between 2011 and 2015, most were males (sex ratio = 1 male:0.79 female). The LWRs of each season were significant (P < 0.05), and all coefficients of determination (r 2) were higher than 0.95. The values of the slope (b) estimated for each LWR regression varied from 2.947 to 3.277 and were lower in spring than those in other seasons, especially in females. The LLRs calculated as the regression of TL on SL and vice versa were linear. The values of K ranged from 0.791 to 2.981 in males (n = 407) and from 0.752 to 2.681 in females (n = 322), and the highest value was found in spring. The present results provide baseline information on biological data of LWRs, LLRs, and K for S. schlegelii in this area, and will be useful in further studies on stock management of S. schlegelii.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The weight of fish varies in relation to its length. Length-weight relationships (LWRs) are the most important biological parameters for understanding fish survival, growth rate, reproduction stock biomass, and other aspects of population’s dynamics (Martin-Smith 1996; Muchlisin et al. 2010). They enable us to follow seasonal variations in fish growth (Richter et al. 2000) and are useful for predicting fisheries yield (Garcia et al. 1998) and biomass (Martin-Smith 1996). In addition, LWRs can be used to perform morphological comparison among species or populations from different habitats or regions (King 1996). Relationships between different types of length measurements, such as total length-standard length relationships (LLRs), are also important in studies for comparing growth rates (Moutopoulos and Stergiou 2002). Therefore, determining LWRs and LLRs is very important for the management and conservation of natural populations.
Condition factor is a quantitative parameter of the condition state, fatness, or well-being of fish (Tesch 1968), based on the assumption that, for a given length, heavier fish are in better condition. Fulton’s condition factor (K) is used for comparing seasonal changes in fish nutritional condition, and usually increases with sexual maturation.
Sex ratio, the proportion of males and females in a population, is a key demographic parameter and an indicator of the population’s behavior and fecundity, and is a fundamental concept in evolutionary biology (Hardy 2002). Knowledge on a population’s sex ratio across different seasons is essential for understanding the seasonal segregation of sexes and their differential growth. A bias in sex ratio might be due to environmental changes that interfere in the sex determination system, sex-biased mortality, divergent sexual behavior, growth rate, and longevity expectation (Conover and Kynard 1981; Schultz 1996).
The black rockfish, Sebastes schlegelii Hilgendorf, 1880 (Scorpaeniformes: Scorpaenidae: Sebastes), inhabits rocky reefs in the coastal waters of the North Pacific Ocean (Sasaki 2003). It is commercially important, and is an important cultured species for stock enhancement in Japan, Korea, and China (Yoshida et al. 2005; An et al. 2009; Liu et al. 2014). In Japan and Korea, artificially raised S. schlegelii juveniles have been released to enhance fisheries production since the 1980s and middle 1990s, respectively (Yoshida et al. 2005; An et al. 2009), and in China the S. schlegelii stock enhancement started in 1995. In 2010, 3.85 million S. schlegelii juveniles were released in the coastal region of Shandong Province, China (unpublished data), a number that decreased to approximately 1 million juveniles in 2012 (Lü et al. 2014). So far, most studies performed on S. schlegelii have focused on genetic diversity, immunity, growth, protein utilization, and gene function (Lee et al. 2000; Kim et al. 2014; Song et al. 2015). Although a few research papers have dealt with its biological characteristics, including LLRs, LWRs, and condition factor (Xue et al. 2011; Wang et al. 2013, 2016), the information is still incomplete and limited to the fish inhabiting a few areas within the Yellow Sea. Although it is known that biological characteristics may vary temporarily and spatially, there is a lack of comprehensive seasonal biological data for male and female S. schlegelii. Lidao Bay is a typical costal bay located in the northern coast of the Yellow Sea, Shandong Province, China. S. schlegelii is one of the commercially important dominant fish species in this area but there is no biological information about this species in Lidao Bay. Hence, the present study aimed to provide baseline biological data on LWRs, LLRs and condition factors of male and female S. schlegelii populations inhabiting Lidao Bay, which will be useful for the fishery and stock enhancement of this species.
Materials and methods
S. schlegelii specimens were sampled in the lower section of the Lidao Bay, central Yellow Sea, China (between 37°12′-37°24′ N and 122°33′-122°48′ E). Sampling was conducted seasonally in February, May, August and November at each location, from spring 2011 to spring 2015, using stake net. All captured fish were immediately placed on ice and transported to the laboratory for identification, according to Cheng and Zheng (1987), and measurement. All specimens were sexed by visual inspection of the gonads (Baba et al. 2005) and categorized into three groups: males, females, and combined sexes. Total length (TL) and standard length (SL) of each specimen were measured to the nearest 0.1 cm, and total weight (TW) was recorded to the nearest 0.01 g.
