Abstract
Red seaweeds are a rich source of compounds with various bioactive properties, such as antimicrobial, antioxidant, antifouling, antiproliferative, and anticancer activities. In this study, the antioxidant and antibacterial properties of twelve red macroalgae collected from the Tunisian coast were examined. Estimated total phenolic, flavonoid, and tannin contents in methanolic extracts were found to vary among species. Gracilaria gracilis presented the highest concentration of total phenolic compounds (19.2 ± 1.88 mg GAE/g dried biomass), Laurencia obtusa showed the highest tannin content (18.95 ± 0.84 mg ECat/g DB), and Sphaerococcus cornopifolius showed the highest flavonoid content (7.17 ± 0 mg ECat/g DB). Six species showed significant DPPH radical scavenging activities and total antioxidant capacities: Asparagopsis armata, Gracilaria gracilis, Hypnea musciformis, Laurencia obtusa, Pterocladiella capillacea, and Sphaerococcus cornopifolius. Antimicrobial activity was observed for five species. This study therefore highlights the potential use of red seaweed species collected from the Tunisian coast as sources of bioactive compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Given the economic and ecological challenges that are currently being encountered worldwide, seaweeds are an important resource to consider in the context of sustainable development. These organisms play an important role in maintaining ecological balance. Moreover, the potential for sustainable seaweed cultivation and bioproduction implies that seaweeds are a highly relevant resource for the “blue growth” strategy (Buschmann et al. 2017). Approximately 12 million tonnes of algae are cultivated per year, around 85% of which is used in food products and for human consumption (FAO 2018).
Several studies have shown that macroalgae can be used in the pharmacological sector, as they produce unusual secondary metabolites with various biological properties, such as cytotoxic, antibiotic, antiviral, anti-inflammatory, and antiparasitic activities (Kolanjinathan 2014; Kumar et al. 2017; Al-Enazi et al. 2018).
The accumulation of large doses of reactive oxygen species (ROS) can disrupt the normal functioning of plant cells (Yalcinkayaa et al. 2019). Thus, plants regulate the physiological production of ROS by producing considerable quantities of several enzymatic and nonenzymatic compounds with antioxidant activities, such as superoxide dismutase enzyme, catalases, peroxidases, vitamins C and E, carotenoids, tannins, phenols, and flavonoids (Xiulan et al. 2019). A large number of potent antioxidant compounds (including phlorotannins, sulfated polysaccharides, carotenoids, and sterols) have already been detected in various macroalgae, making these marine organisms a valuable source of compounds with neuroprotective effects that are useful for treating neurodegenerative diseases such as Alzheimer’s or Parkinson’s disease (Pangestuti 2011) as well as numerous other health benefits (Fernando et al. 2020).
The Mediterranean Sea is characterized by high seaweed biodiversity. More than 415 seaweed species have been found along the 1300 km of coast in Tunisia, and more than 60% of this coastal vegetation belongs to the phylum Rhodophyta (Ben Maiz 1995). Rhodophyta are commonly called red algae because of the predominance of the red pigment R-phycoerythrin in these organisms. They contain large amounts of fiber, protein, and minerals, and are a great source of vitamins. Red algae have been used in phytotherapy due to their medicinal properties; for instance, they exhibit various bioactive properties such as antimicrobial activity against pathogenic strains (Kumar et al. 2017), antioxidant activity (Bouhlal et al. 2013), antiproliferative activity (Neethu et al. 2017), and cytotoxic and anticancer activities (Al-Enazi et al. 2018). These properties of red algae aroused our interest, so various common species of red macroalgae were collected from the north coast of Tunisia to evaluate their potential as a source of valuable bioactive products. The results of this study improve knowledge of the antioxidant and antibacterial properties of seaweeds collected from southern Mediterranean coasts.
