Abstract
The Draa Sfar Mine is one of the main base-metal mines in the region of Marrakesh (Morocco). The residues of the activities of Draa Sfar Mine can be a source of the groundwater pollution due to heavy metals. The aim of the present study was to evaluate the physicochemical and metallic quality of the groundwater at the vicinity of this mine. In this context, groundwater samples were taken from 17 wells in April 2016. The study was achieved on the following major elements (Cl−, Na+, Ca2+, K+, Mg2+, NO3−, SO42− and HCO3−), Eh, pH, EC and the heavy metals (Cu, Pb, Zn and Fe). The heavy metals results show that all the wells that were examined are under the WHO (2011) standards. From a physicochemical point of view, the study proved that the 17 wells contain a high degree of salinity, especially those located in the downstream of the mine (P10, P11, P12, P13, P15, and P16).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
World water resources are confronted with quality and quantity problems. Groundwater is the main source of water supply and is currently heavily consumed (over 70%) as a valuable natural resource for various human activities (Prasad and Narayana 2004). Therefore, groundwater quality deterioration is the most common problem for arid and semi-arid areas (Liu et al. 2015; Carballo et al. 2016), mainly due to mining operations. The impact of base-metal mining activities on groundwater have been widely studied around the world (Rosner 1998; Lottermoser et al. 1999; Blowes et al. 2003; El Amari et al. 2014; Buzatu et al. 2016; Han et al. 2017; Srivastas et al. 2017).
Nowadays, Morocco faces a critical period of shortage in water resources, which reaches less than 1000 m3/inhabitant/year. Multiple studies predict that in the end of 2025 the situation will get worse (less than 500 m3/inhabit/year) (Agoumi and Naji 1998; Tazi et al. 2001). The mine of Draa Sfar and of Hajjar are the main base metal mines still in operation in the Marrakesh region, both of which are generators of acid mine drainage AMD (El Adnani et al. 2007a, b ) [AMD naturally occurs when sulfide tailing (pyrite for example) oxidize on contact with water and oxygen and generate acidic leachate]. The Draa Sfar mining complex is located a few hundred meters from Tensift River. These drainage waters are also conveyed, without any purification, to this river whose waters are reused by the local residents for irrigation. They are characterized by high values of electrical conductivity, which is expressed by very high levels of major ions and fairly high in heavy metals. This effluent exceeds the limit values of the proposed Moroccan standards of direct discharges, therefore it requires treatment before distribution in the receiving media (El Adnani et al. 2007a, b). Furthermore, many studies have been done in this area to determine the heavy metal concentration around mine areas and they detected a negative impact on the environment as the pollution of soil, surface, and groundwater (Boulanouar 1995; Hakkou et al. 2001; El Gharmali et al. 2004; El Gharmali 2005; El Adnani et al. 2007a, b; Ait lamkademe 2010; Oufline et al. 2012; Avila et al. 2012; Barkouch and Pineau 2015; El-Fadili et al. 2015). Actually, there is an actual impact of this mine on the quality of groundwater. This study aims to evaluate the impact of the Draa Sfar mining activity on groundwater resources, based on the study of physicochemical and metallic parameters.
Materials and methods
Research area
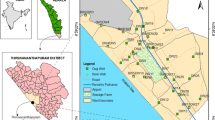
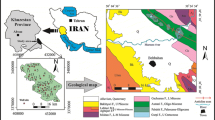
The Draa Sfar mine is located 300 m from Tensift River, in the northern part of the Haouz plain, on the Jebilet, about 16 km northwest of Marrakesh (Fig. 1). It is near a rural community of about 5790 ha, and about 65% of it is farmland. The climate of the place is Mediterranean, almost arid and semi-arid with an average annual of precipitation of 231 mm (10 years). The temperature is characterized by great daily and seasonal variation, with an average value of 11.5 °C in January and 36.8 °C in July (Barkouch et al. 2015). The Draa Sfar Mine is the second producing base of metals mine in Morocco after the Hajjar Mine. It is a volcanogenic massive sulfide (VMS) deposits (Belkabir et al. 2008). The deposit ore from the mine is situated in a series of Sharlef shales, which is localized on the central part of Jebilet, whose land is Visean-Namurian age (Hibti 2001). This mining sector appears as a spike of about 2 km/700 m, which emerges from the Mio-Pliocene and Quaternary cover.