Length-weight relationships were calculated using the equation W = aLb (Ricker 1975), where W is the total weight of the fish in g, L is the total length of the fish in cm, a is the intercept, and b is the slope of the above linear regression. This equation was logarithmically transformed into W = log a + b log L, which fitted a least squares regression using W as the dependent variable. The parameters a and b were estimated by linear regression, after the logarithmic transformation of weight and length data. Slope b provides valuable information on fish growth, which is isometric when b = 3, positive allometric when b > 3, and negative allometric when b < 3 (Morey et al. 2003). Log-log plots of length-weight pairs were performed to exclude extreme outliers (Froese 2006) before regression analysis. Additionally, 95% confidence limits (CL) were estimated for a and b, along with the coefficient of determination (r 2) in LWRs. A similar linear regression was also used to determine LLRs. Fulton’s condition factor was calculated according to K = (W/L3) × 100.
The three indices, LWRs, LLRs, and K, were calculated for each length class and season for males, females, separately, and for all specimens combined. A Chi-square test helped to identify the sex-ratio (male, M: female, F) using SPSS 16.0. All statistical analyses were considered significant for P < 0.05.
Results
Among the 729 specimens of S. schlegelii collected in Lidao Bay from 2011 to 2015, 407 were males (55.83%) and 322 were females (44.17%). The sex ratio of the total population was 1:0.79 (M:F), which was significantly different (P < 0.05). Sex ratio varied among seasons (1:0.62 to 1:1.39), showing a predominance of males in spring, summer, and autumn. A summary of the data is shown in Table 1. When individuals were classified according to TL (Fig. 1), males were more abundant than females in all TL classes between 5 and 20 cm, with sex ratios of 1:0.75 in the 5–10 cm class, 1:0.71 in the 10–15 cm class, and 1:0.81 in the 15–20 cm. Female proportion gradually increased with increasing length and for TL > 20 cm there were more females than males.
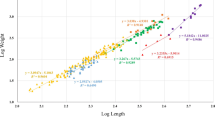
All the parameters estimated for the LWRs of S. schlegelii in Lidao Bay are shown in Table 2. All length-weight regressions were significant (P < 0.05), with r 2 values ranging from 0.951 to 0.991, and slopes (b) ranging from 2.947 to 3.277, with a mean value of 3.169. Thus, LWRs indicated a positive allometric growth in both males (W = 0.0086 L3.234, r 2 = 0.979) and females (W = 0.0089 L3.217, r 2 = 0.981) (Fig. 2). Seasonal evaluation of LWRs evidenced that b varied from 3.039 (spring) to 3.277 (autumn) in males, and from 2.947 (spring) to 3.229 (summer) in females.
The LLRs estimated for S. schlegelii in Lidao Bay, along with their estimated parameters and r 2 values, are given in Table 3. All LLRs were significant (P < 0.05), presenting r 2 values greater than 0.950 and b ranging from 0.797 to 0.885.
K values ranged from 0.791 to 2.981 in males (n = 407) and from 0.752 to 2.681 in females (n = 322). Within each season, there were no significant differences in K values between males and females. In males, the lowest average K was found in autumn (K = 1.459) and the highest in spring (K = 1.846); in females, the lowest average K was found in summer (K = 1.463) and the highest in spring (K = 1.849) (Fig. 3). Average K values in relation to size class are shown in Fig. 4 and, overall, males and females showed a similar trend. Values increased with increasing TL (5–25 cm TL) in both males and females, and maximum values were observed in the 20–25 cm TL. The lowest value was observed in the 5–10 cm TL class in both males and females and individuals of both sexes > 25 cm TL displayed a downward trend in K.