Materials and methods
Algae sampling and extraction
Twelve species were collected manually in shallow water (< 2 m depth) from March to April 2014: Asparagopsis armata Harvey [Bonnemaisoniales], Laurencia obtusa (Hudson) J.V. Lamouroux, Palisada perforata (formerly Laurencia papillosa) (C. Agardh) Greville, Ceramium ciliatum (J. Ellis) Ducluzeau [Ceramiales], Peyssonnelia squamaria (S.G. Gmelin) Decaisne [Peyssonneliales], Sphaerococcus coronopifolius Stackhouse, Hypnea musciformis (Wulfen) J.V. Lamouroux [Gigartinales], Corallina officinalis (Linnaeus), Jania rubens (Linnaeus) J.V. Lamouroux, Jania longifurca Zanardini [Corallinales], Pterocladiella capillacea (S.G. Gmelin) Bornet [Gelidiales], and Gracilaria gracilis (S.G. Gmelin) M. Steentoft, L.M. Irvine & W.F. Farnham [Gracilariales]. All samples were collected from Cap Zebib (37°15′49.66″N, 10°04′02.85″E) except for the G. gracilis, which was collected from Bizerte Lake (37°10′60″N, 9°52′E). In the laboratory, the algae were washed with seawater followed by fresh water in order to remove epiphytes and excess salt. The algae were then dried at ambient temperature in a dry and dark place for 3 days. Next, they were taxonomically identified by morphological characterization according to Fischer et al. (1987) and Cabioch et al. (2006), and a voucher specimen of each species was kept in 2% formaldehyde solution. For each species, a 10 g sample was ground and extracted with MeOH (80%) for 12–16 h at room temperature. All solvents used were of analytical grade.
Determination of the total phenolic content
The total phenolic content of the extracts was assessed using the yellow Folin-Ciocalteu reagent (Dewanto et al. 2002) consisting of phosphotungstic acid (H3PW12O40) and phosphomolybdic acid (H3PMo12O40). This reagent generates blue tungsten and molybdenum oxides when it is reduced during phenol oxidation. An aliquot of 125 µl of the algae extract was mixed with 500 µl distilled water and 125 µl Folin-Ciocalteu reagent. Following agitation and 3 min of incubation in the dark at room temperature, 1250 µl Na2CO3 (7%) were added, and then the extract was incubated for a further 90 min in the dark. Next, the absorbance of the sample was measured using a Jenway 6405 UV/Vis spectrophotometer at 760 nm. This test was performed in triplicate. In parallel, a standard reference curve was established under the same experimental conditions using gallic acid as a positive control. Gallic acid was used as a reference standard for the calibration curve, and the total phenolic content was expressed in mg gallic acid equivalent per g dried biomass (mg GAE/gDB) via the equation
where C is the total phenolic content (mg GAE/g DB), c is the concentration of gallic acid determined from the calibration curve (mg/L), V is the volume of the extract (L), and m is the mass of dry material used (g).
Determination of the flavonoid content
The flavonoid content of the methanolic extract was determined by the aluminum chloride colorimetric method (Dewanto et al. 2002). An aliquot of 250 μl of extract was mixed with 75 μl of a 5% NaNO2 solution. After 6 min, 150 μl of 10% AlCl36H2O were added, and 500 μl of 1 N NaOH were added 5 min later. The final volume was then rounded to 2.5 ml. The last step in this assay was the measurement of absorbance at 510 nm. Catechin was used as a reference standard for the calibration curve, and the flavonoid content was expressed in mg equivalents of catechin per g of dry biomass (mg ECat/g DB). This test was done in triplicate.
Determination of the tannin content
The tannin content of the methanolic extracts was determined by the colorimetric method described by Price et al. (1978). In this method, 50 μl of extract were mixed with 3 ml of 4% vanillin and 1.5 ml of concentrated HCl. After 15 min, the absorbance at 500 nm was measured. This test was done in triplicate. Catechin was used for the calibration curve. Results are expressed in mg equivalents of catechin per g of dry biomass (mg ECat/g DB).
DPPH radical scavenging
The free-radical scavenging capacity of each extract was analyzed using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) test according to the method of Farasat et al. (2013). Briefly, 100 μL of each extract at various dilutions (20, 10, 5, 1, 0.5 mg/ml) were mixed with 100 μL of 0.16 mM DPPH solution. The solution was kept at room temperature for 30 min, after which the absorbance was measured in an automated microplate reader at 517 nm. The amount of DPPH radical scavenging performed by the extract was calculated as follows:
where A0 is the absorbance of the control and Ai is the absorbance of the sample.
The half-maximal inhibitory concentration (IC50) was calculated by linear regression analysis. Ascorbic acid was used as a positive control. This test was done in triplicate.
