Abstract
This study focused on the diversity of lichens present in selected regions of northern Tunisia and assessed their resistance to atmospheric pollution. Twenty-five species of lichen were identified, divided into 13 families and 17 different genera. The results show that the Cladoniaceae and Parmeliaceae families predominate. Foliose and complex lichens are the most abundant. The main objective of this study was to examine, via atomic absorption spectrometry, the bioaccumulation of heavy metals (lead, zinc, copper, and cadmium) in the lichens Cladonia rangiformis, Flavoparmelia caperata, Parmotrema perlatum, and Evernia prunastri, which were selected due to the presence of similar lichens at the sites. The results showed high accumulations of lead, copper, and zinc at all stations (Nefza, Babouche, Oued Zen) characterized by intense road traffic and/or industrial activity, while cadmium levels were low at all stations. Flavoparmelia caperata proved to be a species tolerant of metal stress, making it a promising candidate for air quality biomonitoring programs aimed at assessing air pollution (17.53 mg/g DW of Pb and 89.8 mg/g DW of Zn in Nefza, and 2.36 mg/g DW of Cd and 10.13 mg/g DW of Cu in Oued Zen). These results highlight the importance of lichens as biological indicators for assessing pollution, the need to carefully monitor heavy metal concentrations in urban environments, and to provide future projects with funding for their potential transplantation to polluted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to growing urbanization, industrial activity, rising traffic emissions, and a lack of urban planning, air pollution is one of the most significant environmental problems in many cities in the world (Benítez et al. 2019; Shahi Khalaf Ansar et al. 2022). The urban region is also acknowledged as the primary source of air pollution (Azimi-Yancheshmeh et al. 2021); it is responsible for around 78% of the region’s major air pollutants and carbon emissions (Abas et al. 2022). The overabundance of heavy metals has become one of the major causes of environmental pollution. Also, the use of organic contaminants such as poly- and perfluoroalkyl substances poses serious environmental problems for soil and water (Kavusi et al. 2023). This pollution negatively affects the ability of the ecosystem to support life (Syed Salleh and Abas 2023). Previous research has demonstrated that levels of potentially toxic elements (PTEs) in soil, water, and air are above environmental quality standards (Fry et al. 2021; Abas et al. 2022). The most prevalent elements in total suspended particle matter are primarily Cu, Co, V, Ni, Fe, and Zn (Abas et al. 2022).
Air quality can only be saved by detecting and measuring air pollution. Although it is possible to assess air quality using measuring devices, their high cost will never allow complete coverage of the territory (Zahra et al. 2014). In addition to physical–chemical methods, there is another simple and less costly alternative for studying environmental conditions. This is biomonitoring, or biological monitoring, which uses animal or plant species to report environmental conditions according to their sensitivity (Asgari Lajayer et al. 2019). Lichens are good examples; they can be used for assessing air quality and to directly assess the environmental impacts of pollutants (Déruelle and Richard 1983). They are a suitable alternative for studying how various contaminants affect the environment. Their types, variety, existence or absence, and species are the results of the integration of these pollutants over time (Lawal et al. 2023). Lichens are symbiotic associations of a fungus (mycobiont) and an algal or cyanobacterial partner and/or cyanobacteria (photobiont) (Sharma and Mohammad 2020). They are lichenized fungi (Grimm et al. 2021). Lichens do not have roots—they receive most, if not all, of their nutrients from the atmosphere as dry or wet deposits (Anderson et al. 2022). Furthermore, lichens grow in all climates and are very resistant (Boustie and Grube 2005). About 20,000 species have been described (Grimm et al. 2021). Most lichens are sensitive to changes in their environment and are generally sensitive to atmospheric pollution (Anderson et al. 2022). They have been widely used as indicators of changes in the environment as well as for air quality monitoring (Seed et al. 2013; Chahloul et al. 2022, 2023; Lawal et al. 2023). Studies and research on Tunisian lichen flora remain negligible to this day, although Tunisia offers a great diversity of lichens. Most of the work has focused on the secondary metabolites of lichens and their biological proprieties (Khadhri et al. 2019; Mendili et al. 2019, 2021a, 2021b, 2022a, 2022b). However, studies that have touched on the atmospheric pollution of lichens remain rare (Chahloul et al. 2022, 2023).