The lithostratigraphy of the Draa Sfar shows two units separated by a horizon with sulfide mineralization. The first is calcarenitic represented by a sandstone and pelitic facies with an intercalation of the limestone. The second is a volcanic and volcano-sedimentary unit. It consists of a sandstone pelitic facies with the base surmounted by rhyolites, pyroclastics, and vesiculated tuffs (Hibti 2001) (Fig. 2).

Modified from Hibti (2001)
a Geological map of the study area and b stratigraphic log of the Draa Sfar Mine.
Draa Sfar consists of a deposit of pyrite discovered in 1953 and had not been until 1979, producing 60 Mt of product in the first 2 years (1979 and 1980) (Ministry of Energy and Mine 1996). Industrial activity stopped in March 1981, although it restarted in 1999 due to its great resource of polymetallic components (Cu, Fe, Pb, and Zn).
The sulfide mineralization is in the form of several lenses of pluralistic dimensions. The mineralogy consists essentially of pyrrhotite (75–90 of the mineralized volume), which is associated with sulfides, sulfo-arsenides, sphalerite, galena, chalcopyrite, arsenopyrite, pyrite, and stannite, as well as oxides (magnetite and cassiterite). Other rare minerals have been observed, namely cobaltine, native bismuth, bismuthinite, paraguanajuatite, and electrum (Hibti 2001). During its exploitation, tailings were discharged all around the mine, posing a risk to the environment.
The hydrogeological system of Jebilet massive is represented by two reservoirs (El Mandour 1990), an upper layer of weathered schist, semi-permeable and capacitive (Transmissivity T 9 × 10−4 m2/s and storage coefficient S 5 × 10−2), and a deeper reservoir, where water circulates in cracks and fractures.
Sample stations
Seventeen groundwater samples were taken in April 2016 in the vicinity of the Draa Sfar Mine. The well samples were selected according to the general flow direction of the groundwater (Fig. 3).
Physicochemical analyses
Water analyses, including sampling and preservation, were carried out according to the standard ISO methods and the analytical procedures recommended by the French Association for Normalisation (AFNOR 1997). Analyses were performed on the water samples after filtration using 0.45-µm acetate cellulose membranes.
The physical parameters of hydrogen potential (pH) and temperature (T) are measured in situ using a pH/mV/°C meter type ADWA AD111. The electrical conductivity (EC) is determined by a conductivity meter type HANN HI 8733. The depth of the water level is calculated via a sound piezometric probe of 200 m electric. The major chemical elements are determined by ion chromatography, using DIONEX ICS-1100 at the two laboratories (Center of Analysis and Characterization of the Faculty of Sciences Semlalia as well as the National Center for Research on Water and the Environment of the Faculty of Science and Technology of Marrakech). The heavy metals (Fe, Cu, Zn, and Pb) were determined by inductive coupled plasma atomic emission spectrometer (ICP-AES) of the Perkin-Elmer Optima 3100RL type at the REMINEX laboratory of the mining company Guemassa (all samples exhibit charge balance errors less than 10%).
Results and discussion
Piezometric level
According to the results that were carried out in April 2016 at 17 wells, the depth of the water varies between 8.1 m (P12) and 34.4 m (P2). The analysis of the piezometric map (Fig. 2) shows that the direction of the groundwater flow is from the southwest to northeast.
Physicochemical quality of water
The quality of water for potability use
Results of analysis of the physicochemical parameters of the wells are shown in Table 1.