Total antioxidant capacity (TAC)
The total antioxidant capacity of each seaweed extract was determined by the phosphomolybdenum method described by Prieto et al. (1999). This assay is based on the reduction of Mo(VI) to Mo(V) by the antioxidant compounds and the formation of a green phosphate/Mo(V) complex. Briefly, in triplicate, an aliquot of 0.2 ml (10 mg/ml) of each methanolic (80%) extract was combined with 2 ml of the reagent solution (28 mM sodium phosphate and 4 mM ammonium molybdate in 0.6 M sulfuric acid). Samples were capped and incubated in a thermal block at 95 °C for 90 min. After cooling at room temperature, the absorbance of each solution was measured using a UV–visible spectrophotometer at 695 nm. 0.2 ml of 80% methanol were added as a blank to 2 ml of the reagent solution, which was then incubated under the same conditions as the other samples. Ascorbic acid was used as a reference standard for the calibration curve. The total antioxidant capacity was expressed in mg equivalents of ascorbic acid per g of dry biomass (mg EAA/g DB).
Antimicrobial activity
Seven indicator microorganisms were used for the assay: six bacteria (Staphylococcus aureus, Streptococcus B, Pseudomonas cepacia, Pseudomonas fluorescens, Enterococcus faecalis, and Aeromonas hydrophila) and one yeast (Candida albicans). These are the standard reference bacteria used in the laboratory. Antibacterial tests were performed by the disc diffusion method in agar-plated Petri dishes according to Ismail et al. (2016). Extracts were tested at 1 mg and applied to sterile filter paper discs (6 mm). After evaporating the solvent, the discs were placed on trypto-casein-soy agar (TSA, Bio-Rad) plates inoculated with a strain cultured for 18 h (106 bacteria ml−1) in tryptone soy broth (TSB, Bio-Rad). A disc loaded with solvent was simultaneously prepared as a control. Plates were incubated overnight at 30 °C. The test was done in duplicate. Antimicrobial activity was evaluated by measuring the diameter (in mm) of the inhibition zone around the disc after 24 h of incubation. The biocide CuSO4 was used as a positive control at 5 ppm (Hellio et al. 2001).
Statistical analysis
Statistical analysis of the total phenolic content, flavonoid content, tannin content, DPPH radical scavenging capacity (IC50), total antioxidant capacity (TAC), and antimicrobial activity was performed with the XLSTAT 2016.1 software. One-way ANOVAs were performed and significant differences (p < 0.05) were determined via Duncan's test. Principal component analysis (PCA) was used to determine correlations between antioxidant activity (DPPH and TAC) and the total phenolic, flavonoid, and tannin contents of the species. The PCA was implemented with SPSS 15.0.
Results
Total phenolic, flavonoid, and tannin contents
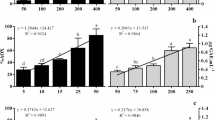
The results for the total phenolic contents are given in Fig. 1. They ranged between 0.20 ± 0 and 19.29 ± 1.8 mg GAE/g DB. The extract from G. gracilis had a significantly higher (p < 0.05) total phenolic content (19.29 ± 1.8 mg GAE/g DB) than any other extract. The total phenolic content of the extract from A. armata was the second highest (14.95 ± 0.5 mg GAE/g DB), followed by the extract from P. perforata (12.45 ± 0.4 mg GAE/g DB). The lowest total phenolic contents were observed for members of the order Corallinales: C. officinalis, J. rubens, and J. elongata (0.59 ± 0.08; 0.20 ± 0.15 and 1.24 ± 0.10 GAE/g DB, respectively).
The flavonoid contents of the seaweed extracts are given in Fig. 2. Flavonoid contents varied from 0.77 ± 0.1 to 7.17 ± 0 mg ECat/g DB, with S. coronopifolius and A. armata presenting the highest levels (7.17 ± 0 and 4.64 ± 0.63 mg ECat/g DB, respectively). Extracts from members of Corallinales presented significantly lower levels of flavonoids, ranging between 0.77 ± 0.1 and 0.9 ± 0.08 mg ECat/g DB.
Figure 3 presents the tannin contents of the seaweed extracts. The values obtained ranged between 5.90 ± 3.1 and 18.95 ± 0.8 mg ECat/g DB.
Among the species analyzed, the extract from L. obtusa contained a significantly higher (p < 0.05) amount of tannin (18.95 ± 0.8 mg ECat/g DB) than the other extracts. The extracts from A. armata, H. musciformis, and S. cornopifolius also presented high tannin contents (17.50 ± 0.5, 17.16 ± 1.7, and 16.62 ± 0 mg ECat/g DB, respectively). However, the extracts from C. ciliatum, P. squamaria, and J. rubens contained significantly lower levels of tannin than the other extracts (6.62 ± 0.01, 5.98 ± 0.8, and 5.90 ± 0.6 mg ECat/g DB, respectively).
DPPH radical scavenging activity and total antioxidant capacity
The radical scavenging activities and total antioxidant capacities of the seaweed extracts are given in Figs. 4 and 5, respectively.
