Abstract
The present study was aimed to survey the eastern Red Sea region of Saudi Arabia for selecting suitable cage farming sites. Nine stations along the Red Sea coast were selected for this study. Water, sediment, and biological samples were collected from the stations for the analysis of physical, chemical, and biological parameters. Results indicate that the environmental parameters such as water temperature, salinity, pH, and dissolved oxygen content of the coastal waters were within the normal range reported for a suitable cage fish farming site. Most of the stations are located near to residential or industrial areas and hence not suitable for a cage farming project. Two stations which are far away from the industrial and residential areas and showed optimal environmental conditions are recommended for possible cage farming after further feasibility studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Saudi Arabia has a tremendous potential for aquaculture sector, and the Ministry of Agriculture has identified aquaculture for intense focus and proposes to develop it in all coastal and interior areas. Aquaculture development in Saudi Arabia started during 1980 with the establishment of fish culture project at the Saudi Arabian National Center for Science and Technology (now, King Abdulaziz City for Science and Technology). A national Fish Farming center was then started in Jeddah during 1982. These institutions provided extension services to the increasing population of local fish farmers. The demand for fresh fish shows an increasing trend even though it is not a true stable diet in Saudi Arabia. A major portion of Saudi Arabian fish supplies is provided by marine capture fisheries. The capture fisheries played a major role in sea foods which increased from 49,080 t in 2000 to 68,000 t in 2008. However, commercially important species such as groupers, snappers, emperors, Spanish mackerel, and tunas have recorded a reduction or stagnation in landings due to overfishing by traditional fisheries (Jin et al. 2012). There are also reports of increasing catches per unit effort (Tharwat and Al-Gaber 2006). This restricted supply may become more severe based on United Nations’ estimation of a 60 million population by 2050. Aquaculture production has been increasing at a rate of 25 % per annum over the last 10 years, whereas fisheries recorded only a 7 % increase. The combined fish production at 10 % per annum is still inadequate. Thus, the kingdom currently imports 70 % of its annual seafood requirements. The kingdom’s total seafood imports for 2007 were 150,378 t with a total value of SAR899 million (USD240 million).
Due to the increase in demand for fish in Saudi Arabia and the reduction of fish stocks in the world’s oceans, there is a need for the development of the aquaculture sector to meet the food production. Among the different aquaculture systems, offshore cage culture has been recommended for increasing Saudi Arabia’s aquaculture production for various reasons. This includes increasing the competition faced by the sector for available resources, the need for economies of scale, and the drive for increased productivity per unit area. The marine, commercial cage culture was pioneered in Norway with the development of salmon farming (Beveridge 2004). The cage aquaculture sector has reported to a rapid growth during the past two decades (Tilman et al. 2002; Foley et al. 2005). Globally, the decline of fish stocks has been a motivating factor for expanding the role of aquaculture in the fishing industry (Baldwin et al. 1999). Nowadays, the trend demonstrates that while the capture fisheries remain stable (or is in decline, in some cases), production from aquaculture sector has increased (FAO 2002). In this case, the system of cage farming (mariculture) has had an important role in meeting the global demand for fish products (Fredriksson et al. 1999). Open sea cage culture technology is developing rapidly in order to maximize the utilization of area available off the coast.
Selection of suitable site is the key factor for any aquaculture practice for success and sustainability (Perez et al. 2003). To start an aquaculture project, it is necessary to consider geographic regions with adequate water quality and exchange (Perez et al. 2003). Cage farming, like other methods, need good water quality, i.e., the site should be far away from the industrial units, major cities, possible agricultural input areas, harbors, etc. The water quality parameters such as temperature, salinity, pH, turbidity, dissolved oxygen, and concentration of nutrients should be within the normal range to support the growth of cultured species (Perez et al. 2003). Also, it is necessary to observe the regulations established by authorities for any installations in coastal zones. Fish cages represent installations based on offshore and must satisfy some requirements in order to maintain a good relationship with the natural environment (Uriarte Villalba 2001). Physical restrictions on open sea cage farming include wave conditions of certain coastal zones, lack of adequate current to replenish the water inside the cages, or strong currents that may affect the cage structures (Turner 2000). Another aspect to be considered is the existence of pollutant sources in the coastal zones which can cause low growth or health problems in cultured organisms. The accumulation of organic materials will lead to the formation of H2S which is lethal to the aquaculture species (Pillay 1992).