Groundwater pH mean value of all samples was relatively close to neutrality, while the temperature varied between 19.5 (well P17) and 24.4 °C (well P7) (Table 1). This temperature is almost in equilibrium with that of the atmosphere because the depth of the wells is low. The temperature and pH values measured are according to the standards of water potability (WHO 2011).
Well electrical conductivity values varied from 1100 to 5000 μS/cm. EC values exceeded the guideline value set by WHO 2011 (180 and 1000 µs/cm). These conductivity values are expressed by very high levels of major elements, especially Cl−, Mg2+, Ca2+, Na+, HCO3− and SO42−. The variations of EC show a significant increase from the mine’s upstream to its downstream. The high EC of the wells could be explained by dissolving sedimentary minerals from Triassic saliferous and calcareous formations (Boulanouar 1995).
Chlorides are important inorganic anions contained in varying concentrations in natural water, usually in the form of sodium (NaCl) and potassium (KCl) salts. They are often used as a pollution index (Abdoulaye et al. 2014). In our samples, the chloride value ranged from 113 in P17 to 1564 mg/l in P16. The water samples upstream of the mine meet the standards for chloride ions (with the exception of well P2 501 mg/l). In contrary, the water points downstream exceeded the standards water of potability determined by WHO (2011) (< 250 mg/l). These variations are mainly due to the infiltration of the mine discharge water from the mine and the lithological nature of the Plio-Quaternary aquifer.
Groundwater sodium values are generally low, with no value exceeding 150 mg/l in the various wells. Sodium levels ranged from 40 in P4 to 141 mg/l in P13 (no value exceeding the WHO limit), and therefore the water examined is eligible for human consumption. As for potassium element, all the water samples have concentrations lower than 6 mg/l and which do not exceed the standards determined by WHO.
The nitrate concentration also varied from one well to another (lower than detection limit in well P17 and 55 mg/l in well P2). The mentioned values are according to the standards of water potability (WHO 2011) (< 50 mg/l), except well P2, which exceeds the recommended standard. These variations may be due to the use of nitrogenous fertilizers from agricultural areas to the wells studied.
The sulfate value of the studied wells varies widely from 97 in P17 to 559 mg/l in P10. High concentrations are placed on the following wells P1, P8, P10, P13, P15, and P16, as they exceed the WHO standard (< 250 mg/l). However, other wells show acceptable values by WHO (2011). High sulfate levels would be related to the dissolution of evaporates minerals (gypsum and anhydrite) (Ait lamkademe 2010).
The bicarbonate value of the points studied varies between 318 in P4 and 440 mg/l P13. The magnesium concentrations ranged from 44 in P6 to 375 mg/l in P16, and the calcium value of the scrutinized water differs between 60 and 657 mg/l. These concentrations confirm the high conductivity observed in the groundwater sample (Fig. 4). These results are similar to those obtained by El Adnani et al. (2007a, b).
Physicochemical analysis results of the water show a significant variability according to well location (a progressive increase in the upstream–downstream direction). The wells located upstream (P1, P2, P3, P4, P5, P6, P7, and P17) generally present a mineralization below the standard of the WHO for water quality. On the other hand, the downstream wells are characterized by high mineralization with conductivities of up to 5 mS/cm and exceeding the WHO (2011) standard. The high conductivities are caused by the high concentrations of Cl−, SO42−, Na+, HCO3−, Ca2+ and Mg2+, mainly due to the lithological nature of the area. The enrichment of sulfate wells could be linked to the dissolution of evaporates minerals (gypsum and anhydrite) by meteoric water before its infiltration into the Plio-Quaternary aquifer (Ait lamkademe 2010). Maybe the high value of SO42− is due to soil leaching or oxidation of sulfides materials present in abundance in this area (Bakalowicz 1974). In addition, the origin of the carbonates is also endogenous due to the calcareous nature of the aquifer matrix, which explains the high Ca and Mg concentrations. The high salinity of the wells is due to the dissolution of saline rocks (rock salt) from Triassic saliferous formations (that enrich water with chloride and sodium ions) (Boulanouar 1995; El Adnani et al. 2007a, b) as well as the infiltrate of excessively saline untreated mine water from the mine.