Extracts of H. musciformis, S. coronopifolius, A. armata, C. ciliatum, and P. capillacea had significantly lower (p < 0.01) IC50 values (between 13.9 ± 0.3 and 18.6 ± 0.2 mg/ml) than the other extracts, indicating that they had significantly enhanced radical scavenging capacities. The extract from H. musciformis displayed the lowest IC50 value of all (13.9 ± 0.3 mg/ml); however, a statistical analysis indicated that there was no statistically significant difference between the IC50 values obtained for the five most active species.
The highest total antioxidant capacity, 2.02 ± 0.1 mg EAA/g DB, was obtained for the H. musciformis extract (Fig. 5). Three other extracts also presented nonnegligible total antioxidant capacities: L. obtusa, A. armata, and P. capillacea (1.22 ± 0.0, 1.05 ± 0.1, and 0.90 ± 0.2 mg EAA/gDB, respectively). The lowest antioxidant capacities were observed for the methanol extracts of J. longifurca and P. perforata (both 0.07 ± 0.1 mg EAA/gDB).
The results from the principal component analysis (PCA) of the total phenolic (TPC), flavonoid (FC), and tannin (TC) contents and the antioxidant activities (DPPH, TAC) of the twelve seaweed extracts are presented in Fig. 6. The first two principal components, F1 and F2, explained 61.4% and 16.4% of the total variance of the data set, respectively. Analysis of the first component (F1) highlighted strong negative correlations of TPC, FC, and TC (on the right) with DPPH (on the left), revealing that the species with the highest DPPH radical scavenging potencies (the lowest IC50 values, i.e., A. armata, H. musciformis, P. capillacea, L. obtusa, and G. gracilis) also showed the highest TPCs, FCs, and TCs. TPC, FC, and TC also showed positive correlations with the total antioxidant capacity (TAC), indicating that the species with the highest gallic acid equivalent contents and catechine equivalent contents were also those with the highest TACs. This analysis confirmed that A. armata, H. musciformis, P. capillacea, L. obtusa, and G. gracilis are the species with the strongest antioxidant activities.
Principal component analysis (PCA) of the total phenolic, flavonoid, and tannin contents and the antioxidant activities (DPPH, TAC) of seaweed extracts. Each species is distributed according to its variance. A.a Asparagopsis armata, L.o Laurencia obtusa, P.p Palisada perforata, C.c Ceramium ciliatum, P.s Peyssonnelia squamaria, H.m Hypnea musciformis, C.o Corallina officinalis (Linnaeus), J.l Jania longifurca, P.c Pterocladiella capillacea, G.g Gracilaria gracilis. The two species S. coronopifolius and J. rubens were not taken into account in the PCA analysis due to missing data
Antimicrobial activity
Table 1 shows the antimicrobial activities of algae extracts. Data are only shown for algae with antimicrobial activities toward at least one pathogenic strain. The results show that only three pathogenic strains were sensitive to algal extracts (Pseudomonas cepacia, Streptococcus B, and the yeast Candida albicans); Staphylococcus aureus, Pseudomonas fluorescens, Enterococcus faecalis, and Aeromonas hydrophila were all resistant to all of the tested extracts. Among the twelve algae species from which extracts were obtained, only five displayed antimicrobial activity: P. perforata, S. coronopifolius, J. longifurca, H. musciformis, and P. capillacea. Only weak activities were observed, with inhibition zone diameters ranging from 6 to 10 mm. Streptococcus B was the most sensitive strain, as it was inhibited by 41% of the extracts. Three extracts inhibited Candida albicans—those of P. perforata, H. musciformis, and P. capillacea. Only two extracts—those of J. longifurca and P. capillacea—displayed activity against Pseudomonas cepacia.