The impact of environmental pollution on our planet has become a major concern. The principal goal of this paper is to examine the use of lichens as an indicator and accumulator of environmental contaminants, with a focus on the accumulation of some metal contaminants in Northern Tunisia. In addition, this study aimed to evaluate the collection sites available and to select the lichen that accumulates the most pollutants in the cleanest areas, to contribute to their transplantation in polluted areas in future projects.
Materials and methods
Chemicals and equipment
The chemicals and equipment used in the experimental work are listed below.
Chemicals: potassium hydroxide (KOH), sodium hypochlorite (NaOCI), 1-phenylenediamine, and hydrochloric acid.
Equipment: silica crucible, oven (Protherm Furnaces) for calcination, and an atomic absorption spectrometer (AANALYST 400).
Characteristics of the study areas
The study areas are all located in the humid bioclimatic zone, characterized by a high rainfall of 1000 mm. The Bizerte Governorate, located in the north of Tunisia, is characterized by a mixture of coastal, urban, and industrial environments, making it a region prone to pollutant emissions from a variety of human activities (Table 1).
The Beja Governorate, also located in northern Tunisia, is characterized by its rural landscape and agricultural activities, making it an environment potentially exposed to agricultural pollutants (Table 1).
The Jandouba Governorate, located in the north of Tunisia, has an environment characterized by forested and humid zones. There is a limited presence of industrial activities but a certain influence of road traffic and forest fires on air quality in this governorate (Table 1).
The distribution maps of sulfur dioxide (SO2), ozone (O3), nitrogen dioxide (NO2), carbon monoxide (CO), and the aerosol index in the governorates of Bizerte, Beja, and Jandouba in 2019 shown in Fig. 1 provide the maximum values for each pollutant in the three zones. For all pollutants, the maximum values are obtained in the Bizerte area. Therefore, we can conclude that the most polluted of the three zones is the Bizerte zone. It has the highest concentrations of SO2, O3, NO2, CO, and aerosols. We used data collected from the Google Earth Engine (GEE) platform and the Sentinel 5P satellite.
Lichen sampling
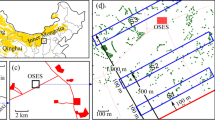
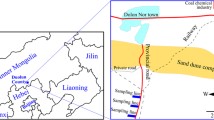
Lichen samples were collected in February, March, and April 2019 from the Bizerte [Ichkeul National Park: IC (37° 07′ 43" N: 9° 39′ 50" E) and Bazina: B.Z (36° 57′ 36" N: 9° 17′ 45" E)], Beja [Nefza: NF (37° 02′ 11" N: 9° 05′ 12" E)], and Jandouba [Babouche: B.B (36° 48′ 08″ N, 8° 40′ 36″ E) and Oued Zen: O.Z (36° 24′ 0″ N, 9° 30′ 0'' E)] regions of Tunisia (Fig. 2). Voucher specimens have been deposited in the lichenological herbarium of the Department of Biology, Faculty of Sciences of Tunisia.
Lichen identification
On the one hand, the identification of lichens was carried out by macroscopic observations to determine the general morphological characteristics of different species of lichens, such as the type of thallus (leprose, crustose, squamous, foliose, fruticose, composite, or gelatinous), the color of the thallus (yellow, orange, green, blue-green, brown), the orientation of the tips, and their habitat. The samples were analyzed in the area. On the other hand, the identification of lichen was carried out by performing chemical tests with certain reagents which give specific colorations when in contact with the thallus (Van Haluwyn and Asta 2013; Van Haluwyn et al. 2014; Asta et al. 2016).
The main reagents used were as follows:
-
K test: 10% KOH solution in water
-
C test: NaOCI, an aqueous calcium hypochlorite solution
-
KC test: K and C solutions applied one after the other
-
P test: 1% 1-phenylenediamine in a 10% aqueous sodium thiosulfate solution.
For the addition of each reagent, a + followed by the observed color is noted if the reaction is positive, and a – is indicated if no color has appeared (a negative reaction).