The high concentrations of sulfates, calcium, sodium, magnesium, and chlorides are explained by an infiltrate leachate often coming from tailings deposited into open area and near the mine (El Adnani et al. 2007a, b).
Hydrochemical facies
Piper diagrams are widely used for classifying hydrogeochemical facies of water basing on major ion analysis. The deferral of the results of the Plio-Quaternary Aquifer water analyses to the triangular piper diagram (Fig. 5) shows an abundance of the major ions already reported. By use of the diagram, it is observed that all the water points present a calcium and magnesium chloride and sulfate facies with the exception of the P17 well water, which has a calcium and magnesium bicarbonate facies.
Heavy metals
The results of the heavy metals analysis of the sampled wells are shown in Table 2. Iron is one of the most abundant metal elements in Earth’s crust. It is found in fresh natural waters at levels ranging from 0.5 to 50 mg/l. All the water samples display iron contents lower than 20 µg/l and therefore below the threshold of 50 mg/l, with the exception of well P17, which has iron concentrations of 97.4 µg/l. The high iron concentrations may be explained by chemical and microbial oxidation of pyrite and pyrrhotite (gangue minerals of the Draa Sfar mine ore) (Boularbah et al. 2006; Hakkou et al. 2008).
Zinc levels in groundwater normally do not exceed 0.05 mg/l (WHO 2011). All analyzed wells have a zinc concentration below 5 mg/l, with the exception of well P8, which has a zinc content of 0.058 mg/l (P8 is the nearest well to the mine, this could be an indication of the mine effect on groundwater). As for lead concentrations, all points have levels below the threshold determined by WHO. As for copper, all the water samples have concentrations lower than 3.6 µg/l and which do not exceed the standards determined by WHO.
The quality of water for irrigation use
The method used to test the irrigation of water of the Plio-Quaternary aquifer is that of Riverside-Wilcox (from diagram software). It is based on the water salinity represented by the electrical conductivity and the degree of soil alkalinity expressed by the sodium adsorption ratio (SAR) (Wilcox 1948).
The transfer of the results of the analysis of the studied area to the Wilcox diagram shows that the water points are distributed between the C3-S1 and C4-S1 classes (Fig. 6). For the first class, water is suitable for the irrigation of salt-tolerant crops and well-drained soils, however salinity must be monitored. Water of the C4-S1 class, in particular the wells P10, P13, P15, and P16, have very high mineralization and conductivity and are therefore not recommended for irrigation.
Conclusions
This study enabled evaluating the physicochemical and metallic quality of water of the Plio-Quaternary aquifer at the vicinity of the Draa Sfar Mine. The analysis of the results revealed that water of controlled points downstream is negatively affected by the geological and anthropogenic origin’s pollution. The presence of limestone, saline, leachate, and tailings of mining residues may be the main cause affecting groundwater quality, as well as the infiltration of the mine discharge water. The groundwater in the mine’s vicinity is of poor quality for the major elements. Therefore, the assessment of contamination by heavy metals in the all wells studied showed that there are too low values, therefore consumption would be safe. The result in 2016 confirmed once again that the pollution of the groundwater downstream of the Draa Sfar Mine is a noticeable issue. This explains why no environmental management approach has been taken since 1995. The overall quality of groundwater within the study area is saline in nature and not suitable for direct use for drinking and agricultural purposes, so water treatment must be required prior to its use.