Discussion
Total phenolic, flavonoid, and tannin contents
Results showed that the amount of phenolic compounds in the extract depended on the species considered. Natural antioxidants are found in algae as phenolic compounds (flavonoids, xanthones, coumarins, carotenoids, phenolic acid, tannins, anthocyanins, etc.). Polyphenols are a major class of secondary metabolites in macroalgae. Marine polyphenols, or phlorotannins (which are particularly common in brown algae), are a group of a molecules with various structures and degrees of polymerization, and therefore different biological activities (Neethu et al. 2017; Al-Enazi et al. 2018). These metabolites have the capacity to improve food quality and stability, and can also be used as nutraceuticals to terminate free-radical chain reactions in biological systems, providing additional health benefits for humans (Fernando et al. 2020). In this study, several seaweed species such as G. gracilis, A. armata, H. musciformis, S. coronopifolius, P. capillacea, and L. obtusa were found to contain significant amounts of phenolic compounds. The highest phenolic content (19.29 ± 1.8 mg GAE/g DB), which occurred in the G. gracilis extract, is comparable to the content (21.63 mg GAE/g DB) found by Widowati et al. (2014) for the same species collected from aquaculture ponds in Indonesia. Furthermore, the MeOH extract of H. musciformis exhibited a relatively high phenolic content (11 ± 0.7 mg GAE/g DB), which is in agreement with Chakraborty et al. (2015), who observed a content of 9.84 mg GAE/g DB for the same species. Dellai et al. (2013) reported 19.21 mg GAE/g DB of total phenolics for L. obusta, which is slightly higher than the content determined in the present study.
We found the amounts of polyphenols in the members of Corallinales to be low. Comparable levels were obtained by Rico et al. (2012), who noted a total phenolic level of 4.64 mg GAE/g DB for Corallina elongata collected from the Canary Islands.
Only a few studies have analyzed the flavonoid contents of red macroalgae. Sarojini et al. (2012) investigated the flavonoid contents of fifteen seaweeds, including seven members of Rhodophyta. They found that this taxonomic group gave lower levels of flavonoids (6.03–20.91 mg/g DB) than brown algae (20.72–32.89 mg/g DB) and green algae (8.43–33.39 mg/g DB). The flavonoid content of C. officinalis as determined by Ismail (2017) using the aluminum chloride colorimetric technique was 3.48 ± 0.822 mg ECat/g DB, whereas we obtained a value of 0.776 ± 0.1 mg ECat/g DB for this species in the present study. These observed differences between studies may be due to interstudy differences in environmental parameters such as pH, salinity, temperature, geographical location, and biological parameters, which could influence the production of secondary metabolites by these organisms (Heo 2006; Xie et al. 2019).
Tannins are generally defined as high molecular weight polyphenolic compounds (over 1000 kD), and phlorotannins are mostly found in brown algae, where they are used as antioxidative components to overcome oxidative stress (Wei et al. 2003; Gupta and AbuGhannam 2011; Gamze et al. 2014; Creis et al. 2018).
DPPH radical scavenging and total antioxidant capacity
In the present study, the methanol extracts of H. musciformis, A. armata, S. coronopifolius, and P. capillacea gave the lowest IC50 values. In particular, the MeOH extract of H. musciformis showed the highest DPPH antiradical scavenging potency, with an IC50 value of 13.9 ± 0.3 mg/ml; this is comparable to the value obtained by Chakraborty et al. (2015) for this species, 15.4 mg/ml. The antioxidant potential of this alga was also highlighted by Chakraborty et al. (2015), who isolated three substituted aryl meroterpenoids with potential antioxidative activities. In addition, the two compounds phloretin and (−)-epicatechin were extracted from the red algae H. musciformis; these compounds have high potential applicability in the human food and well-being industries (Rozo et al. 2019). Fellah et al. (2017) showed that seasonal variation had a significant effect on the antioxidant activity of Sphaerococcus coronopifolius, with the highest DPPH activity occurring in summer.
Pterocladiella capillacea collected from Tunisian coasts showed significant antiradical activity, and the associated data were in accordance with those reported by Alencar et al. (2018). Those authors demonstrated that fatty acids were responsible for the DPPH radical scavenging capacity.
Antimicrobial activity
The present data for H. musciformis and P. perforata indicate that extracts of these species inhibit Streptococcus B and Candida albicans and were in accordance with previous data from Shanab (2007).
Additionally, the present study indicated that Pseudomonas cepacia and Streptococcus B were inhibited by the methanol extract from P. capillacea, which is accordance with the results reported by Mohy El-Din and El-Ahwany (2016), who found that extracts from the same two species acted against those pathogens. El Kassas and Attia (2014) demonstrated that the observed antibacterial activity against the Gram-positive bacterium B. subtillus in P. capillacea was due to alkaloid compounds.
According to the present data, the Sphaerococcus coronopifolius extract only exhibited activity against Streptococcus B. A broader activity spectrum (antimicrobial and antitumor) was described for this species by Rodrigues et al. (2015) and Pinteus et al. (2015). In contrast to our findings, Bouhlal et al. (2012) reported that S. coronopifolius presented antibiotic activity against E. faecalis. Two tetracyclic diterpenes, ioniols I and II, which possess antibacterial activities against a panel of Staphylococcus aureus strains, were extracted from this species collected from the rocky coasts of the island of Corfu in the Ionian Sea (Smyrniotopoulos et al. 2008).