Previously, experiments were conducted on the production of rabbit fish Siganus rivulatus in floating cages at the fish farming center, Jeddah coast of Saudi Arabia (Lichatowich et al. 1984; Bukhari 2005). In this study, we have carried out a feasibility study for selecting suitable cage farming sites along the eastern Red Sea coast of Saudi Arabia. We have collected samples from the coastal sites for the analysis of physical, chemical, and biological parameters. The sampling strategy comprises a series of procedures aimed at selecting the most favorable locations and determining the appropriate site for cage culture. As specific site details suitable for cage farming along the eastern Red Sea coast of Saudi Arabia are lacking at present, details collected from this preliminary study will be useful for the marine aquaculture industry in Saudi Arabia. As this study is a preliminary one, further investigation on hydrographical parameters will add more information for selecting a suitable cage aquaculture site in the Saudi Arabian Red Sea coast.
Materials and Methods
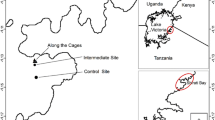
The study was conducted along the eastern Red Sea region of Saudi Arabia from May to September 2013. Nine stations along the Red Sea coast were selected for this study (Fig. 1). The sampling was carried out during May 2013 at station 1 and 2, in June 2013 at station 3 and 4, and September 2013 at stations 5–9. The samples from all the stations were collected during the summer season (mid-May–mid-September) to avoid seasonal changes in environmental parameters.
Station 1 is located on the East coast of the Red Sea in Saudi Arabia near to Rabigh, approximately about 150 km North of Jeddah (22.797386° N and 38.957306° E). Station 2 is near to Thuwal (22.271194° N and 39.059° E), a fishing village belonging to the governorate of Jeddah in the Makkah Province of Saudi Arabia about 80 km north of Jeddah on the coast of the Red Sea. Thuwal had long been a fishing center until the Royal Saudi Navy re-constructed the village. This region is experiencing an increase in business activities due to its proximity to the King Abdullah Economic City. Station 3 is on the South Corniche of the Red Sea approximately 25 km south of Jeddah (21.319242° N 39.102203° E). The near shore zone, extending between the shoreline and the fringing reef, is a shallow water area of small but variable depth and width. The bottom is rocky and is composed of hard reef limestone structures covered by a more or less thin layer of unconsolidated reef sediments. Station 4 is located near to the Albudhae coast guard center of the Red Sea, approximately 35 km south of Jeddah (21.243389° N 39.145528° E).
The station 5 is located about 40 km south of Jeddah (21.116111° N and 39.193222° E). The nearest land mark for this station is the Sarom coast guard center. This station is also near to the industries such as SAWACO seawater desalination plant and also Suido Kiko Middle East.
Station 6 is located near to Al Shuaibah, on the Red Sea approximately 70 km south of Jeddah (20.915° N and 39.2601° E). This region contains a variety of marine organisms, including bivalves and algae. A portion of the bottom surface of the soil rigid bottom and the other part is sandy clay. Station 7 is selected near to Al Masnaa coast guard center of the Red Sea, located approximately 245 km south of Jeddah (19.974° N and 40.5266° E) and south of Al Lith city.
Khawr Al Zawher lagoon of the Red Sea, located about 290 km south of Jeddah (19.7977° N and 40.7828° E), is selected as station 8. This area is protected from waves and good diversity of marine organisms, including algae, seagrass, and mangroves. Station 9 is located near to Ras Mohaisen head coast guard center of the Red Sea about 320 km south of Jeddah (19.6964°N and 40.8136°E). This area also possesses a good diversity of algae, seagrass, and mangroves. It is protected from strong waves due to the mangroves.