References
Abdoulaye DN, Khadijettoumint MMS, Mohamed B, Mohamed OSAO, Michel B (2014) Contribution à l’étude de l’évolution Spatio-temporelle de la qualité physicochimique de l’Eau de la Rive droite du fleuve Sénégal. J Mater Environ Sci 5(1):320–329
AFNOR (1997) Qualité de l’eau, recueil des normes françaises, vol 2, 2nd edn. Association Française de Normalisation, Paris
Agoumi A, Naji A (1998) Changements climatiques et ressources en eau dans les pays du Maghreb. Rapport établi dans le cadre du projet PNuD-FEM RAB/94/g31
Ait lamkademe A (2010) Origine de la salinité de l’eau dans les schistes profonds dans la région de Marrakech. Thèse de doctorat, de l’Université Paris Sud 11, pp 120
Avila M, Perez G, Esshaimi M, Mandi L, Ouazzani N, Brianso JL, Valiente M (2012) Heavy metal contamination and mobility at the mine area of Draa Lasfar (Morocco). Open Environ Pollut Toxicol J 3(Suppl 1-M2):2-12.11
Bakalowicz M (1974) Géochimie des eaux aquifères karstiques I. Relation entre minéralisation et conductivité. Ann Spéléol 29:267–282
Barkouch Y, Pineau A (2015) Evaluation of the impact of mine activity on surrounding soils of Draa Lasfar mine in Marrakech-Morocco. Afr J Environ Sci Technol 10(1):44–49
Barkouch Y, El Fadeli S, Lakmichi H, Khadiri ME, Pineau A (2015) Characterization of five heavy metal fractions in agricultural soils around the mine area of Draa Lasfar Marrakech-Morroco. Asian J Sci Technol 6:1588–1594
Belkabir A, Gibson H, Marcoux E, Lenz D, Rziki S (2008) Geology and wall-rock alteration at the Hercynian Draa Sfar Zn-Pb-Cu deposit Morocco. Ore Geol Rev 33:280–306
Blowes D, Ptacek C, Jambor J, Weisener C (2003) The geochemistry of acid mine drainage. Treatise Geochem 9:149–204
Boulanouar M (1995) Faune aquatique des puits et qualité de l’eau dans les régions de Marrakech et des Jbilet. Statut et dynamique d’une population de Proasellus coxalis africanus (Crustacés Isopodes) des Jbilet. Thèse de doctorat, D’Etat Fac Sc Semlalia Marrakech Morocco, p 207
Boularbah A, Schwartz C, Bitton G, Aboudrar W, Ouhammou A, Morel JL (2006) Heavy metal contamination from mining sites in south Morocco: 2. Assessment of metal accumulation and toxicity in plants. Chemosphere 63:811–817
Buzatu A, Dill H-G, Buzgar N, Damian G, Maftei A-E, Apopei A-I (2016) Efflorescent sulfates from Baia Sprie mining area (Romania)—acid mine drainage and climatological approach. Sci Total Environ 542:629–641
Carballo MA, Macias F, Nieto JM, Ayora C (2016) Long-term fluctuations of groundwater mine pollution in a sulfide mining district with dry Mediterranean climate: implication for waters resources management and remediation. Sci Total Environ 538:327–334
El Adnani M, Rodriguez-Maroto JM, Sbai ML, Idrissi Loukili L, Nejmeddine A (2007a) Impact of a polymetallic mine (Zn, Pb, Cu) residues on surface water, sediments and soils at the vicinity (Marrakech, Morocco). Environ Technol 28(9):969–985
El Adnani M, Ait Boughrous A, Yacoubi Khebiza M, El Gharmali A, Sbai ML, Erraouane S, Loukili Idrissi L, Nejmeddine A (2007b) Impact of mining wastes on the physicochemical and biological characteristics of groundwater in a mining area in Marrakech (Morocco). Environ Technol 25(1):71–82
El Amari K, Valera P, Hibti M, Pretti S, Marcello A, Essarraj S (2014) Impact of mine tailings on surrounding soils and ground water: case of Kettara old mine Morocco. J Afr Earth Sci 100:437–449
El Gharmali A (2005) Impact des résidus miniers et des eaux résiduaires sur la contamination métallique des écosystèmes aquatiques et terrestres de la région de Marrakech. Thèse doctorat d’Etat Université Cadi Ayyad Faculté des Sciences Semlalia Marrakech Morocco, p 166