Discussion
Fish LWRs are affected by a series of factors including season, habitat, gonad maturity, diet, stomach fullness, and sex (Tesch 1971; Bagenal and Tesch 1978). Some fish species exhibit dimorphic growth according to sex, so their LWRs might be significantly different between sexes. In such cases, LWRs should be presented separately for males, females, and both sexes (Froese 2006). The value of a may vary daily, seasonally, or between habitats, but the value of b does not vary significantly throughout the year (Bagenal and Tesch 1978) generally ranging from 2.5 to 3.5 in LWRs (Froese 2006). In fact, the use of length-weight relationships should be strictly limited to the length ranges used in the linear regression. In the present study, all b values were within the expected normal range, so LWRs can be used to predict S. schlegelii weight in the length range 5–35 cm, which was the range of TLs sampled within this study. The values of b obtained in this study revealed a positive allometric growth for all sample categories within each season, except for females in spring (Table 2), indicating an increasing in relative body thickness or plumpness. In this study, b was lower on spring than on other seasons, especially for females. These low values are probably related to black rockfish’s viviparous reproduction in which gestation starts in April and parturition occurs in June (Mori et al. 2003). Thus, some spring specimens might be collected just after spawning season, which might be the reason why spring had lower b values than other seasons. Wang et al. (2013) and Xue et al. (2011) also reported a positive allometric growth for S. schlegelii in Haizhou Bay (n = 334) and Jiaozhou Bay (n = 147), which are located along the central coast of the Yellow Sea, China (Table 4). For S. schlegelii inhabiting Korea’s south coast, the value of b was 3.01, considering all individuals (n = 322), and 2.98 for females and 3.02 for males (n = 140 and n = 182, respectively; Baeck et al. 2012).
Condition factor is a useful index for the monitoring of feeding intensity and growth rates in fish (Oni et al. 1983). It is strongly influenced by both biotic and abiotic conditions and can therefore be used as an index to assess the status of the aquatic ecosystem in which fish live (Anene 2005). The K values of all specimens were constant during summer, autumn, and winter, and increased in spring, possibly due to high food availability and increased water temperatures. While, females K decreased after parturition in June (early summer). Females tend to have larger and heavier gonads, which would increase their K in comparison to males of identical length, but average K values were not significantly different between sexes. The lack of seasonal data on prey availability prevents us from examining K variation relative to food-production cycles. As there are no other studies on S. schlegelii K, the data presented here constitute the baseline for future work regarding condition factors’ variation in relation to biotic and abiotic variables.
Understanding variation in sex ratio is especially important, as it is a key parameter of population fecundity. Unbalanced sex ratios can drive sexual selection, affect mating system, and influence population persistence and conservation status, owing to the effect of sex ratio on effective population size (Emlen and Oring 1977; Clutton-Brock 2007). The overall sex ratio of S. schlegelii (1:0.79, M:F) significantly deviated different from the expected 1:1. Significant deviations were also found in other fish species of the genus Sebastes, but with no consistent trend. In southern Newfoundland waters, the overall M:F ratio in beaked rockfishes (S. fasciatus and S. mentella) was 1:0.94, and in the golden redfish S. marinus was 1:1.33 (Ni and Wilfred 1985). In the Gulf of Alaska, S. polyspinis M:F ratio was nearly 1:1, while females predominated in the Aleutian Islands (57 females to 43 males) (Clausen and Heifetz 2002). Seasonal and size class variations in sex ratio found in the present study indicated sexual segregation in S. schlegelii according to both variables. Males predominated in spring, summer, and autumn, and there was an increase in female abundance in winter, which might be due to vitellogenesis being completed in March (Mori et al. 2003).
Examining sex ratios across TL classes showed that female proportion gradually increased with increasing length, which was consistent with Baba et al. (2005) results. For S. schlegelii with TL ranging from 5 to 20 cm, there were more males than females, but for TL > 20 cm, female ratio increased. This increase in female ratio in relation to body size might be due to different longevity or growth rates between sexes (Schultz 1996). S. schlegelii females are known to grow faster than males, and their asymptotic mean length is also larger than that of males (Baba et al. 2005). Variations in sex ratio at different growth stages or ages were documented in other rockfishes, such as S. melanops. The representation of S. melanops females in age categories falls from about 50% to 10–20% from 10 to 20 years-old fish (Ralston and Dick 2003). Sex ratio bias could be caused by several mechanisms, such as offspring sex ratio, sex differences in mortality and migratory rates, and differ according to age at maturity (Donald 2007). Thus, in addition to hereditary factors, the sex ratio of S. schlegelii might be influenced by environmental factors including temperature and toxicants, which either affect sex determination or induce sex-biased mortality. In fish, both males and females might be the least represented sex (Székely et al. 2014). Increased male mortality is typically associated with mate search and courtship or with male-male competition for territories and mating opportunities (Le Boeuf 1974; Lodé et al. 2004). A recent meta-analysis concluded that reports on biased sex ratios may often be false due to small sample size or sampling bias (Wehi et al. 2011), which will erroneously make one sex appear more abundant than another. However, the lack of adequate information about S. schlegelii sex ratio in other populations restrains further considerations, indicating that more studies are required to confirm a sex ratio bias in this species.
