Abstract
Carbonate aquifers provide large parts of the water supply for more than a quarter of the world’s population. The geochemical assessment of these heterogeneous aquifers is a valuable endeavor to ensure a rational management and protection of groundwater resources. To secure its durability, regard to increasing water demands and climate challenges make the problem of prevalence of water scarcity, vulnerability, and drought conditions extremely complicated. In Tebessa–Kasserine basin (Tuniso-Algerian international border), karst aquifers are receiving increasing interest, as the area has typical karst landscape and the hydrogeological system mostly consists of carbonates formation. Thus, a thorough understanding of aquifer behavior and water mineralization origin using geochemical and statistical tools can lead to relevant information regarding karst processes, groundwater chemistry, and protection. Subsequently, this study represents a pioneer baseline of the hydrogeochemical characterization of karst features in this international border area and it aims to identify the origin of karst water mineralization, its spatial distribution, and factors influencing water composition. The hydrogeochemical assessment of the sampled waters shifts from low mineralized Ca-HCO3 waters to Ca-SO4 and Na–Cl water types. TDS values range from 10 to 490.66 mg/l. A Gibbs diagram indicates that karst waters have been recently recharged by direct rainfall infiltration. Water quality gets modified along pathways and dissolution, formations weathering, and ion exchange processes seem to be the predominant geochemical factors influencing water mineralization. The PCA confirms the spatial variability of water types and indicates that it largely depends on aquifer lithology and on geographical position of water points.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbonate hydrogeological aquifers constitute a unique underground ecosystem and provide important water resources for about a quarter of the world’s population (Ford and Williams 1989, 2013; Hartmann et al. 2009). Nevertheless, karst features exhibit a high degree of variability caused by heterogeneous anisotropic distribution of porosity, permeability, and flow patterns related to the complex geological and hydrochemical context of karst processes (Panagopoulos et al. 2005; Hamed et al. 2014, 2017b; Nicolini et al. 2016; Khaska et al. 2013; Demdoum et al. 2015). Hence, there is still limited knowledge about their recharge conditions, their vulnerability to pollution, as well as their sensitivity to climate change challenges. The drought conditions, with considerable decrease in natural water supply, are expected to have significant unpredictable impacts on groundwater availability and suitability for different uses. In this regard, a number of papers have approached the karst recharge assessment and simulation (White 1988; Woo and Moore 1996; Williams 2008). It strongly depends on the morpho-structural context of fractures and karst discontinuity and on carbonates layers permeability (Sen 1995) related to seals and beds extension, carbonates dissolution, groundwater flows, and residency that vary dramatically in kart environment (Kacaroglu 1999; Worthington et al. 2000; White 2002). Assessing spatial distribution of karst features, physico-chemical characterization of groundwater in carbonates reservoirs and monitoring of the anisotropic aquifers proprieties constitute a dynamic field of research (Lopez-Chicano et al. 2001; Aquilina et al. 2003, 2005, 2006; Barbieri et al. 2005, 2017; Tuccimei et al. 2005; Moore et al. 2009; Barberá and Andreo 2012; Bicalho et al. 2012; Hamed et al. 2014, 2017b; Mokadem et al. 2016). In most well-studied cases, aquifer geomorphological aspects or karst environmental issues have long been assessed (Klimchouk et al. 1996; Calaforra and Pulido-Bosch 1999; Johnson and Neal 2003; Gutiérrez et al. 2008; Cooper and Gutiérrez 2013). Scarcely, few works focused on monitoring of water mineralization and on evaluation of the predominant geochemical processes influencing the spatial variability of groundwater composition (dissolution, mixing,…) (Raines and Dewers 1997; Kaçaroglu et al. 2001; Günay 2002; Land 2003; Nader et al. 2003; Lamont-Black et al. 2005; Yechieli et al. 2006; Omelon et al. 2006; Moore et al. 2009; Wu et al. 2009; Chiesi et al. 2010; Fidelibus et al. 2011; Apaydin and Aktas 2012), or its possible relation to different hydrogeological factors and water–rock interactions (Jiménez-Torrecilla et al. 2004; Moral et al. 2008; Sánchez et al. 2015; Nicolini et al. 2016; Nguyet et al. 2016; Besser et al. 2017; Hamed et al. 2017a). Chemical assessment of karst groundwater requires careful studies for the evaluation of their vulnerability, their protection, and their pollution especially in recharge zones (Barbieri et al. 2017; Hamed et al. 2017b).
In this regard, as in the study area, the hydro-geochemical characterization of karst hydrogeological systems has never been addressed in an exhaustive way in any previous survey. This study represents a pioneer baseline hydrogeochemical investigation of carbonate reservoirs in the studied region. It aims to obtain a refined comprehension of the hydro-geological and hydro-geochemical behavior of the studied basin. It attempts an assessment of groundwater chemistry, an evaluation of karst system recharge conditions, and water mineralization processes. Subsequently, the geochemical composition of number of sampled waters (springs, wells, boreholes) are carefully examined using multivariate analyses, ionic ratios, and mineral solubility evaluation together with its possible relations to different hydrogeological variables. Consequently, a general hydro-chemical model based on field observations and the obtained data is proposed to conduct a platform for further studies aiming for a detailed survey and more efficient water resource management in Tebessa–Kasserine basin. The Tebessa–Kasserine karst system includes different groundwater reservoirs as they are characterized by rapid water infiltration and groundwater circulation, related to the development of complex networks of preferential pathways provided by opened joints and karst evolution (Hamed et al. 2014; Hadji et al. 2014).