El Gharmali A, Rada A, El Adnani M, Tahlil N, El Meray M, Nejmeddine A (2004) Impact du drainage minier acide sur les écosystèmes aquatiques superficiels dans la région de Marrakech Maroc. Environ Technol 25:1431–1442
El Mandour A (1990) Actualisation des connaissances hydrogéologiques du massif des Jebilet, Meseta Occidentale. Thèse 3ème cycl, Université Cadi Ayyad, Marrakech, Maroc
El-Fadili S, Bouhouch RR, Benmazhar H, Barkouch Y, Zimmerman MB, Sedki A (2015) Caractérisation de la qualité physico-chimique et minéralogique de l’eau de consommation de quatre zones de la ville de Marrakech-Maroc. J Mater Environ Sci 6(9):2437–2445
Hakkou R, Wahbi M, Bachnou A, El Amari K, Hanich L, Hibti M (2001) Impact de la décharge publique de Marrakech (Maroc) sur les ressources en eau. Bull Eng Geol Environ 60:325–336
Hakkou R, Benzaazoua M, Bussière B (2008) Acide mine drainage at the abandoned Kettara mine Morocco: 1: Environmental characterization. Mine Water Environ 27:145–159
Han Y-S, Youm S-J, Oh C, Cho Y-C, Ahn J-S (2017) Geochemical and ecotoxicological characteristics of stream water and its sediments affected by acid mine drainage. Catena 148(1):52–59
Hibti M (2001) Les amas sulfurés des Guémassa et des Jebilet (Mesta sud-occidentale, Maroc): témoins de l’hydrothermlisme précoce dans le bassin mesetien. Thèse de doctorat, D’Etat. Fac. Sc. Semlalia Marrakech, Morocco, p 301
Liu F, Song Yang L, Han D, Zhang Y, Ying Ma HBu (2015) The role of antropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subel Lake basin, Ordos energy base, Northwestern China. Sci Total Environ 538:327–334
Lottermoser BG, Ashley PM, Lawie DC (1999) Environmental geochemistry of the Gulf Creek copper mine area, north-eastern New South Wales, Australia. Environ Geol 39:61–74
Ministry of Energy and Mine (1996). Regional delegation of Marrakesh. Marrakesh region monography (geology, mine, energy)
Oufline R, Hakkou R, Hanich L, Boularbah A (2012) Impact of human activities on the physico-chemical quality of surface water and groundwater in the north of Marrakech (Morocco). Environ Technol 33:2077–2088
Prasad BG, Narayana TS (2004) Subsurface water quality of different sampling stations with some selected parameters at Machilipatnam Town. Nat Environ Pollut Technol 3:47–50
Rosner U (1998) Effects of historical mining activities on surface water and groundwater—an example from northwest Arizona. Environ Geol 33:224–230
Srivastas SK, Yadav HL, Seervi V, Jamal A (2017) Assessment of water quality near vicinity of lignite mine region, Gujarat, India: a case study. IARJSET 4:42–47
Tazi O, Abdelilah F, El-Younoussi S (2001) Impact de la pollution sur l’unique réseau hydrographique de Casablanca, Maroc. Science et Changements Planétaires/Sécheresse 12:129–134
WHO (2011) Guidelines for drinking-water quality. Recommendations, 4th edn, p 518
Wilcox LV (1948) The quality of water for agricultural use. US Dept Agric Tech Bull 962:19
Acknowledgements
The authors would like to thank Ali Bouari, Jaber Hassna, El Azhari Abdellah, and all the staff of the Center for Analysis and Characterization (CAC) and the National Center for Research on Water and the Environment (CNEREE), UCA, Marrakech, for their collaboration during hydro-geochemistry analyses. Many thanks to the editors and reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The present paper is an original work and all the authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Boujghad, A., Bouabdli, A. & Baghdad, B. Groundwater quality evaluation in the vicinity of the Draa Sfar Mine in Marrakesh, Morocco. Euro-Mediterr J Environ Integr 4, 12 (2019). https://doi.org/10.1007/s41207-018-0096-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-018-0096-3