Analysis of Physical, Chemical, and Biological Parameters of the Coastal Waters
The physical, chemical, and biological parameters of the selected sites were assessed in this study. All the measurements and samplings were done at the average water depth of 1 m to maintain uniformity between the sampling stations. The measurements and samples were taken in replicates (n = 3) in each station, and average values were considered. The surface water temperature, salinity, pH, and dissolved oxygen content were measured in situ. The salinity was measured using a Refractometer and the other parameters were measured with the help of a portable dissolved oxygen probe (Hana) and pH meter (Hana). For the estimation of nutrients, surface water samples were collected in plastic bottles (2 L), kept in an icebox, and brought to the laboratory. In the laboratory, water samples were analyzed phosphate, nitrate, nitrite, ammonia, and chlorophyll “a” and “b” concentration. All these parameters were analyzed according to the standard methods compiled by Venugopalan and Paulpandian (1989).
Sediment samples were also collected from all the sites (at 1 m depth) using an Ekman grab sampler (15 × 15 cm, with approximate sample volume of 3 L). The collected sediment samples were divided into two parts (equal volume in each grab sample), and one part was used for the analysis of metal concentration, chlorophyll, and benthic invertebrate communities. The other part was subjected to mechanical analysis to grade grain size. For this, sediment samples were dried in an oven and sieved through a series of mesh sizes and graded according to Krumbein and Sloss (1963).
Element Analysis in Water and Sediment Samples
The concentration of elements such as Cd, Cu, Fe, Mn, Pb, and Zn in water and sediment samples was analyzed. The sediment samples were dried in an oven at 80 °C. The metals were extracted from the sediment samples according to Binning and Baird (2001) and were detected using ICP Optical Emission Spectrometer (Varian 720-ES). The metal concentration is water samples were extracted according to Anoop et al. (2007) and analyzed using ICP Optical Emission Spectrometer (Varian 720-ES).
Benthic Invertebrate Community Analysis
Sediment samples collected were stored in the refrigerator and sieved no later than 1 week following collection. For samples containing dense clumps of silt or clay, the sample bag was filled with water and soaked for a few hours prior to sieving. The sediment samples were sieved using 0.5 mm mesh, and the invertebrate groups visible on the sieve were collected in a separate vial. The invertebrates were identified up to the lowest taxonomic level wherever possible using manuals and field guides.
Results
Physical, Chemical, and Biological Parameters of the Coastal Waters
The salinity, dissolved oxygen content, pH, and temperature of the coastal waters during the study period are given in Table 1. The salinity of the seawater collected from selected stations varied between 39 and 44 (Table 1). The dissolved oxygen content of the coastal waters was low at station 3 (2.4 mg l−1) and high in station 1 (7.17 mg l−1). The temperature of the seawater showed a range of 30.8 and 34.3 °C, and the pH varied between 7.9 and 8.4 (Table 1). In general, the dissolved oxygen content was above 5 mg l−1 and salinity was below 40 psu at stations 8 and 9.
The total ammonia, nitrate, nitrite, phosphate, and chlorophyll a and chlorophyll b content of the seawater collected from the nine stations during this period is given in Table 2. The ammonia content was high at station 8 and station 9 and low in station 7 (Table 2). The nitrite concentration of the coastal waters varied between 0.195 and 0.2284 μg l−1. The lowest value was observed at station 9 and the higher value was at station 4. The nitrate content was high at station 1 (1.073 μg l−1) and low in station 8 (0.041 μg l−1). The phosphate concentration of seawater varied between 0.0021 (station 1) and 1.241 μg l−1 (station 9) during this study period. The chlorophyll a content of the seawater was high at station 7 and low in station 6.