Geological and hydrogeological setting
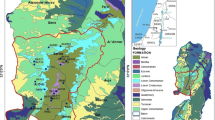
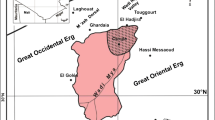
The study area, Tebessa–Kasserine plain, lies between the longitudes 35°33′ to 35°9′E and the latitudes 7°50′ to 8°50′N (Fig. 1). It is bounded by the Atlas Mountains along Algeria-Tunisia confines and is characterized by high mountains with altitude ranging from 670 to 1700 m. Geologically, this area has a typical karst landscape. The synthetic stratigraphic sequence, from Cretaceous to Tertiary (Albian to Eocene), is dominated by limestones, dolomites, clayey limestones, and clayey dolomites. The Cretaceous carbonates are outcropping across the area. Climate conditions enhance the dissolution of limestones and dolomites rocks resulting in an intense karstification of carbonates units from Mesozoic and Cenozoic times, except Albian carbonates formations. These carbonate massifs include diverse reservoirs related to the development of complex networks of preferential pathways provided karst evolution (Lapiaz, gaves, chasm, fractures…) (Fig. 2) and of ineffectiveness of forest cover. Tectonically, the study area is a part of the intensely deformed domain of Oriental Saharan Atlas. It is characterized by a development of various compressional and extensional attributed to Plio-Quaternary deformations inherited from early Cretaceous tectonic phase. The main NE-SW fault systems have a dip roughly 60° (Addoum 1995; Ahmadi et al. 2006). This trend is also expressed by Cretaceous limestones NE–SW folds often pierced by Triassic salt deposits while the Eocene carbonates from the core of the synclinal structures. Tectonic features, glacis morphology, and alluvial sediments play a crucial role in the spatial distribution of the hydrographic network and in the intense karstification of carbonates reservoirs in the area (Fig. 2) (Kowalski 1997).
Map of the main axis of Bouakkous cave of and the underground river system of Hammamet, Tebessa-Algeria (Quinif 1975); modified; a front view of Bouakkous cave, b the access to the Bouakkous cave; c the emergent part of the Bouakkous cave; d the underground lake of the Bouakkous cave
The hydrogeological system of the Tebessa–Kasserine basin is represented by five carbonate reservoirs formed by:
-
Albian carbonate formations admitting frequent intercalations of clays, marls, and dark marly limestones;
-
Cenomanian karst unit (limestones);
-
Karstified Turonian limestones;
-
The principal karst aquifer logged in Maastrichtian limestones;
-
Karstified Eocene limestones.
The basin has experienced in recent years an urban, industrial, and agricultural development. The use of chemical fertilizers represents the main causes of water resource pollution in the study area. Nitrate, originating mainly from agriculture, is considered to be the principal contaminant of groundwater in this region. The vegetation types are irrigated fields crops and orchards. This activity resulted in an overexploitation of groundwater, involving a negative water balance in for eseeable future and pollution groundwater.
Materials and methods
Sampling
Different sampling trips were conducted during 2016–2017 period to collect water samples from a number of springs, boreholes, and wells in the study area. A preliminary exploration of the studied region led to relevant information about appropriate locations of the chosen samples carefully identified, in gained insights from the assessment of geological and structural context of the region of interest evaluated based on both a literature review (regional geological map) and field observations. A total of 47 water points referring to one well, six boreholes, and 40 springs have been selected across the study area. Field data (depth, well and borehole productivity, discharge mechanism, spring debit, etc.) is used to conduct a spatial database of the studied region using GIS platform.
Field measurements of pH, TDS, EC, and temperature of the sampled waters were recorded using a multi-parameter portable device (conductometer, Ph/Eh meter, temperature sensor, etc.). Special material has been used for a critical evaluation of groundwater quality. For major and minor element concentration evaluations, the samples were kept in 50, 500, and 1000 polyethylene bottles, carefully sampled, stored in wet ice at constant temperature, and transferred to CERAD laboratory (Tebessa-Algeria) where geochemical analyses were carried out.
Analyses
The sampled water for classical dissolved mineral analyses have been filtered and conserved in glass flacons of 60 ml for anion analyses in Nalgene bottles for both trace element analyses and major cation concentration analyses after acidification. Special materials have been used during analyses (HACH for Alcalimetric dosage, 0.45-µm filters, sealed water circulation cells for separation from the atmospheric system, ultra-pure HNO3 for acidification, etc). Concentrations of major and minor elements have been assessed using standard methods: spectrometric technique, chromatography liquid phase, and flame photometer. All samples exhibit charge balance errors less than 10%.