The state of the air quality in the areas
The Van Haluwyn and Lerond (1986) method was used to compare air quality in the areas studied; this formed the basis for classifying the lichens according to their degree of resistance to pollution.
Mineralization
The amounts of the four trace elements Zn (zinc), Pb (lead), Cu (copper), and Cd (cadmium) were determined by following the methods reported by Adjiri et al. (2018). In addition, three replicates were used for each treatment.
Preparing samples for mineral analysis
The samples were stored in a refrigerator at 4 °C and dried at 70–80 °C until a constant mass was obtained. The samples were then ground to a powder using a grinder and stored in pillboxes. The determination of the elements required the sample to be placed in solution. The organic matter was destroyed in a dry process.
Calcination
The first step in calcination was to weigh 0.5 g of lichen powder into a silica crucible. The crucible was placed in an oven (Protherm Furnaces) and the temperature was gradually raised to 450 °C and maintained at that for 4–6 h, depending on the nature of the sample. It was then allowed to cool. Next, the ash was moistened with 3 mL of distilled water and 1 mL of concentrated HCl was added. Finally, evaporation was performed on a hot plate.
Atomic absorption spectrometry assay
For the determination of the four trace elements (Pb, Zn, Cu, and Cd), we used the atomic absorption spectrometry technique. The instrument used was an AANALYST 400 atomic absorption spectrometer.
Statistical analysis
The data were analyzed using statistical software. A two-way permutation ANOVA was performed to test for differences between species and sites, using Duncan's test for post hoc comparisons. Statistical analysis was performed using SPSS software (SPSS, 20.0). The data for the pair chart and heat map were generated using Python Pandas Dataframe and Google Colab.
Calculations and graphical representations were made using Microsoft Excel and Google Colab. The safety intervals of the means were calculated at the 95% level.
Results
Lichen identification
Lichen identification is challenging in Tunisia due to limited knowledge about the lichen flora. As a result, thallus reactions and macroscopic observations were carried out to identify the lichens. Indeed, the collection stations (Bazina, Ichkeul National Park, Nefza, Babouche, and Oued Zen) show substantial lichen diversity.
Our work involved chemical tests with various reagents for which contact with the thallus results in a range of colors (Table 2). Therefore, they are distributed as shown in Table 3.
Depending on their habitats, the lichens collected are corticolous, muscicolous, saxicolous, and terricolous lichens. The morphological characteristics of the lichens collected enabled us to distinguish seven types of thallus: complex, crustose, foliose, fruticose, gelatinous, leprose, and squamulose (Table 4).
The first results show a high biodiversity of lichens, with 25 species. Of these lichens, 32% are foliose, 24% are complex, and 20% are fruticose, while leprose constitute only 4% (Fig. 3).
In Fig. 4, we can see that terricolous and corticolous lichens are the most dominant lichens in the three governorates in which lichens were sampled.
The systematic classification of all the identified lichen species is shown in Table 5. The results obtained reveal 25 species of lichenized fungi, which are divided into 13 families and 17 genera (Fig. 5). These species are important bioindicators of atmospheric SO2 content and heavy metal accumulation.
Sulfur dioxide and air pollution
Lichen species are distributed according to specific ecological conditions (Abas and Din 2020). Some species only grow on acidic substrates (rocks, bark, etc.). Others will only grow on a neutral or basic substrate. Some require very high light conditions and others do not, depending on the humidity, dryness, temperature, and even the degree of air pollution (Van Herk 2001). As shown in Table 6, some lichens have been found to be bioindicaters of the approximate atmospheric SO2 content (in µg/m3 of air) (Tiévant 2001).
The state of the air quality
The most widely used method for assessing the air quality of a given ecosystem is that of Van Haluwyn and Lerond (1986), which is based on classifying lichens according to their degree of resistance to pollution. This method was used to compare air quality in the areas studied. As shown in Table 7, the species most sensitive to pollution (Anaptychia ciliaris, Parmotrema perlatum, and Ramalina fraxinea) are found in Nefza, Oed Zen, and Babouche. However, the predominance of tolerant lichens in Bazina and Ichkel suggests that some parts of these areas may be exposed to high levels of pollutants (Table 7).