Metal Concentration in Seawater
The concentration of elements such as cadmium, copper, iron, manganese, lead, and zinc in the coastal waters are presented in Table 3. The cadmium concentration varied between 0.21 and 7.86 μg l−1. The highest concentration was measured from the water samples collected at station 1 and the lowest was in station 8. Cadmium content in the seawater collected from the station 9 was below detectable limits. The concentration of copper in water samples showed a range between 4.067 and 52.094 μg l−1 during the study period. The values were low at stations 8, 1, and 9 high at stations 2 and 3. The iron content of the coastal waters showed a higher value of 5080.371 mg l−1 at station 2 and lower value of 198.211 μg l−1 at station 6. The Mn concentration in coastal waters was high at station 2 and low at station 1 and station 3. The coastal waters of the study area showed the lead content range of 2.341 and 17.468 μg l−1. The Pb content was below detectable limit from the stations 5 to 9. The Zn content showed a higher value of 93.252 μg l−1 at station 3 and lower content of 7.49 μg l−1 at station 1.
Benthic Community Composition
The marine invertebrate communities of the study region mainly comprise of gastropods, bivalves, and polychaetes (Table 4). Gastropods were the dominant groups in most of the stations except station 6. In station 6, bivalves and polychaetes were more abundant than other groups during the sampling period. The gastropods abundance showed a maximum of 673 ind. m−2 at station 3 and the minimum of 10 ind. m−2 at station 7. Polychaetes abundance was high at station 6 and all the other stations, only low numbers were observed. Bivalves showed a maximum abundance of 185 ind. m−2 at station 6 and were not found at stations 1 and 2. The other common invertebrate groups observed in the study area include amphipods, crabs, and echinoderms (Table 4).
Sediment Analysis
Chlorophyll Content in Sediment
The chlorophyll a and chlorophyll b content of the sediment samples collected from the study stations are given in Table 2. Chlorophyll a content was high at the station 7 and low in station 4. The chlorophyll a content of sediment samples collected from station 8 and station 9 were found to be 2.711 and 1.672 mg kg−1. The chlorophyll b content varied between 1.236 and 3.15 mg kg−1. The highest value was observed in station 7 and the lowest was at station 4.
Metal Analysis
The concentration of major elements such as cadmium, copper, iron, manganese, lead, and zinc in the sediment samples are given in Table 5. The cadmium content was detected in the sediment samples collected from only three stations such as stations 3, 4, and 5. The station 3 recorded a higher cadmium content of 1.831 μg g−1. The copper content was high at station 7 (27.562 μg g−1) and low in station 5 (3.1 μg g−1). The Fe content of the sediment samples varied between 17581.68 and 126.78 μg g−1. Mn content of the sediment was high at the station 7 and low in station 4. The sediment samples collected from station 7 showed higher lead content, and the lower value was observed at station 9. The zinc content of the sediment varied between 3.954 and 0.36 μg g−1. The highest value was observed at station 2, and the lower content was in station 6.
Grain Size Analysis
In the present study, sediment samples were categorized into very coarse sand, coarse sand, medium sand, fine sand, and very fine sand. The grain size of the sediment samples collected from the study stations are given in Table 6. The percentage of very coarse sand in the sediment varied between 12.084 and 3.657. The coarse sand percentage showed a range of 16.987 and 4.407 in the study area. The medium sand composition in the sediment samples varied between 25.984 and 6.448. The fine sand percentage was high at station 6 (48.677) and low in station 3 (23.982). The percentage of very fine sand composition in the sediment samples varied between 47.644 and 9.308.
Discussion
The environmental parameters such as water temperature, salinity, pH, and dissolved oxygen content of the coastal waters were within the normal range reported for the Red Sea (Al-Farawati 2010). Generally, most of the tropical species prefer the normal salinity strength of the seawater and they cannot survive in low salinities such as below 15 psu (Loka et al. 2012). The suitable pH for most of the species is between 7.5 and 8.5, and extreme pH values can directly affect gill surfaces and leading death (McDonald 1983). Also, the ammonia level should be less than 0.5 ppm in the area. This indicates that the water samples collected from the study region showed the values within the acceptable levels for a cage farming site.