Principal component analysis (PCA)
PCA is widely used as a multivariate statistical method for chemical and physical groundwater characterization. It reduces the complexity and deciphers patterns within large sets of data (Wold et al. 1987; Farnham et al. 2003). It examines the underlying patterns or relationships for a large number of inter-correlated variables and summarizes information in a smaller set of factors or components that account the greatest amounts of variance. This aggregation in a limited number of factors permits the representation of the original data in a standardized test score space, facilitating the interpretation of the data with a minimum of information loss (Saporta 2006). When PCs are plotted together, similarities and disparities are expressed by closely grouped points referring to different processes influences. For this study, PCA is applied to normalized matrix variables from 47 water points. The interpretation and the integration of the obtained data using different geochemical ratios and the most common representations is obtained by the use of XLSTAT (version 2014), Diagramme 4.0, Phreeq C, and ArcGis software.
Results and discussion
Hydrochemistry
In situ measurements
All karst groundwater, in the study area, is neutral with pH ranging from 6.7 to 8.3, within the normal limits for natural groundwater. The average pH value for the five Turonian boreholes is about 7.41 lower than that of the deep thermal water of Boulaâba (Kasserine), which exhibits a pH of about 7.6 (Fig. 3a). The highest pH value is found at the Varconian-Albian Salhi water in Bou Roummane Jebel (Tebessa), which reveals a pH slightly basic (8.3), related to sulfur dominance.
a Spatial distribution of pH values in Tebessa–Kasserine basin; b spatial distribution of pCO2 values in Tebessa–Kasserine; c spatial distribution of TDS values (mg/l) in Tebessa–Kasserine basin; d spatial distribution of Ca concentrations (meq/l) in Tebessa–Kasserine basin; e spatial distribution of Mg concentrations (meq/l) in Tebessa–Kasserine basin; f spatial distribution of HCO3 concentrations (meq/l) in Tebessa–Kasserine basin
Correspondingly, the calculated pCO2 varies between 5. 10−4–4.44 10−2 atm. The lowest values are attributed to thermal waters of Hammamet and Boulaâba and to Serdiesse spring water expressed by 5.10−4, 8.10−4, and 7.10−4 atm, respectively, suggesting the influence of karst–non-karst contact and leaching of marl deposits in pCO2 spatial variation. In contrast, higher values, compared to 10−3.5 atm of precipitations, characterize the Maastrichtian, Turonian, Cenomanian, and Eocene reservoirs (Fig. 3b) at high altitude of Jebel Doukkane (1712 m), Mestiri, Mizeb, Tenoukla (1556 m) and Jebel Dyr (1472 m), respectively, indicating additional sources of CO2 in groundwater among them; root respiration and soil organic matter (Van der Weijden and Pacheco 2003; Emblanch et al. 2003; Hamed et al. 2013). Correspondingly, an increase pCO2 induces an obvious clear decrease in pH values.
The measured TDS values range between a minimum of 10 mg/l (Gattara spring in Maastrichtian formations) and a maximum of 490.66 mg/l in the thermal water of the Turonian (Hammame) with a mean value of 130.86 mg/l (Fig. 3c). The highest TDS values are found at Mestiri Jebel, Doukkane and Hammamet, in the West of Tebessa and in the bordering region at Bouderiesse Jebel, indicate long residence time and enhanced dissolution of water-bearing friable limestones starta. Similarly, at Lajred Jebel, in the eastern side, the increasing TDS values are explained by the interaction between water and the hosted rocks admitting frequent intercalations of marls. The average values are found at Sammama, Boulaâba, and Chaâmbi Jebels (Kasserine region thermal water), while the low mineralized water is mainly obtained at the central part of the studied area, in Dyr Jebel, in the south of Bouroumane, Mizeb, and Tenoukla Jebels as well as in the west of Jebel Troubia (Fig. 3c).
Major elements
Ca, Mg, and HCO3 are the dominant elements in groundwater chemistry in the studied area. The Ca concentrations vary between 1.8 and 11.75 meq/l (Fig. 3d). The highest calcium concentrations are found at Chaâmbi Jebel (thermal water of Boulaâba borehole) with 11.75 meq/l. This value can be explained by the enhanced dissolution of carbonates units by the thermal waters (increasing of permeability). Similarly, these values are obtained for Dyr Jebel related mainly to the leaching of the intensively fractured Eocene formations (Kamgang and Ekodeck 1991; Vicat et al. 2002). The lowest calcium concentrations are measured in Bouromane Jebel waters (0.9 meq/l) hosted in Vraco-Albian formations, as well as close to Doukkane Jebel, Troubia, and Mestiti in the Maastrichtian formations.
Magnesium concentrations vary between 0.22 and 3.1 meq/l (Fig. 3e). The highest magnesium concentrations are measured from the south of Mizeb Jebel (Turonian; boreholes T9, T5, T8) towards Dyr Jebel (Samaia, Baida, Trab 1, Trab 3; Eocene), at Hammamet and with slight increase at Bouderiass Jebel probably due to the existence of Turonian limestone formations and Eocene carbonate units characterized by high Mg content. However, the lower concentrations are attributed to Troubi and Gouraye Jebels, indicating ion exchange process with Maastrichtian limestones admitting intercalations of marl units; while the decrease of Mg concentrations in Bourommane Jebel (0.2 meq/l) may be attributed to the aquifer lithology or to the saturation or over-saturation status of these waters with respect to dolomite minerals in the Vraco-Albian aquifer (Nader et al. 2003). Similarly, for spring waters of Lajred, Sammama, and Boulaâba Jebels, the lower measured values can be explained by the Turonian facies dominated by marly limestones or by the hydrothermal nature of these springs.