Using PCA, two clusters of bioactive red algae were clearly identified, in agreement with previous conclusions drawn from data analysis. The first cluster consists of Hypnea musciformis and Laurencia obtusa, which present large amounts of tannins and significant total antioxidant capacities. The second cluster consists of Pterocladiella capillacea, Gracilaria gracilis, and Asparagopsis armata, which present large amounts of total polyphenols and flavonoids and weak DPPH radical scavenging activities (IC50 values). Among these species, A. armata seems to be particularly rich in flavonoids, tannins, and total polyphenols, and it possesses significant antioxidant activity. Individuals of this species collected from other locations have also been reported to produce these bioactive compounds (Zubia et al. 2009; Pinteus et al. 2015; Neethu et al. 2017). This species is considered to be invasive in the Mediterranean Sea (Pinteus et al. 2016), and should therefore be considered for industrial applications.
The results obtained in this study highlight that G. gracilis has a substantial polyphenol content. This species is widely cultivated in Asian countries (FAO 2018), and cultivation data for Tunisia (Ben Said et al. 2018; Chebil Ajjabi et al. 2018) indicate that this species has high potential for development in the southern Mediterranean Sea. In addition, the two species Hypnea musciformis and Pterocladiella capillacea represent potential candidates for the development of the seaweed-based industry in Tunisia, as these two genera are also cultivated in other parts of the world (FAO 2018).
Conclusions
This work enhances knowledge of the potential uses of seaweeds from southern Mediterranean coasts, which is important because such information is lacking for this region. The results highlight the importance of selecting certain species for biotechnological development applications in Tunisia. The present study found that the levels of certain target molecules such as polyphenols, tannins, and flavonoids varied from one species to another. Thus, five red macroalgae species of interest were identified (Asparagopsis armata, Gracillara gracili, Hypnea musciformis, Pterocladia capillacea, and Laurencia obtusa). The results obtained indicated that H. musciformis and L. obtusa present high tannin levels and significant total antioxidant capacities, whereas P. capillacea, G. gracilis, and A. armata possess large amounts of total polyphenols and flavonoids as well as weak DPPH radical scavenging potencies (IC50). It is worth noting that most of the species studied did not exhibit any significant antimicrobial activity.
Seaweeds are a renewable marine resource with multiple possible recovery methods (e.g., CO2 sequestration, wastewater treatment, and bioremediation in integrated multitrophic aquaculture systems). Their cultivation and exploitation in various industrial and innovative sectors could be a key element of the development of a sustainable “blue economy.”
References
Al-Enazi NM, Awaad AS, Alqasoumi SI, Alwethairi MF (2018) Biological activities of the red algae Galaxaura rugosa and Liagora hawaiiana butters. Saudi Pharmaceutical J 26:25–32
Alencar DB, Diniz JC, Rocha SAS, Pires-Cavalcante KMS, Lima RL, Sousa KC, Freitas JO, Bezerra RM, Baracho BM, Sampaio AH, Viana FA, Saker-Sampaio S (2018) Fatty acid composition from the marine red algae Pterocladiella capillacea (S. G. Gmelin) Santelices & Hommersand 1997 and Osmundaria obtusiloba (C. Agardh) R. E. Norris 1991 and its antioxidant activity. An Acad Bras Cienc. 90(1):449–459
Ben Maiz N (1995) Étude nationale sur la diversité biologique de la flore marine et aquatique en Tunisie. Projet de coopération: MEAT/PNUE/GEF. Minister de l’Environment, Tunis
Ben Said R, Mensi F, Majdoub H, Ben Said A, Ben Said B, Bouraoui A (2018) Effects of depth and initial fragment weights of Gracilaria gracilis on the growth, agar yield, quality and biochemical composition. J Appl Phycol 30(4):2499–2512
