Abstract
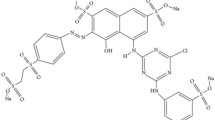
Wastewater treatment is a growing concern of environmentalists around the world. The textile industry, being the biggest contributor of wastewater, generates a huge amount of effluent every day. The objective of this study was to analyze the discoloration and degradation of the commercially used Procion Red H-3B textile dye (CI. Reactive Red 3), using a bacterial SPB-4 consortium (containing Bacillus sp. Stenotrophomonas sp., Pseudomonas sp. and Alcaligenes sp.). The parameters included the varying effects of pH, temperature and inoculum size, the initial concentration of the dye, and the initial concentration of a carbon and nitrogen source. The maximum decolouration (94%) by the consortium, was observed at an initial concentration of 30 mgL−1 of dye, after 6 h of incubation. Optimal conditions for decolouration, were pH 6, temperature 32 °C, 10% inoculum (v/v), glucose (0.1% w/v), and (0.3% w/v) yeast extract concentration. When the dye concentration was increased up to 300 mgL−1, the decolorization percentage reduced to 82%, within 28 h of incubation. The gene sequence and identification of strain, were carried out, using16S rRNA gene amplification technique. The considerable decrease in the COD of the dye (above 80%), clearly revealed the transition of the complex dye to simple oxidizable products. The impact of degradation, was confirmed by the analysis of peaks in the HPTLC and FTIR analysis report. This result demonstrates the potential of microbial processes in the successful bio-remedial treatment of textile wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The textile sector plays a vital role in the global economy, but it also adversely affects the environment. This industry produces a variety of liquid effluents, which are often contaminated with noxious or toxic substances. The volume of water, used throughout the operating process, is enormous. The application of water includes washing fibers for bleaching, mercerizing, dyeing, printing and washing of finished products. Wastewater from the textile industry, is a complex mixture of many polluting substances, ranging from anthraquinone, polycyclic, triphenylmethane and oligochloride-based pesticide waste to heavy metals, associated with dyes and the dyeing process [1]. To treat 1 kg of cotton, nearly 150 L of water is consumed, and the waste generated after the treatment of fabric, is a mixture of different chemicals with large amounts of dye molecules [2]. The textile industry is the largest consumer of these dyes [3]. The annual global production of azo dyes, is estimated at around one million tonnes, of which, more than 2000 structurally different azo dyes, are currently in commercial use [4]. Azo dyes find application in a variety of industries, such as textile coloring, food, cosmetics and printing on paper. Reactive Azo dyes, are one of the most problematic compounds in textile effluents. They are difficult to remove, because of their high solubility in water, and their low depletion rate. Leaving these highly colored industrial effluents, directly into the water resources, is very dangerous. The dye molecules in the effluent water absorb sunlight, which decreases the intensity of light, absorbed by aquatic plants and phytoplankton, resulting in a reduction in the chemical process and oxygenation of reservoirs of water [5]. In addition, the physical appearance of colored water has a negative impact on its aesthetic value. These dyes are generally xenobiotic in nature, and in some cases, are severely mutagenic and carcinogenic [6]. They adversely affect water resources, soil fertility, aquatic organisms, and the integrity of the ecosystem. Allergic effects of these dyes have also been reported by scientists [7, 8]. Various physicochemical methods, are already used for the removal of azo dyes from wastewater. Likewise, color removal from wastewater can be accomplished by absorption, coagulation, flocculation, oxidation, and electrochemical methods [9]. Some of these methods are effective, but most of them are quite expensive. They generate significant amounts of chemical sludge waste, and their disposal in a secure landfill, increases the cost of the process [10]. The effluents discharged by these industries also contain a wide range of physicochemical parameters, such as temperature, pH, conductivity, hardness, alkalinity, chemical oxygen demand (COD), total suspended solids (TSS), nitrates, nitrites, cations (Na+, K+, Ca2+ and Mg2+) and anions (Cl-, CO32-, HCO3-, SO42-) [11]. Therefore, in such situations, biological treatment is the real hope. These methods have the advantages of being