As seen in Fig. 3f, based on bicarbonate concentrations ranging from 0.57 and 5.21 meq/l, three separated groups were identified; the east and west sectors are characterized by low bicarbonate concentrations (Troubia, Mestiri, and Doukkane Jebels). The obtained values in the west are mainly related to water circulation within the karst system and the transit time while in the eastern side, in Sammama Jebel, they can be attributed essentially to thermal waters of Boulaâba and Chaâmbi Jebels boreholes. The central part of the study area reveals increasing values of HCO3, especially at Dyr Jebel, composed of intensively fractured friable Eocene limestones, similarly, at Turonian formations of Mizeb Jebel, Tenouka (Cenomanian) and Hamame (Turonian). A sudden variation in HCO3 concentrations is observed at Gouray and Bouroumane Jebels. These particular measures exhibit the tectonic control in the lateral interconnection of different lithologies (Fig. 3f).
Hydrochemical facies
Chemical composition from 47 sampling sites is shown in a Piper diagram (Piper 1944). Three different groups are identified relative to the three abundant water types for karst systems (Fournillon 2012), suggesting that karst waters are dominated different contribution of fresh water, deep flows paths, and mixing of them (Barbieri et al. 2017). Groundwater shows a shift from low mineralized Ca-HCO3 water type referring to the majority of sampled waters, to high mineralized Ca–Cl waters, attributed to thermal waters (Boulaâba borehole) with 21% of the total analyzed samples. Serdiess spring, hosted in Maastrichtian limestones admitting frequent intercalations of marl and gypsum deposits (Fig. 4), and Kourbil groundwater, logged in Vraco-Albian limestones, exhibit Na–Cl hydrochemical facies and correspond to 4% of the total sampled waters. The spatial distribution of different water types leads to consideration of different salinity sources and a variety of mineralization processes.
Mineralization processes
As seen in Fig. 5, the Gibbs diagram, a useful tool for identifying mechanisms controlling groundwater chemistry, applied on the sampled waters, indicates that hydrochemical composition of karst groundwater in the study area is mainly controlled by rainfall infiltration as well as the dissolution of the hosted aquifers, especially for Eocene and Maastrichtian reservoirs.
In this connection, the involvement of carbonate dissolution in groundwater mineralization is evaluated by different correlations; pH-pCO2, Ca-TDS, Mg-TDS, and HCO3-TDS. According to the scatter plots shown in Fig. 6, these elements are apparent, obviously, in relative correlation with groundwater salinity and chemical weathering. pCO2-pH scatter plot reveals a coefficient of 0.604 (Fig. 6a) and shows a clear correlation with thermal springs and for marl-limestones contact (Boulaâba, Hammame boreholes and Serdiess spring) and non-proportional relation (non proportional) with spring related to karstified and fissured aquifers (Youkous, El Annba, Samaia, Elkarma, etc). Correspondingly, the binary diagrams of major elements (Fig. 7) confirm that dissolution and rock–water interactions seem to be the dominant factors in groundwater chemistry control. Figure 7a shows that Ca and HCO3 correlate linearly with a slope close to 1:2, indicating a common origin of these elements. Calcite dissolution largely contributes to groundwater mineralization. The sampled waters shift to the left relative to an excess of HCO3 over Ca, suggesting an additional source of bicarbonate related to dolomite dissolution (Fig. 7b). Besides carbonate dissolution, an additional source of Ca and Mg concentration should be accountable for Ca–Mg extra over HCO3 (Fig. 7c) probably explained by evaporates dissolution. In this regard, Fig. 7d, e illustrates the correlation between Ca, Mg, and SO4 concentrations and reveals that the involvement of evaporates formations weathering in water mineralization especially for Boulaâba thermal water that shows a clear dominance of sulfate (Fig. 7d, e) relative to the leaching of gypsum formations. Additionally, the oxidation of plant-derived organic matter leads to increasing SO4 and HCO3 concentrations expressed by a relative excess of SO4 over Ca (Fig. 7d) while the Ca excess may be attributed, furthermore, to a reduction of dissolved sulfates H2S.
Based on the comparison between Ca/Mg and Ca/HCO3 (Fig. 7a, b, f), it seems that there is a notable difference in kinetic of calcite and dolomite dissolution (Mudry 1987; Emblanch 1997; Emblanch et al. 1998; Celle-Jeanton et al. 2001). Two different processes occur successively; first, a congruent dissolution of calcite with marginal contribution of dolomite until saturation with respect to calcite and its subsequent precipitation and second, an incongruent dissolution of dolomite (Mudry 1987; Emblanch 1997; Emblanch et al. 1998; Celle-Jeanton et al. 2001). Otherwise, regard to the difference in solubility products of two minerals, water initially dissolves calcite. Once saturated, the system continues to be driven irreversibly by the dissolution of dolomite and concomitant precipitation of calcite. The dedolomitization induces a gradual enrichment in magnesium and gradual depletion of calcium (Fig. 7f) related to affect rock’s porosity and density (Lumsden and Chimahusky 1980; Raines and Dewers 1997; Nader et al. 2003). Hence, the Mg/Ca ratio is often used as a good indicator of residence time. Calcite dissolves more quickly than dolomite and shows a rapid equilibrium with the hosted rock while Mg concentrations reveal a progressive increase with flow paths and residency (Fig. 8a).