Phosphorus is a limiting nutrient necessary for the growth of algae and other aquatic plants (Prema 2013). This indicate that excess amount of phosphorus content in the coastal waters may enhance the algal growth leading to algal blooms. Normally, the total phosphorus content of the marine waters for fish farming should be <0.015 mg l−1 (Prema 2013). In the present study, phosphate concentration of seawater showed a range of 0.0021 and 1.241 μg l−1 indicating that the total phosphorus content is at the optimum level for cage farming. The total inorganic nitrogen content of the seawater suitable for aquaculture is <0.1 mg l−1. For a cage farming site, the nitrate and nitrite content of the coastal waters should not exceed 4 and 200 mg l−1, respectively (Tiensongrusmee 1986). Results showed that the nitrite and nitrate content of the water samples collected from the study sites were <0.1 mg l−1 indicating the suitability of the sites for cage farming.
Heavy metals and other trace elements originate mainly from industrial and anthropogenic pollutants. Hence, the sites near to industrial sites are normally avoided for fish farming activities. Water samples collected from the study stations for metal analysis showed higher values near the industrial zones compared to others. The toxicity of most of the metals depends mainly on the other factors such as pH, hardness, and alkalinity of the seawater (Prema 2013). The concentration of copper and lead in the seawater of the culture site should be below 0.1 and 0.02 mg l−1. Results indicated that the metal concentration of the seawater of the study stations is within the normal range reported for fish farming. For cage culture, a firm substrate with a combination of fine gravel, sand, and clay will be highly productive (Loka et al. 2012). Mud or rock bottom may cause difficulties for the safe anchorage of cages. From the results, it is obvious that the bottom of all the stations are sandy with largest proportion of fine sand.
As the physical, chemical, and biological parameters did not show much variation between the stations except few cases, it is necessary to observe the other factors for site selection. For example, most the stations are located near to industrial units or residential areas. Mangroves are widespread in station 2 especially Avicennia marina. Also, good diversity of seaweeds and few seagrass species are observed in the region. Station 1 has several industries such as a factory of Arabian Cement, an electricity station that supports Makkah and Madinah, and a large refinery belonging to Saudi Aramco. The refining operations in Rabigh have increased significantly as Aramco, in joint venture with Sumitomo Chemical of Japan, has built a new petrochemical plant, Petro Rabigh. Due to the presence of large industrial units, this site is not appropriate for cage culture in commercial scale. Station 2 (Thuwal) is also considered as a fast developing city due to the increased construction activities. Biodiversity of this area is good with the presence of seagrass and mangroves. Sediment color is black may be due to the presence of high organic matter. While topography of the site looks suitable for selection of cage culture, this region is not suitable due to conflict with industries and recreational users.
The station 3 area of Jeddah receives through Al-Kumra effluent equivalent of 300,000 m3 of semi-treated sewage (Basaham et al. 2009). Due to the pollution and the presence of industrial units in nearby regions, this area is also not suitable for aquaculture practices. The station 4 is noticed with fishing activities. Mangroves, mainly Avicennia marina, are abundant in this region. Due to the possible conflict with other users particularly fishing, this area is not recommended for cage culture. The station 6 region showed abundance of marine organisms, including algae and seagrass. However, desalination plant and power station located in this region are one of the major criteria to exclude this site for cage culture. Station 5 also receives industrial waste through SAWACO seawater desalination plant and also Suido Kiko Middle East effluent. The presence of industrial units near this site indicates the possible pollution in this region so not suitable for aquaculture.
The station 8 located near to Khawr Al Zawher lagoon of the Red Sea is protected from waves and showed the good diversity of marine organisms including algae, seagrass, and mangroves. This area is far away from industrial units, and conflicts with other users are limited compared to other regions. Another region suitable for possible cage culture is the station 9 located near to Ras Mohaisen coast guard center.