environmentally friendly and economically feasible. The effectiveness of these treatment systems depends on the survival and adaptability of microorganisms, during treatment processes [12]. There are reports, indicating the ability of different groups of bacteria, fungi, actinomycetes, and algae to cause azo dyes to fade [13]. They have matured enzyme systems for the discoloration and mineralization of azo dyes, under defined environmental conditions [14]. The application of the pure culture system ensures the generation of data, and the restitution of the elaborate mechanism of degradation of the dye [15]. However, a higher degree of biodegradation and mineralization can be achieved, when the metabolic activities of mixed cultures within a microbial community complement each other [16].The advantage of mixed culture over pure culture, is its ability to biodegrade more efficiently and in less time [17]. The use of the microbial consortium as a biodegradation system, serves a promising and affordable application in the treatment of wastewater, in polluted environments. This article reports the use of a novel bacterial SPB-4 consortium (containing Bacillus sp. Stenotrophomonas sp., Pseudomonas sp. and Alcaligenes sp.) In the degradation of an azo textile dye Procion Red H-3B. The consortium's ability to rid itself of chemical oxygen demand, was measured to verify the consortium's ability to convert a complex dye into a simple, non-toxic compound. The degraded product, after treatment with the SPB-4 consortium, was analyzed by HPTLC and FTIR.
Materials and methods
Chemicals and dyes
Procion Red H-3B (CI. Reactive Red 3) is an azo dye that can easily dissolve in water, and has an acidic character. Initially, a 1000 mgL−1 stock solution was prepared, followed by serial dilutions, according to the concentrations required from various experiments. All chemicals used were of analytical grade. Bushnell and Haas (BHM) medium (Hi-Media), containing the following elements in gL−1: MgSO4: 0.2; KHPO4: 1.0; CaCl2: 0.02; FeCl3: 0.05; NH 4NO3: 1.0 supplemented with glucose (0.1% w/v), yeast extract (0.3% w/v), and 100 (mgL−1) dye, was used as the growth medium.
Collecting the samples
Soil and water samples, were collected from the Pandesara area of the textile dye effluent discharge sites in Surat, Gujarat, India, using the spot and grab method. After examining the temperature and pH, using a laboratory thermometer and pH meter, the samples were transferred to the laboratory, for further evaluation. The sample was stored at 4 °C, and was then used for screening for bleaching bacteria.
Enrichment of dye-degrading organisms
Enrichment was carried out using a BHM medium, with 100 mgL−1 of reactive red dye, added to a 250 mL erlenmeyer flask. The dye served as the exclusive source of carbon and energy, no additional co-substrate was added. The flasks, were then inoculated with 10 mL of soil suspension (10% w/v), and kept for incubation under static condition (37 °C). The soil suspension was prepared by adding 10 g of air dried soil into 50 mL deionised water. The stable dye decolourization culture, was obtained by performing repeated transfers at different dye concentrations, until a consistent growth was achieved. The selected isolated colonies, are picked up and transferred onto fresh medium to ensure purity. The same procedure was repeated for the water samples to be tested as control in the later experiments.
Identification by 16SrRNA
The 16S rRNA sequencing technique, was used for the identification of cultures with constant discoloration. Samples were submitted to Genei, Bangalore, India.
Reduction of COD during decolourization
The chemical oxygen demand of the bleaching medium, was observed at different time intervals. To determine the change in COD, the titrimetric procedure was followed, in which the supernatant was refluxed with potassium dichromate, in the presence of silver sulfate, mercury sulphate, and concentrated H2SO4 was titrated with ferrous ammonium sulfate. (FAS), using a ferroin indicator [18].
Optimization of the consortium culture
5 mL of culture broth, was collected in each flask at different time intervals, during the period of incubation. The test samples, were then centrifuged at 8000 rpm for 10 min, and the supernatant was analyzed by UV–Vis spectrophotometer (Shimadzu UV-1800, Japan) at λ max of the respective dye (λmax of Reactive Red 3 is 530 nm). The cell pellet, was resuspended in 5 mL of distilled water, obtained after centrifugation of the culture (5 mL) at an absorbance of 660 nm. The unseeded flask, containing the dye (Procion Red H-3B), was used as a reference to correct the disappearance of the color.