Saturation indexes
The calculated saturation indexes with respect to different minerals, using Phreeq C software in the basis of the measured major ions concentrations for the analyzed water, are presented in Table 1. Karst groundwater in limestone and dolomite aquifers is saturated to over-saturated with respect to calcite and dolomite minerals as the saturation index (SI) calcite ranges for both between − 1 and 1 for the majority of waters samples (Fig. 8b, c). Waters from Hammem thermal resources and Kourbil spring are plotted largely below the equilibrium line, for SIcalcite of about − 3.1 and − 2.05, respectively. For the latter, the calcite under-saturation is mainly attributed to the recent recharge of fresh rainfall water and it rapid circulation within karst conduits while for the thermal water in Hammame area, the mixing of two fluids saturation with calcite, the fresh water coming from recharge areas and groundwater from deeper circuits can result in a fluid under-saturated with calcite (Ward and Halley 1985; Barbieri et al. 2017). In this connection, Fig. 8c illustrates two different groups; the first is saturated to oversaturated with respect to dolomite mineral while the second is largely below the equilibrium line. This can be attributed to the kinetic of dolomite dissolution function of water residency. Additionally, the well-fitting linear line SIcalcite and SIdolomite, shown in Fig. 8d, indicates the weathering process of carbonate rocks within the aquifer with a rapid dissolution of calcite. This is inconsistent with obtained results from the previous section.
The involvement of evaporate formation dissolution in groundwater chemistry is evaluated by the calculated gypsum and halite saturation indexes. As seen in Fig. 8e, f, the analyzed waters are plotted largely below the equilibrium line with SIgypsum and SIhalite ranging respectively from − 4.8 and − 0.55 and − 10.28 and − 5.71. The highest values for both minerals are attributed to Boulaâba thermal water.
Statistical analyses: principal components analyses (PCA)
The PCA applied for 47 water points of karst groundwater displays nine factors explaining 100% of total variance. The three first factors account for 80.12% of variance or 45.16, 22.67, and 12.29%, respectively (Tables 2, 3). By plotting PC1 and PC2 together, differences in loadings are presented by two separated clusters (Fig. 9a). Cl, Na, Ca, and SO4 indicate strong positive loadings on PC1. The first factor represents different sources of groundwater salinization; dissolution, ion exchange, weathering of the hosted rock, while HCO3 and Mg show a strong positive loading on PC2 explaining carbonates dissolution, the principal mineralization processes within karst aquifers. pH and EC show moderate loadings for F1 and F2, respectively.
In consistence with obtained data from geochemical characterization of the sampled waters, the multivariate analyses indicates that marl and gypsum dissolution largely contributes to groundwater mineralization. The evaporitic chloride-sulfate minerals represent an additional source of groundwater salinity, in addition to limestone and dolostone dissolution. Otherwise, groundwater mineralization seems to be influenced by two principal factors; aquifer lithology and geographic position of the sampled water. Thus, the interpretation of the obtained data is reevaluated (Fig. 9b) by a more detailed examination sector by sector based essentially in the dominant carbonate lithology expressed by HCO3, Mg and Ca to gain additional insights from limestone, dolomite, and evaporitic (80% limestones and 20% marls) dissolution assessment.
Conclusions
In Tebessa-Kasserine transboundary basin, limestone and dolomite aquifers are characterized by low mineralization and a dominant calcium-bicarbonate facies with the exception of samples attributed to thermal waters, essentially Boulaâba borehole, groundwater from different fracture zones and permanently in contact with non-karst formations where salinity levels up to more than 400 mg/l and the hydrochemical facies shifts to Ca–Cl and Na–Cl water types. The majority of sampled waters are saturated to oversaturated with respect to calcite and dolomite minerals. Besides to carbonates dissolution, ion exchange and marl, gypsum and evaporitic formations weathering and CO2 partial pressure variations seem to be the key factors influencing groundwater mineralization.
In regards to their recent recharge par rainfall infiltration and their rapid circulation within karst systems, the analyzed waters are, generally, drinkable, except the Sahli and Kourbil springs, regard to high sulfur concentrations. The results of these works provide valuable information for groundwater exploration in the studied region. However, and taking into account the high degree of variability and heterogeneity of karst aquifers, additional insights for the protection and the reasonable management of these freshwater resources can be gained from a systematic physical and chemical monitoring of these resources. The increasing temperature and the decrease of natural supplies will add another dimension for the issues of water availability and quality in the study area.