According to the distribution maps of SO2, O3, NO2, CO, and the aerosol index, the maximum values for all pollutants are obtained in the Bizerte area. Therefore, we can conclude that the most polluted of the three governorates is the Bizerte zone. It has the highest concentrations of SO2, O3, NO2, CO, and aerosols (Fig. 1).
The areas situated in the governorate of Bizerte have a low diversity of species. They are characterized by important industrial activities and a high density of road traffic. However, Beja and Jandouba are characterized by limited industrial activity.
For this reason, the selection of lichens is based on the presence of similar lichens at the less polluted sites.
Heavy metal contents
Our study focuses on the accumulation of zinc, lead, copper, and cadmium by the selected lichens (Cladonia rangiformis, Flavoparmelia caperata, Parmotrema perlatum, and Evernia prunastri) collected from the selected sites in Babouche, Oued Zen, and Nefza. The selection of lichens is based on the presence of similar lichens at the sites.
Duncan's test reveals non-significant differences between these four lichens in the levels of the four bioaccumulated PTEs at the same site.
Zinc content
Figure 6 shows that the Zn levels vary between the sites and that the species collected at Nefza have the highest Zn levels of all the species examined. The concentration of Zn shows significant differences between Babouche and Nefza; on the other hand, the Oued Zen site does not show any significant difference from those two sites in the Zn concentration (p > 0.05). The levels are lower at the Babouche and Oued Zen sites. The highest zinc contents are on the order of 39.8 mg/g DW (in Flavoparmelia caperata obtained from Nefza) and 32.26 mg/g DW (in Flavoparmelia caperata collected from Oued Zen).
Lead content
Pb concentration shows significant differences (p < 0.05) between Nefza and the other two sites (Babouche and Oued Zen) (p > 0.05). The results in Fig. 7 show that higher levels of lead were observed at the Nefza site compared to the other sites. The lead content varies from 24.6 mg/g DW in Cladonia rangiformis to 10.35 mg/g DW in Parmelia perlata (Fig. 7).
For Oued Zen, the highest content was recorded in Flavoparmelia caperata (12.1 mg/g DW); for Babouche, the highest content (11.85 mg/g DW) was recorded in Parmotrema perlatum.
Copper content
The analysis reported in Fig. 8 shows that the species at the sites in Oued Zen and Babouche contain the highest levels of copper. The Cu concentrations at the three sites are not significantly different (p > 0.05). For the Oued Zen and Babouche sites, Flavoparmelia caperata shows the highest content (11.5 mg/g DW), followed by Parmotrema perlatum (8.9 mg/g DW). For the site in Nefza, the highest content is about 6.8 mg/g DW, in Flavoparmelia caperata.
Cadmium content
The results obtained when determining cadmium are shown in Fig. 9. The Cd contents at Nefza are significantly different (p < 0.05) bto those at the other two sites (Babouche and Oued Zen) (p > 0.05). The Cd contents are very similar and generally low (ranging from 0 to 2.36 mg/g DW). Figure 9 shows that the species harvested in Babouche have rather high cadmium contents: on the order of 2.36 mg/g DW for Flavoparmelia caperata, 0.86 mg/g DW for Evernia prunastri, and 0.65 mg/g DW for Parmotrema perlatum. At the Nefza site, the cadmium content is only about 0.16 mg/g DW in Evernia prunastri.
Correlation analysis
The correlation heat map (Fig. 10) shows the pairwise correlations between Zn (zinc), Pb (lead), Cu (copper), and Cd (cadmium). We find that Zn shows moderately positive correlations with Pb and Cu, with r = 0.61 and r = 0.27. This suggests that higher values of Zn tend to be associated with higher values of Pb and Cu (Table 8).
Cadmium shows negative correlations with Zn and Pb (r = – 0.48 and r = – 0.33, respectively). This means that there may not be a strong linear relationship between Cd and the other elements (Table 8).
The pair chart shown in Fig. 11 allows us to visualize the relationships between all the heavy metals at once. It confirms the observations made based on the correlation heat map.
The diagonal of the pairwise diagram displays histograms showing the distributions of the heavy metals.