References
An HS, Park JY, Kim MJ, Lee EY, Kim KK (2009) Isolation and characterization of microsatellite markers for the heavily exploited rockfish Sebastes schlegeli, and cross-species amplification in four related Sebastes spp. Conserv Genet 10:1969–1972
Anene A (2005) Condition factor of four Cichlid species of a man-made lake in Imo State, Southeastern Nigeria. Turk J Fish Aquat Sci 5:43–47
Baba K, Sasaki M, Mitsutani N (2005) Estimation of age composition from length data by posterior probabilities based on a previous growth curve: application to Sebastes schlegelii. Can J Fish Aquat Sci 62:2475–2483
Baeck GW, Jeong JM, Yeo YM, Huh SH, Park JM (2012) Length-weight and length-length relationships for 10 species of scorpionfishes (Scorpaenidae) on the south coast of Korea. J Appl Ichthyol 28:677–679
Bagenal TB, Tesch FW (1978). Age and growth In: Bagenal T (eds) Methods for assessment of fish production in Fresh waters, 3rd edn. IBP Handbook No. 3. Blackwell Science Publications, New York, p 101–136
Cheng Q, Zheng B (eds) (1987) Systematic synopsis of Chinese fishes. Science Press, Beijing, pp 460–463
Clausen DM, Heifetz J (2002) The northern rockfish, Sebastes polyspinis, in Alaska: commercial fishery, distribution, and biology. Mar Fish Rev 64:1–28
Clutton-Brock T (2007) Sexual selection in males and females. Science 318:1882–1885
Conover DO, Kynard BO (1981) Environmental sex determination: interaction of temperature and genotype in a fish. Science 213:577–579
Donald PF (2007) Adult sex ratios in wild bird populations. Ibis 149:671–692
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Froese R (2006) Cube law, condition factor and weight-length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22:241–253
Garcia CB, Duarte JO, Sandoval N, Schiller DV, Melo G, Navajas P (1998) Length-weight relationships of demersal fishes from the Gulf of Salamanca, Colombia. Naga 21:30–32
Hardy ICW (2002) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge
Kim HN, Chae YS, Shim WJ, Park CI, Jung JH (2014) Combined effects of Iranian heavy crude oil and bacterial challenge (Streptococcus iniae) on biotransformation and innate immune responses in rockfish (Sebastes schlegeli). Bull Environ Contam Toxicol 93:199–203
King RP (1996) Length-weight relationships of Nigerian coastal water fishes. Naga ICLARM Q 19:53–58
Le Boeuf BJ (1974) Male-male competition and reproductive success in elephant seals. Am Zool 14:163–176
Lee SM, Hwang UG, Cho SH (2000) Effects of feeding frequency and dietary moisture content on growth, body composition and gastric evacuation of juvenile Korean rockfish (Sebastes schlegeli). Aquaculture 187:399–409
Liu H, Xu Q, Xu Q, Zhang Y, Yang H (2014) The application of stereo-video technology for the assessment on population change of black rockfish Sebastes schlegeli in a vessel reef area in Haizhou Bay, China. Chin J Oceanol Limnol 33:1–7
Lodé T, Holveck MJ, Lesbarreres D, Pagano A (2004) Sex-biased predation by polecats influences the mating system of frogs. Proc R Soc B Biol Sci 271:399–401
Lü H, Zhang X, Xi D, Gao T (2014) Use of calcein and alizarin red S for immersion marking of black rockfish Sebastes schlegelii juveniles. Chin J Oceanol Limnol 32:88–98
Martin-Smith KM (1996) Length/weight relationships of fishes in a diverse tropical fresh-water community, Sabah, Malaysia. J Fish Biol 49:731–734
Morey G, Moranta J, Massutí E, Grau A, Linde M, Riera F, Morales-Nin B (2003) Weight-length relationships of littoral to lower slope fishes from the western Mediterranean. Fish Res 62:89–96
Mori H, Nakagawa M, Soyano K, Koya Y (2003) Annual reproductive cycle of black rockfish Sebastes schlegeli in captivity. Fish Sci 69:910–923
Moutopoulos DK, Stergiou KI (2002) Length-weight and length-length relationships of fish species from the Aegean Sea (Greece). J Appl Ichthyol 18:200–203