References
Addoum B (1995) L’Atlas Saharien Sud-oriental : Cinématique des plis-chevauchements et reconstitution du bassin du Sud-Est Constantinois (confins algéro-tunisiens). Thèse Doc. Ès Sci. Univ. Paris XI Orsay
Ahmadi R, Ouali J, Mercier E, Mansy JL (2006) The geomorphologic responses to hinge migration in the fault-related folds in the Southern Tunisian Atlas. J Struct Geol 28:721–728
Apaydin A, Aktas SD (2012) Assessment of groundwater quality of the Tatlicay aquifer and relation to the adjacent evaporitic formations (Cankiri, Turkey). Environ Monit Assess 184:2337–2357
Aquilina L, Ladouche B, Doerfliger N, Bakalowicz M (2003) Deep water circulation residence time and chemistry in a karst complex. Ground Water 41(6):790–805
Aquilina L, Ladouche B, Doerfliger N (2005) Recharge processes in karstic systems investigated through the correlation of chemical and isotopic composition of rain and spring-waters. Appl Geochem 20:2189–2206
Aquilina L, Ladouche B, Dorfliger N (2006) Water storage and transfer in the epikarst of karstic systems during high flow periods. J Hydrol 327:472–485
Barberá JA, Andreo B (2012) Functioning of a karst aquifer from Spain under highly variable climate conditions, deduced from hydrochemical records-Environ. Earth Sci 65:2337–2349
Barbieri M, Boschetti T, Petitta M, Tallini M (2005) Stable isotope (2H,18O and 87Sr/86Sr) and hydrochemistry monitoring for groundwater hydrodynamics analysis in a karst aquifer (Gran Sasso, central Italy). Appl Geochem 20:2063–2081
Barbieri M, Nigro A, Petitta M (2017) Groundwater mixing in the discharge area of San Vittorino Plain (Central Italy): geochemical characterization and implication for drinking uses. Environ Earth Sci 76:393
Besser H, Mokadem N, Redhaouania B, Rhimi N, Khelifi F, Ayadi Y, Omar Z, Bouajila A, Hamed Y (2017) GIS based model evaluation of groundwater quality and estimation of soil salinization and land degradation risks in arid Mediterranean site (SW Tunisia). Arab J Geosci 10:350. https://doi.org/10.1007/s12517-017-3148-0
Bicalho CC, Batiot-Guilhe C, Seidel JL, Van Exter S, Jourde H (2012) Geochemical evidence of water source characterization and hydrodynamic responses in a karst aquifer. J Hydrol 450–451:206–218
Calaforra JM, Pulido-Bosch A (1999) Gypsum karst features as evidence of diapiric processes in the Betic Cordillera, southern Spain. Geomorphology 29:251–264
Celle-Jeanton H, Travy Y, Blavoux B (2001) Isotopic typology of the precipitation in the Western Mediterranean region at three different time scales. Geophys Res Lett 28:1215–1218
Chiesi M, Waele JD, Paolo F (2010) Origin and evolution of a salty gypsum/anhydrite karst spring: the case of Poiano (Northern Apennines, Italy). Hydrogeol J 18:1111–1124
Cooper AH, Gutiérrez F (2013) Dealing with gypsum karst problems: hazards, environmental issues and planning. In: Shroder J (ed) Treatise on geomorphology, vol 6. Karst Geomorphology. Elsevier, New York, pp 451–462
Demdoum A, Hamed Y, Feki M, Hadji R, Djebbar M (2015) Multi-tracer investigation of groundwater in El Eulma Basin (northwestern Algeria), North Africa. Arab J Geosci 8(5):3321–3333
Emblanch C (1997) Les équilibres chimiques et isotopiques du carbone dans les aquiferes karstiques. Etude en région méditerranéenne de montagne sur le bassin expérimental de la Fontaine de Vaucluse (Doctoral dissertation)
Emblanch C, Puig JM, Zuppi GM, Mudry J, Blavoux B (1998) Comportement particulier lors des montées de crues dans les aquifères karstiques, mise en évidence d’une double fracturation et/ou de circulation profonde: Exemple de la Fontaine de Vaucluse. Eclogægeol. Helv 92:251–257
Emblanch C, Zuppi GM, Mudry J, Blavoux B, Batiot C (2003) Carbon 13 of TDIC to quantify the role of the unsaturated zone: the example of the Vaucluse karst systems (Southeastern France). J Hydrol 279(1):262–274
Farnham IM, Johannesson KH, Singh AK, Hodge VF, Stetzenbach KJ (2003) Factor analytical approaches for evaluating groundwater trace element chemistry data. Anal Chim Acta 490(1):123–138
Fidelibus MD, Gutiérrez F, Spilotro G (2011) Human-induced hydrogeological changes and sinkholes in the coastal gypsum karst of Lesina Marina area (Foggia Province, Italy). Eng Geol 118:1–19
Ford DC, Williams PW (1989) Karst geomorphology and hydrology, vol 601. Unwin Hyman, London
Ford D, Williams PD (2013) Karst hydrogeology and geomorphology. Wiley, New York
Fournillon A (2012) Modélisation géologique 3D et hydrodynamique appliquées aux réservoirs carbonatés karstiques: caractérisation des ressources en eau souterraine de l’Unité du Beausset (SE France) (Doctoral dissertation, Aix-Marseille Université)