Bouhlal R, Riadi H, Martínez J, Bourgougnon N (2012) The antibacterial potential of the algae (Rhodophyceae) of the Strait of Gibraltar and the Mediterranean coast of Morocco. Afr J Biotech 9(38):6365–6372
Bouhlal R, Riadi H, Bourgougnon N (2013) Antioxidant activity of Rhodophyceae extracts from Atlantic and Mediterranean coasts of Morocco. Afr J Plant Sci 7(3):110–117
Buschmann A, Camus C, Infante RJ, Neori A, Israel A, Hernández-González M, Pereda S, Gomez Pinchetti JL, Golberg A, Tadmor Shalev N, Critchley A (2017) Seaweed production: overview of the global state of exploitation, farming and emerging research activity. Eur J Phycol 52:391–406
Cabioch J, Floch Y, Le Toquin A, Boudouresque CF, Meinesz A, Verlaque M (2006) Guide des algues des mers d’Europe. Delachaux et Niestlé, Paris
Chakraborty K, Joseph D, Joy M, Raola VK (2015) Characterization of substituted aryl meroterpenoids from red seaweed Hypnea musciformis as potential antioxidants. Food Chem 212:778–788
Chebil Ajjabi L, Abaab M, Segni R (2018) The red macroalga Gracilaria gracilis in co-culture with the Mediterranean mussels Mytilus galloprovincialis: productivity and nutrient removal performance. Aquacult Int 26:253–266
Creis E, Gall EA, Potin P (2018) Ubiquitous phlorotannins prospects and perspectives. In: La Barre S, Bates SS (eds) Blue biotechnology: production and use of marine molecules. Wiley VCH, Weinheim, pp 67–116
Dellai A, Laajili S, Le Morvan V, Robert J, Bouraoui A (2013) Antiproliferative activity and phenolics of the Mediterranean seaweed Laurencia obtusa. Ind Crops Prod 47:252–255
Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50(10):3010–3014
El Kassas HY, Attia AA (2014) Bactericidal application and cytotoxic activity of biosynthesized silver nanoparticles with an extract of the red algae Pterocladiella capillacea on the HepG2 cell line. Asian Pac J Cancer Prev 15(3):1299–1306
FAO (2018) The global status of seaweed production, trade and utilization. Globe Fish Res Program 124:120
Farasat M, Khavari-Nejad RA, Bagher Navari SM, Namjooyan F (2013) Antioxidant properties of two edible green seaweeds from northern coasts of the Persian Gulf. Iran J Pharm Res 13(1):163–170
Fellah F, Louaileche H, Dehbi-Zebboudj A, Touati N (2017) Seasonal variations in the phenolic compound content and antioxidant activities of three selected species of seaweeds from Tiskerth islet, Bejaia. Algeria J Mater Environ Sci 8(12):4451–4456
Fernando IPS, Ryu B, Ahn G, Yeo I-K, Jeon Y-J (2020) Therapeutic potential of algal natural products against metabolic syndrome: a review of recent developments. Trends Food Sci Technol 97:286–299
Fischer W, Bauchot ML, Schneider M (1987) Fiches FAO d’identification des espèces pour les besoins de la pêche, révision 1. Méditerranée et mer Noire. Zone de pêche 37. Volume I. Végétaux et Invertébrés. FAO, Rome, 1:760
Gamze Y, Egemen D, Sukran D (2014) Comparison of the antioxidative components of some marine macroalgae from Turkey. Pak J Bot 46(2):753–757
Gupta S, Abu-Ghannam N (2011) Bioactive potential and possible health effects of edible brown seaweeds. Tre Food Sci Technol 22:315–326
Hellio C, Broise D, Dufossé L, Gal Y, Bourgougnon N (2001) Inhibition of marine bacteria by extracts of macroalgae: potential use for environmentally friendly antifouling paints. Marine Environ Res 52:231–247
Heo SJ, Cha SH, Lee KW, Jeon YJ (2006) Antioxidant activities of red algae from jeju island. Algae 21(1):149–156
Ismail GA (2017) Biochemical composition of some Egyptian seaweeds with potent nutritive and antioxidant properties. Food Sci Technol Campinas 37(2):294–302
Ismail A, Ktari L, Ahmed M, Bolhuis H, Boudabbous A, Stal LJ, Cretoiu MS, El Bour M (2016) Antimicrobial Activities of bacteria associated with the brown alga padina pavonica. Front Microbiol 7
Kolanjinathan K, Ganesh P, Saranraj P (2014) Pharmacological importance of seaweeds: a review. World J Fish Marine Sci 6(1):01–15
Kumar PS, Mubarak AD, Saratale RG, Saratale GD, Pugazhendhi A, Gopalakrishnan K, Thajuddin N (2017) Synthesis of nano-cuboidal gold particles for effective antimicrobial property against clinical human pathogens. Microb Pathog 113:68–73