Muchlisin ZA, Musman M, Siti Azizah MN (2010) Length-weight relationships and condition factors of two threatened fishes, Rasbora tawarensis and Poropuntius tawarensis, endemic to Lake Laut Tawar, Aceh Province, Indonesia. J Appl Ichthyol 26:949–953
Ni I-H, Wilfred T (1985) Reproductive cycles of redfishes (Sebastes) in southern Newfoundland waters. J Northwest Atl Fish Sci 6:57–63
Oni SK, Olayemi JY, Adegboye JD (1983) Comparative physiology of three ecologically distinct fresh water fishes, Alestes nurse Ruppell, Synodontis schall Bloch and S schneider and Tilapia zilli Gervais. J Fish Biol 22:105–109
Ralston S, Dick EJ (2003) The status of black rockfish (Sebastes melanops) off Oregon and northern California in 2003. Pacific Fishery Management Council, Portland, p 7
Richter HC, Luckstadt C, Focken U, Becker K (2000) An improved procedure to assess fish condition on the basis of length-weight relationships. Arch Fish Mar Res 48:255–264
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Department of Environment, Fisheries and Marine Service, Ottawa, p 382
Sasaki M (2003) Black rockfish. In: Mizushima T et al (eds) Fisheries and aquatic life in Hokkaido (in Japanese). Hokkaido Shimbun, Hokkaido, pp 188–193
Schultz H (1996) Drastic decline of the proportion of males in the roach (Rutilus rutilus L) of Bautzen Reservoir (Saxony, Germany): result of direct and indirect effects of biomanipulation. Limnologica 26:153–164
Song H, He Y, Ma L, Zhou X, Liu X, Qi J, Zhang Q (2015) Characterisation of kisspeptin system genes in an ovoviviparous teleost: Sebastes schlegeli. Gen Comp Endocrinol 214:114–125
Székely T, Weissing FJ, Komdeur J (2014) Adult sex ratio variation: implications for breeding systems. J Evol Biol 27:1500–1512
Tesch FW (1968) Age and growth. In: Ricker WE (ed) Methods for assessment of fish production in fresh waters. Blackwell Scientific Publications, Oxford, pp 93–123
Tesch FW (1971) Age and growth. In: Ricker WE (ed) Methods for assessment of fish production in fresh waters. Blackwell Scientific Publications, Oxford, pp 99–130
Wang X, Xue Y, Ren Y (2013) Length-weight relationships of 43 fish species from Haizhou Bay, central Yellow Sea. J Appl Ichthyol 29:1183–1187
Wang LJ, Wu ZH, Nie MM, Liu MX, Liu W, You F (2016) Length–weight relationships and length–length relationships of 13 fish species in Rongcheng Bay, China. J Appl Ichthyol 32:737–739
Wehi PM, Nakagawa S, Trewick SA, Morgan-Richards M (2011) Does predation result in adult sex ratio skew in a sexually dimorphic insect genus? J Evol Biol 24:2321–2328
Xue Y, Ren Y, Xu B, Mei C, Chen X, Zan X (2011) Length-weight relationships of fish species caught by bottom trawl in Jiaozhou Bay, China. J Appl Ichthyol 27:949–954
Yoshida K, Nakagawa M, Wada S (2005) Multiplex PCR system applied for analysing microsatellite loci of Schlegel’s black rockfish, Sebastes schlegeli. Mol Ecol Notes 5:416–418
Acknowledgments
This research was funded by the National Special Research Fund for Non-Profit Marine Sector (No. 201305043), Special Program for Basic Research of the Ministry of Science and Technology of China (No. 2014FY110500), the Scientific and Technological Innovation Project Financially Supported by Qingdao National Laboratory for Mairne Science and Technology (No. 2015ASKJ02), the National Key Basic Program of Science and Technology-Platforms of Aquaculture Stock Resources and the IOCAS funding (No. 2012IO060102). We would like to express many thanks to all colleagues and students in our laboratory for their assistance in field sampling and sample analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, L., Wu, Z., Liu, M. et al. Length-weight, length-length relationships, and condition factors of black rockfish Sebastes schlegelii Hilgendorf, 1880 in Lidao Bay, China. Thalassas 33, 57–63 (2017). https://doi.org/10.1007/s41208-017-0021-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-017-0021-6