Günay G (2002) Gypsum karst, Sivas, Turkey. Environ Geol 42:387–398
Gutiérrez F, Guerrero J, Lucha P (2008) A genetic classification of sinkholes illustrated from evaporite paleokarst exposures in Spain. Environ Geol 53:993–1006
Hadji R, Limani Y, Boumazbeur A, Demdoum A, Zighmi K, Zahri F, Chouabi A (2014) Climate change and their influence on shrinkage–swelling clays susceptibility in a semi-arid zone: a case study of Souk Ahras municipality, NE-Algeria. Desalin Water Treat 52(10–12):2057–2072
Hamed Y, Awad S, Ben Sâad A (2013) Nitrate contamination in groundwater in the Sidi Aïch-Gafsa Oasis region, Southern Tunisia. Environ Earth Sci, Journal. https://doi.org/10.1007/s12665-013-2445-5
Hamed Y, Ahmadi R, Hadji R, Mokadem N, Dhia HB, Ali W (2014) Groundwater evolution of the Continental Intercalaire aquifer of Southern Tunisia and a part of Southern Algeria: use of geochemical and isotopic indicators. Desalin Water Treat 52(10–12):1990–1996
Hamed Y, Hadji R, Redhaounia B, Bâali F, El Gayar A (2017) Climate impact on surface and groundwater in North Africa-A Global Synthesis of Findings and Recommendations. The 1st International Symposium (WREIANA 2017) March 24-25-26, 2017 Gafsa-Tunisia
Hamed Y, Redhaounia B, Ben Sâad A, Hadji R, Zahri F, Zighmi K (2017b) Hydrothermal waters from karst aquifer: case study of the Trozza basin (Central Tunisia). J Tethys 5(1):033–044
Hartmann J, Jansen N, Dürr HH, Kempe S, Köhler P (2009) Global CO2-consumption by chemical weathering: What is the contribution of highly active weathering regions? Global Planet Change 69(4):185–194
Jiménez-Torrecilla N, Galve JP, Asta MP, Gómez L, Fuentes J (2004) Los humedales salinos del entrono de Zaragoza: una singularidad hidrogeomorfológica. GeoTemas 6:115–118
Johnson KS, Neal JT (2003) Evaporite karst and engineering/environmental problems in the United States. Oklahoma Geological Survey circular, 109
Kačaroğlu F (1999) Review of groundwater pollution and protection in karst areas. Water Air Soil Pollut 113(1):337–356
Kaçaroglu F, Degirmenci M, Cerit O (2001) Water quality problems of a gypsiferious watershed: upper Kizilirmak Basin, Sivas, Turkey. Water Air Soil Pollut 128:161–180
Kamgang KB, Ekodeck GE (1991) Altération et bilans géochimiques des biotites des gneiss de Nkolbisson (NW de Yaoundé, Cameroun). Géodynamique 6(2):191–199
Khaska M, La Salle CLG, Lancelot J, Mohamad A, Verdoux P, Noret A, Simler R (2013) Origin of groundwater salinity (current seawater vs. saline deep water) in a coastal karst aquifer based on Sr and Cl isotopes. Case study of the La Clape massif (southern France). Appl Geochem 37:212–227
Klimchouk A, Lowe D, Cooper A, Sauro U (1996) Gypsum karst of the World. Int J Speleol 25(3–4):12
Kowalski WM (1997) Les stades d’effondrement du graben de Tébessa (confins Algéro-Tunisien) et la tectonique plicative Plio-Quaternaire. Bull Soc His Nat, Pays de Montbéliard, France
Lamont-Black J, Baker A, Younger PL, Cooper AH (2005) Utilising seasonal variations in hydrogeochemistry and excitation-emission fluorescence to develop a conceptual groundwater flow model with implications for subsidence hazards: an example from Co., Durham (UK). Environ Geol 48:320–335
Land AL (2003) Evaporite karst and regional groundwater circulation in the Lower Pecos Valley of Southeastern New México. In: Johnson KH, Neal JT (eds) Evaporite karst and engineering/environmental problems in the United States, 109. Oklahoma Geological Survey Circular, Norman, pp 227–232
Lopez-Chicano A, Bouamama M, Vallejos A, Pulido-Bosch A (2001) Factors which determine the hydrogeochemical behaviour of karstic springs: a case study from the Betic Cordilleras, Spain. Appl Geochem 16:1179–1192
Lumsden DN, Chimahusky JS (1980) Relationship between dolomite nonstoichiometry and carbonate facies parameters. In: Zenger DH, Dunham JB, Ethington RL (eds) Concepts and Models of Dolomitization. SEPM Special Publication, 28, pp 123–137
Mokadem N, Demdoum A, Hamed Y, Bouri S, Hadji R, Boyce A, Laouar R, Sâad A (2016) Hydrogeochemical and stable isotope data of groundwater of a multi-aquifer system: Northern Gafsa basin–Central Tunisia. J Afr Earth Sci 114:174–191
Moore PJ, Martin JB, Screaton EJ (2009) Geochemical and statistical evidence of recharge, mixing, and controls on spring discharge in an eogenetic karst aquifer. J Hydrol 376(3):443–455
Moral F, Cruz-Sanjulián JJ, Olías M (2008) Geochemical evolution of groundwater in the carbonate aquifers of Sierra de Segura (Betic Cordillera, southern Spain). J Hydrol 360(1):281–296