Discussion
Due to both natural occurrence and human activity, heavy metal contamination is a significant environmental issue on a global scale. The release of harmful heavy metals into ecosystems has grown considerably because of anthropogenic processes such as mining, industrial waste disposal, urbanization, and agricultural practices (Asgari Lajayer et al. 2019). In addition, air, water, and soil pollution have considerable consequences for ecosystems and the loss of biodiversity (Shahi Khalaf Ansar et al. 2022).
This work aimed to provide a better understanding of the lichens living in the selected regions and to determine their resistance to environmental pollution. The lichens were collected from different types of substrates (soil, moss, tree trunks, rocks, etc.).
The results enabled us to identify 25 lichens. They were divided into 13 families and 17 genera. The results show the dominance of the Cladoniaceae and Parmeliaceae families in terms of the number of species. Analysis of the physiognomic types of the lichens identified showed a dominance of foliose and complex thalli, followed by fruticose, then squamulose, and finally leprous and gelatinous thalli, which were the least represented.
The lichens collected during our studies were distributed according to the ecological conditions, such as the climatic conditions and substrate characteristics. Indeed, most of the species collected were corticolous and foliose. These results agree with those of Déruelle and Richard (1983), who showed that the species most resistant to atmospheric pollution were of the foliose type (Chahloul et al. 2022).
Studies of Tunisia's diversity of lichens are still relatively rare. Indeed, there are not many studies of lichens in Tunisia. However, we can cite the work of El Mokni et al. (2010, 2011).
The Van Haluwyn and Lerond (1986) method for assessing the air quality of a given ecosystem showed that the areas belonging to the Bizerte Governorate are the most polluted. This is explained by satellite data. We can conclude that the most polluted of the three governorates is the Bizerte area. It has the highest concentrations of SO2, O3, NO2, CO, and aerosols. Soleimany et al. (2021) found strong correlations between satellite data and in-situ measurements. For example, they showed strong correlations between the aerosol index and in situ PM2.5 and PM10 concentrations.
As a developing country, Tunisia, particularly in the northern governorates (Bizerte), has experienced a gradual increase in air pollutant emissions. This agrees with Azimi-Yancheshmeh et al. (2021), who indicate that Iran (a developing country) faces high levels of PM2.5.
In this context, the selection of lichens was based on the presence of similar lichens at the least polluted sites. Lichens provide a means of determining the degree of atmospheric pollution without the need for often-complicated chemical methods. In this way, lichen bioindicators can be used to assess air quality. The analysis of trace metal elements (Pb, Cd, Zn, and Cu) in the lichens studied show that the accumulation of these metals depended on the site and the lichen species considered. Based on the results for heavy metal levels, we found that the number and coverage rate of lichen flora are related to the degree of pollution. This enabled us to determine that Flavoparmelia caperata is the most resistant to atmospheric pollution and accumulates more heavy metals.
Lichens can accumulate heavy metals because of their anatomical characteristics (the absence of a cuticle) and their physiological characteristics: continuous activity, the absence of stomata, and regulatory mechanisms (Van Haluwyn and Garrec 2002). The high resistance of lichens to environmental extremes is due to their main ecophysiological and metabolic characteristics (Lorenz et al. 2023).
In our work, Falavoparmelia caperata collected in Babouche was found to have accumulated the highest levels of the most toxic metal, cadmium.
We note that lead and zinc accumulation was high in urban and suburban sites near roads with heavy traffic (in Nefza). The high zinc levels recorded at the Nefza stations are explained by their locations, which are close to road activities.
As a result of our analysis, we found higher levels of lead and cadmium in some areas, particularly in Nefza. These elevated levels can be attributed to several environmental factors, including increased road traffic and the presence of intensive agricultural activities in the area, which can lead to an intensified release of these heavy metals into the atmosphere.
This hypothesis is based on studies that have shown that lichens located in proximity to roads generally have higher concentrations of lead due to vehicle emissions (Benítez et al. 2019; Sujetoviene and Sliumpaite 2013).