For a new cage culture project, it is important to know which part of the coastal zone is appropriate. Once a fish farmer selects a suitable site, it is very difficult to assess all conflicts arising with other users. This fact complicates further the selection of suitable sites for cage farming. Eastern Red Sea coast of Saudi Arabia is experiencing large development activities, and most of the coastal zones are not suitable for cage farming due to the possible conflicts with other industries. An ideal cage farming site should have suitable depth, good tidal inflow, and optimal environmental conditions. The site should also be protected from strong winds and rough weather conditions. In the Red Sea, during the summer SW monsoon, northwesterly winds over the entire basin drive a south flowing surface current, and inflow into the basin is constrained to a weak shallow-subsurface current. This subsurface inflow of cold, nutrient-rich water from the Gulf of Aden extends up to 17–18° N (Jones and Browning 1971; Patzert 1972; Murray and Johns 1997; Smeed 1997). The winter inflow of surface waters and especially the July to September subsurface inflow from the Gulf of Aden supply nutrient-enriched waters to the southernmost Red Sea, boosting biological productivity (Souvermezoglou et al. 1989). Also Murray and Johns (1997) observed that the Red Sea outflow transport is strongly seasonal due to monsoon winds and variations in buoyancy fluxes, with a winter maximum of 0.6 Sv (1 Sv = 106 m3/s) and a summer minimum of 0.05 Sv.
In general, the wind velocity should be less than five knots for stationary cages and ten knots for floating cages. For Red Sea, and Gulf, the velocity of the majority of currents experienced in any direction does not exceed one knot, and only on rare occasions does it exceed two knots. Currents are weakest and most varied in all seasons, hydrographic observations and velocity measurements show that surface circulation consists of a series of cyclonic and anticyclonic gyres that disappear and reappear at preferential locations (Quadfasel and Baudner 1993). It is necessary to allow sufficient depth under the cage in order to maximize water exchange and avoid oxygen depletion and the decomposition of deposited wastes (Loka et al. 2012). Hence, it is important to consider these parameters in addition to conflicts with other users when selecting a suitable site for cage farming.
In conclusion, stations 8 and 9 (Khawr Al Zawher and Ras Mohaisen) are identified as suitable sites for cage farming based on the physical, chemical, and biological parameters. The physical and chemical parameters and heavy metal content were at the optimum level in these stations. Another important aspect is both stations are located away from major industrial units and the possible conflicts with others will be minimum compared to other stations. Further studies particularly depth and water current conditions specific to these areas will provide more details necessary for starting cage farming in these regions.
References
Al-Farawati R (2010) Environmental conditions of the coastal waters of Southern Corinche, Jeddah, Eastern Red Sea: physico-chemical approach. Aust J Basic Appl Sci 4:3324–3337
Anoop AK, Krishnakumar PK, Rajagopalan M (2007) Trichodesmium erythraeum (Ehrenberg) bloom along the southwest coast of India (Arabian Sea) and its impact on trace metal concentrations in seawater. Estuar Coast Shelf Sci 71:641–646
Baldwin K, Celikkol B, Steen R, Michelin D, Muller E, Lavoie P (1999) Open ocean aquaculture engineering: mooring & net pen deployment. Mar Technol Soc J 34:53–58
Basaham AS, Rifaat AE, El-Mamoney MH, El Sayed MA (2009) Re-evaluation of the impact of sewage disposal on coastal sediments of the Southern Corniche, Jeddah, Saudi Arabia. Journal of King Abdulaziz University, Marine Sciences 20:109–126
Beveridge M (2004) Cage aquaculture, Third Editionth edn. Blackwell Publishing, London, pp 111–158