Mudry J (1987) Apport du traçage physico-chimique naturel à la connaissance hydrocinématique des aquifères carbonatés (Doctoral dissertation, Université de Franche-Comté)
Nader FH, Swennen R, Ottenburgs R (2003) Karst-meteoric dedolomitization in Jurassic carbonates, Lebanon. Geologica Belgica 6:3–23
Nguyet VTM, Thanh VP, Hai VD, Roi ND, Tra DTT (2016) Hydrogeochemical characterization and groundwater quality of the Dong Giao karst aquifer in Tam Diep, Ninh Binh, Vietnam. Postojna Acta Carsolog 45(3):233
Nicolini E, Rogers K, Rakowski D (2016) Baseline geochemical characterisation of a vulnerable tropical karstic aquifer; Lifou, New Caledonia. J Hydrol Reg Stud 5:114–130
Omelon CR, Pollard WH, Andersen DT (2006) A geochemical evaluation of perennial spring activity and associated mineral precipitates at expedition Fjord, Axel Heiberg Island, Canadian High Arctic. Appl Geochem 21:1–15
Panagopoulos G, Lambrakis N, Katagas C, Papoulis D, Tsolis-Katagas P (2005) Water–rock interaction induced by contaminated groundwater in a karst aquifer, Greece. Environ Geol 49(2):300–313
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Eos, Trans Am Geophys Union 25(6):914–928
Quinif Y (1975) Contribution à l’étude morphologique des karsts algériens de type hautalpin. Rev Géogr Phys Géol Dyn 18:5–18
Raines MA, Dewers TA (1997) Dedolomitization as a driving mechanism for karst generation in Permian Blaine Formation, Southwestern Oklahoma, USA. Carbonates Evaporites 12(1):24–31
Sánchez D, Barberá JA, Mudarra M, Andreo B (2015) Hydrogeochemical tool applied to the study of carbonate aquifers: examples from some karst systems of Southern Spain. Environ Earth Sci 74:199–215
Saporta G (2006) Probabilités, analyses des données et statistiques, Technip, Paris. SAS
Sen Z (1995) Applied hydrogeology for scientists and engineers. CRC Press, Boca Raton
Tuccimei P, Salvati R, Capelli G, Delitala MC, Primavera P (2005) Groundwater fluxes into a submerged sinkhole area, Central Italy, using radon and water chemistry. Appl Geochem 20:1831–1847
Van der Weijden CH, Pacheco FA (2003) Hydrochemistry, weathering and weathering rates on Madeira island. J Hydrol 283(1):122–145
Vicat JP, Mvondo H, Willems L, Pouclet A (2002) Phénomènes karstiques fossiles et actuels au sein des formations métamorphiques silico-alumineuses de la nappe pan-africaine de Yaoundé (Sud-Cameroun). C R Geosci 334(8):545–550
Ward WC, Halley RB (1985) Pleistocene mixing - Zone Dolomite, Northeastern Yucatan Peninsula. J Sed. Pet. 55:407–442
White WB (1988) Geomorphology and hydrology of karst terrains (No. 551.447 W4)
White WB (2002) Karst hydrology: recent developments and open questions. Eng Geol 65(2):85–105
Williams PW (2008) The role of the epikarst in karst and cave hydrogeology: a review. Int J Speleol 37(1):1
Wold S, Esbensen K, Geladi P (1987) Principal component analysis. Chemom Intell Lab Syst 2(1–3):37–52
Woo KS, Moore CH (1996) Burial dolomitization and dedolomitization of the late Cambrian Wagok Formation, Yeongweol, Korea. Carbonates Evaporites 11(1):104–112
Worthington SRH, Davies GJ, Ford DC (2000) Matrix, fracture and channel components of storage and flow in a Paleozoic limestone aquifer. In: Groundwater flow and contaminant transport in carbonate aquifers. Balkema, Rotterdam, pp 113–128
Wu P, Tang C, Zhu L, Liu C, Cha X, Tao X (2009) Hydrogeochemical characteristics of surface water and groundwater in the karst basin, southwest China. Hydrol Process 23(14):2012–2022
Yechieli Y, Abelson M, Bein A, Crouvi O, Shtivelman V (2006) Sinkhole “swarms” along the Dead Sea coast: reflection of disturbance of lake and adjacent groundwater systems. Geol Soc Am Bull 118(9–10):1075–1087
Acknowledgements
The authors would like to thank the anonymous reviewers for their review. Their gratitude extends to the International Association of Water Resources in the Southern Mediterranean Basin is for the support without forgetting of course the staff of the symposium of WREIANA 2017-Gafsa-Tunisia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The present paper is an original work and all the authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hamad, A., Baali, F., Hadji, R. et al. Hydrogeochemical characterization of water mineralization in Tebessa-Kasserine karst system (Tuniso-Algerian Transboundry basin). Euro-Mediterr J Environ Integr 3, 7 (2018). https://doi.org/10.1007/s41207-017-0045-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-017-0045-6