In addition, the correlations between the elements highlight potential links between certain sources of pollution. The positive correlations between zinc, lead, and copper suggest common sources of pollution, while the negative correlation with cadmium indicates a distinct source for this metal. Overall, our findings conform to those previously reported by Chahloul et al. (2023), indicating that a significant correlation between the elements in lichens was observed.
Based on the rate of accumulation of heavy metals by lichens, we can classify the selected stations into two categories according to the degree of pollution. (1) A heavily polluted station, Nefza: this is the urban station. Intense road traffic and industrial activity are characteristics. (2) Moderately polluted stations: Babouche and Oued Zen. Since urbanization is a consequence of the explosion in industrialization and road traffic, and these activities emit enormous quantities of pollutants into the atmosphere, posing numerous environmental problems, such as air pollution. Llop et al. (2012) indicated that lichens are sensitive to pollution caused by road traffic. These activities lead to a decline in lichens.
Conclusion
In conclusion, this study assessed the potential of lichens as indicators of air pollution and accumulators of heavy metals in northern Tunisia. The results showed a high biodiversity of lichens in the region, with both pollution-sensitive and pollution-tolerant species. The state of the air quality suggests that Bizerte areas are the most polluted, due to intensive industrial and road activities. Among the lichens studied, Flavoparmelia caperata was found to be the most resistant to environmental pollution and to accumulate the highest levels of heavy metals, particularly lead, zinc, and copper. The high levels of these metals in some lichens indicate significant environmental contamination by these pollutants. These results confirm the value of lichens as biological indicators and accumulators of metal contaminants. Further study of more sites and lichens would be enable a more comprehensive assessment of the air pollution in northern Tunisia and their transplantation in polluted areas in future projects.
Data availability
The research data associated with this article is available.
References
Abas A, Din L (2020) Diversity, composition and distribution of lichens along elevational gradients in the tropical mountain forest of Gunung Nuang, Selangor Malaysia. Eco Mont (J Prot Mountain Areas Res) 13(1):4–11. https://doi.org/10.1553/eco.mont-13-1s4
Abas A, Aiyub K, Awang A (2022) Biomonitoring potentially toxic elements (PTEs) using lichen transplant Usnea misaminensis: a case study from Malaysia. Sustainability 14(12):7254. https://doi.org/10.3390/su14127254
Adjiri F, Ramdani M, Lograda T, Chalard P (2018) Bio-monitoring of metal trace elements by epiphytic lichen in the Bordj Bou Arreridj area East of Algeria. Der PharmaChemica 10(3):1–8
Anderson J, Lévesque N, Caron F, Beckett P, Spiers GA (2022) A review on the use of lichens as a biomonitoring tool for environmental radioactivity. J Environ Radioact 243:106797. https://doi.org/10.1016/j.jenvrad.2021.106797
Asgari Lajayer B, Khadem Moghadam N, Maghsoodi MR, Ghorbanpour M, Kariman K (2019) Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: mechanisms and efficiency improvement strategies. Environ Sci Pollut Res 26(9):8468–8484. https://doi.org/10.1007/s11356-019-04241-y
Asta J, Van Haluwyn C, Bertrand M (2016) Guide des lichens de France: Lichens des roches. Belin, Paris, pp 1–235
Azimi-Yancheshmeh R, Moeinaddini M, Feiznia S, Riyahi-Bakhtiari A, Savabieasfahani M, van Hullebusch ED, Asgari Lajayer B (2021) Seasonal and spatial variations in atmospheric PM25-bound PAHs in Karaj city, Iran: sources, distributions, and health risks. Sustain Cities Soc 72:103020. https://doi.org/10.1016/j.scs.2021.103020