Optimization for decolourization and degradation of Reactive Red 3
The decolourization experiments, were performed using 250 mL Erlenmeyer flask, containing 100 mL of pre-cultured SPB-4 consortia. The textile dye effluent, consisting of the textile azo dye Reactive Red 3 (30 mgL−1), was diluted up to 70%, and 50 mL of this effluent was added to the flask. The flasks, were then incubated in shaker at 150 rpm. The aromatic hydrocarbons, formed throughout the course of degradation, were estimated by taking 3 mL of culture supernatant at regular interval of time (3 h). Each time, the samples were centrifuged at 6000 rpm for 15 min, 4±0.2 °C, and the suspended particles were removed by adding equal volume of methanol. The decolourization was examined by measuring the maximum absorbance wavelength between the range 200 and 700 nm at room temperature. The flask, without the dye or bacterial culture, was kept as control flask, and tested under the same conditions. All the experiments were carried in triplets, and the average value was noted for calculations. The decolourization efficiency was expressed as follows
To study the effect of different nitrogen sources, like yeast extract, urea, meat extract, peptone and malt extract (3 g L−1), was added into the bacterial culture medium. The effect of nitrogen source, was studied over a period of time (0–120 h) at 30 mg L−1 dye concentration, 20% inoculums concentration, and temperature 37°C under static conditions. In another experiment, keeping the parameters constant, the effect of glucose, maltose, fructose, mannose, starch, lactose, sucrose (10 g L−1), was analysed for effect of various carbon sources. Decolourization and degradation of Reactive Red 3 at diverse concentrations viz. 0.1–1.0 (w/v), was carried to optimize the effect of carbon and nitrogen sources. To evaluate the effect of different environmental conditions, the same experiment was carried out at different temperature (25–50 °C), pH (4–12), and dye concentrations (30–300 mg L−1). Figure 2 represents the effect of environmental and nutritional factor on decolourization percentage.
HPTLC analysis of the degraded product
Pre-coated TLC silica gel plates were used to monitor the degradation of the dyes. Culture supernatants at different times during the decolourization, were used for analysis. A micro-syringe, was used to locate 10 µL of the sample on TLC plates. The solvents in the system include N-propanol, methyl ethyl ketone, ammonium hydroxide in the ratio4: 3: 3 (v/v). The chromatogram of the dye, was observed under daylight and UV light (254 nm).
FTIR analysis of the degraded product
Biodegradation of Procion Red H-3B, has resulted in the formation of various metabolites. To extract the metabolites, an equal volume of ethyl acetate was used. The extracts were dried over anhydrous Na2SO4, evaporated to dryness on a rotary evaporator and analyzed. Thermo Scientific 6700 set up, a frequency range of 4000 cm−1 to 400 cm−1V, was used to perform the FTIR analysis. A mixture of different dyes is present in industrial effluents; therefore, the ability of the SPB-4 consortium to bleach different dyes, was investigated.
Results and discussion
Screening pattern of dye decolorizing bacterial cultures
Four different bacterial consortia, namely SPB-1, SPB-2, SPB-3 and SPB-4, were isolated by an enrichment culture technique, using Procion Red H-3B dye (100 mg L−1). Additionally, glucose and yeast extract as were added as co-substrates to the enrichment media [19]. The decolourization percentage increased with the addition of co-substrate. The SPB-4 bacterial consortium, among all the other isolated bacterial consortia, was found to be the most efficient with maximum decolourization efficiency under static conditions at 37 °C. Four different bacterial species, were obtained by isolation on plates of stain agar from the mixed consortium SPB-4. Testing of the individual strain was also done, but none of them showed any significant decolourization ability. But, when the four bacterial cultures were mixed and inoculated together in a liquid medium, complete decolourization of Procion Red H-3B was observed, thus suggesting the role of the four bacterial species in the decolourization. Researchers, have reported the synchronized action of microorganisms, for the biodegradation of textile dye [20, 21].