Lavanya B, Narayanan N, Maheshwaran A (2016) Pharmacological studies on Hypnea musciformis (Wulfen) Lamouroux. IJARIIT 2(4)
Mohy El-Din SM, El-Ahwany AMD (2016) Bioactivity and phytochemical constituents of marine red algae (Jania rubens, Corallina mediterranea and Pterocladia capillacea). J Taibah Univ Sci 10:471–484
Neethu PV, Suthindhiran K, Jayasri MA (2017) Antioxidant and antiproliferative activity of Asparagopsis taxiformis. Phcog Res 9(3):238–246
Pangestuti R, Kim SK (2011) Neuroprotective effects of marine algae. Mar Drugs 9(5):803–818
Pinteus S, Alves C, Monteiro H, Araujo E, Horta A, Pedrosa R (2015) Asparagopsis armata and Sphaerococcus coronopifolius as a natural source of antimicrobial compounds. World J Microbiol Biotechnol 31(3):445–451
Pinteus S, Rodrigues AN, Silva J, Lokman C, Lemos MF, Pedrosa R (2016) The marine invasive Asparagopsis armata (Harvey, 1855) as source of bioactive valuable compounds—antioxidant potential enrichment by vacuum liquid chromatography. In: International Meeting on Marine Research (IMMR’18), Peniche, Portugal, 5–6 July 2018. https://doi.org/10.3389/conf.FMARS.2016.04.00067
Price ML, Van Scoyoc S, Butler LG (1978) A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J Agric Food Chem 26:1214–1218
Prieto P, Pineda M, Anguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Rico M, López A, Tangil MS, Rivero A (2012) Phenolic profile of crude extracts derived from a red alga, Corallina elongata Ellis & Solander. In: Krueger D, Meyer H (eds) Algae. Nova Science, New York, pp 135–144
Rodrigues D, Alves C, Horta A, Pinteus S, Silva J, Culioli G, Thomas OP, Pedrosa R (2015) Antitumor and antimicrobial potential of bromoditerpenes isolated from the red alga Sphaerococcus coronopifolius. Mar Drugs 13:713–726
Rozo G, Rozo C, Puyana M, Ramos FA, Almonacid C, Castro H (2019) Two compounds of the Colombian algae Hypnea musciformis prevent oxidative damage in human low density lipoproteins LDLs. J Functional Foods 60:103399
Sarojini Y, Laakshminarayana K, Seshagiri P (2012) Varation in distribution of flavonoids in some seaweed of Visakhapatnam coast of India. Der Pharma Chem 4(4):1481–1484
Shanab SMM (2007) Antioxidant and antibiotic activities of some algae (Egyptian isolates). Int J Agri Biol 9(2):220–225
Smyrniotopoulos V, Quesada A, Vagias C, Moreau D, Roussakis C, Roussis V (2008) Cytotoxic bromoditerpenes from the red alga Sphaerococcus coronopifolius. Tetrahedron 64(22):5184–5190
Wei Y, Li Z, Hu Y, Xu Z (2003) Inhibition of mouse liver lipid peroxidation by high molecular weight phlorotannins from Sargassum kjellmanianum. J Appl Phycol 15:507–511
Widowati I, Lubac D, Puspita M, Bourgougnon N (2014) Antibacterial and antioxidant properties of the red alga Gracilaria gracilis from the north coast of Tava, Semarang, Indonesia. Int J Latest Res Sci Technol 3(3):179–185
Xie X, He Z, Chen N, Tang Z, Wang Q, Cai Y (2019) The roles of environmental factors in regulation of oxidative stress in plant. Biomed Res Int 8(2019):9732325
Xiulan X, He Z, Chen N, Tang Z, Wang Q, Cai Y (2019) The roles of environmental factors in regulation of oxidative stress in plant. Biomed Res Int 8:9732325
Yalcinkaya T, Uzilday B, Ozgur R, Turkan I, Mano J (2019) Lipid peroxidation-derived reactive carbonyl species (RCS): their interaction with ROS and cellular redox during environmental stresses. Environ Exp Bot 165:139–149
Zubia M, Fabre MS, Kerjean V, Deslandes E (2009) Antioxidant and cytotoxic activities of some red algae (Rhodophyta) from Brittany coasts (France). Bot Mar 52:268–277
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Philippe Michaud, Chief Editor.
Rights and permissions
About this article
Cite this article
Hmani, I., Ktari, L., Ismail, A. et al. Assessment of the antioxidant and antibacterial properties of red algae (Rhodophyta) from the north coast of Tunisia. Euro-Mediterr J Environ Integr 6, 13 (2021). https://doi.org/10.1007/s41207-020-00222-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-020-00222-7