Benítez Á, Medina J, Vásquez C, Loaiza T, Luzuriaga Y, Calva J (2019) Lichens and bromeliads as bioindicators of heavy metal deposition in ecuador. Diversity 11(2):28. https://doi.org/10.3390/d11020028
Boustie J, Grube M (2005) Lichens—a promising source of bioactive secondary metabolites. Plant Genet Resour 3(2):273–287. https://doi.org/10.1079/PGR200572
Chahloul N, Khadhri A, Vannini A, Mendili M, Raies A, Loppi S (2022) Bioaccumulation of potentially toxic elements in some lichen species from two remote sites of Tunisia. Biologia. https://doi.org/10.1007/s11756-022-01069-9
Chahloul N, Khadhri A, Vannini A, Mendili M, Raies A, Loppi S (2023) Selecting the species to be used in lichen transplant surveys of air pollution in Tunisia. Environ Monit Assess 195(5):570. https://doi.org/10.1007/s10661-023-11219-4
Déruelle S, Lallemant R (1983) Les lichens témoins de la pollution. Vuibert, Paris
El Mokni R, Mahmoudi MR, El Aouni MH (2010) Aperçu sur les lichens Corticoluss fruticose dans la région de Kroumiria. Révue FSB 8:72–76
El Mokni R, Jouili El-Mokni H, El-Aouni M-H (2011) Les espèces du genre Lobaria (Schreb.) Hoffm. de la région de Kroumirie (Nord-Ouest de la Tunisie): caracterisation morpho-chimique bioindication. Révue FSB 9:114–119
Fiore-Donno A-M (1997) Les lichens épiphytes comme bioindicateurs de la pollution atmosphérique genevoise. Saussurea 28:189–218
Fry KL, Gillings MM, Isley CF, Gunkel-Grillon P, Taylor MP (2021) Trace element contamination of soil and dust by a New Caledonian ferronickel smelter: dispersal, enrichment, and human health risk. Environ Pollut 288:117593. https://doi.org/10.1016/j.envpol.2021.117593
Gavériaux J-P (1999) Lichens et qualité de l’air (initiation). pp 1–10. https://www.afllichenologie.fr/telecharger/Doc/Lichens-et-qualite-Air.pdf
Grimm M, Grube M, Schiefelbein U, Zühlke D, Bernhardt J, Riedel K (2021) The lichens’ microbiota, still a mystery? Front Microbiol. https://doi.org/10.3389/fmicb.2021.623839
Van Haluwyn C, Asta J (2013) Guide des lichens de France: Lichens des arbres. Belin, Paris, pp 1–378
Van Haluwyn C, Garrec JP (2002) Biosurveillance végétale de la qualité de l’air. p117
Van Haluwyn C, Asta J, Boissière J (2014) Guide des lichens de France: Lichens des sols. Humensis, Paris, pp 1–221
Kavusi E, Shahi Khalaf Ansar B, Ebrahimi S, Sharma R, Ghoreishi SS, Nobaharan K, Abdoli S, Dehghanian Z, Asgari Lajayer B, Senapathi V, Price GW, Astatkie T (2023) Critical review on phytoremediation of polyfluoroalkyl substances from environmental matrices: need for global concern. Environ Res 217:1144. https://doi.org/10.1016/j.envres.2022.114844
Khadhri A, Mendili M, Araújo MEM, Seaward MRD (2019) Comparative study of secondary metabolites and bioactive properties of the lichen Cladonia foliacea with and without the lichenicolous fungus Heterocephalacria bachmannii. Symbiosis. https://doi.org/10.1007/s13199-019-00630-6
Lawal O, Ogugbue CJ, Imam TS (2023) Mining association rules between lichens and air quality to support urban air quality monitoring in Nigeria. Heliyon 9(1):e13073. https://doi.org/10.1016/j.heliyon.2023.e13073
Llop E, Pinho P, Matos P, Pereira MJ, Branquinho C (2012) The use of lichen functional groups as indicators of air quality in a Mediterranean urban environment. Ecol Ind 13(1):215–221. https://doi.org/10.1016/j.ecolind.2011.06.005
Lorenz C, Bianchi E, Poggiali G, Alemanno G, Benesperi R, Brucato JR, Garland S, Helbert J, Loppi S, Lorek A, Maturilli A, Papini A, de Vera J-P, Baqué M (2023) Survivability of the lichen Xanthoria parietina in simulated Martian environmental conditions. Sci Rep 13(1):4893. https://doi.org/10.1038/s41598-023-32008-6