Binning K, Baird D (2001) Survey of heavy metals in the sediments of the Swartkops River Estuary, Port Elizabeth South Africa. Water 27:461–466
Bukhari FA (2005) Trials of rabbitfish Siganus rivulatus production in floating cages in the Red Sea. Emirates Journal of Agricultural Sciences 17:23–29
FAO (2002) The state of world fisheries and aquaculture 2002. FAO (United Nations Food and Agriculture Organization. http://www.fao.org/sof/sofia/index_en.htm
Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin SF, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK (2005) Global consequences of land use. Science 309:570–574
Fredriksson D, Swift R, Muller E, Baldwin K, Celikkol B (1999) Open ocean aquaculture engineering: system design and physical modelling. Mar Technol Soc J 34:41–52
Jin DI, Kite-Powell H, Hoagland P, Solow A (2012) A bioeconomic analysis of traditional fisheries in the Red Sea. Mar Resour Econ 27:137–148
Jones EN, Browning DG (1971) Cold water layer in the southern Red Sea. Limnol Oceanogr 16:503–509
Krumbein WC, Sloss LL (1963) Stratigraphy and sedimentation. W. H. Freeman, San Francisco, 660
Lichatowich T, Al-Thobaiti S, Arada M, Bukhari F (1984) Growth of Siganus rivulatus reared in sea cages in the Red Sea. Aquaculture 40:273–275
Loka J, Vaidya NG, Philipose KK (2012) Site and species selection criteria for cage culture. In: Philipose KK, Loka J, Sharma SR, Krupesha, Damodaran, Divu, eds. Handbook on open sea cage culture. Central Marine Fisheries Research Institute, Karwar, India
McDonald DG (1983) The effects of H + up on the gills of freshwater fish. Can J Zool 61:691–703
Murray SP, Johns W (1997) Direct observations of seasonal exchange through the Bab el Mandab Strait. Geophys Res Lett 24:2557–2560
Patzert WC (1972) Seasonal reversal in Red Sea circulation. Publ Cent Natl Exploit Oceans Actes Colloq 2:55–85
Perez OM, Ross LG, Telfer TC, del Campo Barquin LM (2003) Water quality requirements for marine fish cage site selection in Tenerife (Canary Islands): predictive modelling and analysis using GIS. Aquaculture 224:51–68
Pillay TVR (1992) Aquaculture and the environment. Fishing news books. Blackwell Science Publishing Ltd, England, 189p
Prema D (2013) Site selection and water quality in mariculture. CMFRI Bulletin, India, pp 35–43p
Quadfasel D, Baudner H (1993) Grey-scale circulation cells in the Red Sea. Oceanol Acta 16:221–229
Smeed D (1997) Seasonal variation of the flow in the strait of Bab al Mandab. Oceanol Acta 20:773–781
Souvermezoglou E, Metzl N, Poisson A (1989) Red Sea budgets of salanity, nutrients and carbon calculated in the strait of Bab al Mandab during the summer and winter seasons. J Mar Res 47:441–456
Tharwat AA, Al-Gaber AR (2006) Traps (Gargours) in Saudi Territorial Waters of the Arabian Gulf. Journal of King Abdulaziz University: Marine Sciences 17:13–31
Tiensongrusmee B (1986) INS/81/008/Manual/1 - Site selection for the culture of marine finfish in floating netcages.
Tilman D, Cassman KG, Matson PA, Naylor R, Polasy S (2002) Agricultural sustainability and intensive production practices. Nature 418:671–677
Turner R (2000) Offshore mariculture: site evaluation. Options méditerranéennes Série B 30:141–157
Uriarte Villalba A (2001) Environmental considerations for site selection of marine fish farms. In: Uriarte A, Basurco B (eds) Environmental impact assessment of Mediterranean aquaculture farms. CIHEAM, Zaragoza, pp 67–74, Cahiers Option s Méditerranéenn es; n . 55
Venugopalan VK, Paulpandian AL (1989) Methods in hydrobiology. Annamalai University, C.A.S. in Marine Biology, Parangipettai, 134
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University (grant no. 324/150/1433). The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salama, A.J., Satheesh, S., Balqadi, A.A. et al. Identifying Suitable Fin Fish Cage Farming Sites in the Eastern Red Sea Coast, Saudi Arabia. Thalassas 32, 1–9 (2016). https://doi.org/10.1007/s41208-015-0001-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-015-0001-7