Mendili M, Bannour M, Araújo MEM, Aschi-Smiti S, Mark RDS, Khadhria A (2019) Secondary metabolites and antioxidant capacity of the Tunisian lichen Diploschistes ocellatus (Ascomycota). Int Med Mushrooms. https://doi.org/10.1615/IntJMedMushrooms.2019031423
Mendili M, Bannour M, Araújo MEM, Seaward MRD, Khadhri A (2021a) Lichenochemical screening and antioxidant capacity of four Tunisian lichen species. Chem Biodivers. https://doi.org/10.1002/cbdv.202000735
Mendili M, Essghaier B, Seaward MRD, Khadhri A (2021b) In vitro evaluation of lysozyme activity and antimicrobial effect of extracts from four Tunisian lichens: Diploschistes ocellatus, Flavoparmelia caperata, Squamarina cartilaginea and Xanthoria parietina. Arch Microbiol 203(4):1461–1469. https://doi.org/10.1007/s00203-020-02129-x
Mendili M, Seaward MRD, Khadhri A (2022a) Does the lichenicolous fungus Heterocephalacria bachmannii affect the antimicrobial potential of its host Cladonia foliacea? Nat Prod Res 36(12):3095–3099. https://doi.org/10.1080/14786419.2021.1933974
Mendili M, Khadhri A, Mediouni-Ben Jemâa J, Andolfi A, Tufano I, Aschi-Smiti S, DellaGreca M (2022b) Anti-inflammatory potential of compounds isolated from Tunisian lichens species. Chem Biodivers. https://doi.org/10.1002/cbdv.202200134
Seed L, Wolseley P, Gosling L, Davies L, Power SA (2013) Modelling relationships between lichen bioindicators, air quality and climate on a national scale: results from the UK OPAL air survey. Environ Pollut 182:437–447. https://doi.org/10.1016/j.envpol.2013.07.045
Sérusiaux E, Diederich P, Lambinon J (2004) Les macrolichens de Belgique, du Luxembourg et du nord de la France. Ferrantia 40–188
Shahi Khalaf Ansar B, Kavusi E, Dehghanian Z, Pandey J, Asgari Lajayer B, Price GW, Astatkie T (2022) Removal of organic and inorganic contaminants from the air, soil, and water by algae. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-21283-x
Sharma M, Mohammad A (2020) Lichens and lichenology. In: Yusuf M (ed) Lichen-derived products: extraction and applications. Wiley, Hoboken, pp 101–118
Soleimany A, Grubliauskas R, Šerevičienė V (2021) Application of satellite data and GIS services for studying air pollutants in Lithuania (case study: Kaunas city). Air Qual Atmos Health 14:411–429. https://doi.org/10.1007/s11869-020-00946-z
Sujetoviene G, Sliumpaite I (2013) Response of Evernia prunastri transplanted to an urban area in central Lithuania. Atmos Pollut Res 4(2):222–228. https://doi.org/10.5094/APR.2013.023
Syed Salleh SNA, Abas A (2023) Monitoring heavy metal concentrations using transplanted lichen in a tourism city of Malaysia. Sustainability 15(7):5885. https://doi.org/10.3390/su15075885
Tiévant P (2001) Guide des lichens. Delachaux, Colombes, p 304. ISBN: 9782603012116
Van Herk CM (2001) Bark pH and susceptibility to toxic air pollutants as independent causes of changes in epiphytic lichen composition in space and time. Lichenologist 33(5):419–442. https://doi.org/10.1006/lich.2001.0337
Van Haluwyn C, Lerond M (1986) Les lichens et la qualité de l’air, évolution méthodologique et limites. Ministère de l’Environnement, Paris, p 207
Zahra F, Alami O, Ouali Alami FZ, Elabidi A, Mouhir L, Fekhaoui M, & Serghini A (2014) Utilisation des lichens comme bio-indicateurs de la pollution atmosphérique par le plomb, cadmium et zinc de la région de Rabat-Sale-Zemmour-Zaêr (Maroc). Afrique Sci 10(3). http://www.afriquescience.info.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Giulia Guerriero.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mendili, M., Jrad, T.B. & Khadhri, A. Lichen diversity and bioaccumulation of heavy metals in northern Tunisia: a study to evaluate environmental pollution. Euro-Mediterr J Environ Integr 8, 847–862 (2023). https://doi.org/10.1007/s41207-023-00413-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41207-023-00413-y