Optimization of decolorization conditions
In this study, the bacterial consortia SPB-4 was subjected to a range of pH; and it was found that decolourization percentage was optimal at pH 8 and temperature 32±0.2 °C under microaerophilic condition. The decolourization percentage, was remarkably reduced at lower pH (3–5), and higher pH (9–12). This signifies the inhibition of decolourization activity at extreme pH conditions. The strain consortia SPB-4, showed optimum colour removal activity within 15 h of incubation in microaerophilic condition. Moreover, when the temperature was increased (37°, 40°, and 50 °C), or decreased (20 °C), it resulted in significant decline in the decolorization performance. The strain was exposed to different dye concentrations, ranging from 30 to 300 mg L-1 and the maximum decolourization was observed at 30 mg L-1. The culture exhibited optimum decolourization of dye in the presence of carbon and nitrogen source as co-substrate. The different carbon sources, tested in the present study, are glucose, maltose, fructose, mannose, starch, lactose, sucrose (10 g L-1). Maltose was found to be better carbon source, showing maximum decolourization (98%), while glucose (78%), fructose (87%), mannose (53%), starch (43%), lactose (64%), and sucrose (71%), showed decolourization of the dye respectively. The nitrogen sources analysed in the present study, were yeast extract, urea, meat extract, peptone and malt extract (3 g L−1). Peptone with 96% decolourization, showed extra decolourization percentage among all the other nitrogen sources. The decolourization percentage by yeast extract, urea, meat extract, and malt extract, were 69%, 78%, 56% and 48%, respectively. Figure 1 depicts the decolourization percentage at various environmental and nutritional parameters on decolourization percentage.
Change in COD during degradation of dye
The four bacterial consortia isolated by an enrichment culture technique, were studied for change in COD [22]. Mixed results have been obtained with different consortia. The SPB-1 consortium, showed a minimal reduction in COD of 23% and 48% of Procion Red H-3B dye, within 24 h and 48 h of inoculation, respectively. The SPB-4 consortium, showed a maximum reduction in COD of 57% and 93% of Procion Red H-3B dye, within 24 h and 48 h of inoculation, respectively. All other consortia, showed COD reductions between COD reduction by SPB-4 and SPB-1. The high rate of COD reduction, is considered an indicator of mineralization. Dissolved oxygen has a significant impact on the rate of decolourization. The concentration of biomass did not affect the rate of decolourization. The presence of oxygen inhibits the metabolic activity of the bacterial culture, directed by azo reductase, causing a variation in the discoloration of the azo dyes. The controversy in the oxidation of reduced electron carriers (e.g. NADH), with oxygen or azo group as electron receptor, is the main reason behind. Figure 2 shows the reduction of COD in the four bacterial consortia at various time intervals.
Identification of cultures present in consortium SPB-4 by gram staining and 16S rRNA technique
SPB-4 consortium obtained after enrichment culture technique was subjected to gram staining. The consortium, was then preceded for 16SrRNA technique, to identify the organism present in consortium SPB-4 [23]. The consortium contains Bacillus subtilis, Stenotrophomonas maltophilia, Pseudomonas stutzeri, and Alcaligenes faecalis.
UV–Vis spectrophotometric analysis
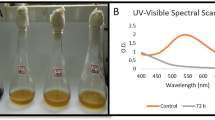
In our study, the textile effluent sample and the treated sample, showed many absorption peaks in the range between 200 and 700 nm, and their maximum absorbance was noted at 295 nm. The effluent sample carried several inorganic compounds, which was evident by the stable peaks absorbance in the UV region. While the bacterial treated sample, exhibited sharp decline in absorbance, signifying the decolourization of dye molecules in the sample. On the basis of our results, it can be stated that the formation of various peaks in UV and visible region, indicated the presence of complex dye molecules along with certain inorganic compounds in the untreated effluent sample, and it got removed in the bacterial treated effluent sample. Figure 3 represents the graph of untreated and bacterial treated effluent, containing Reactive Red 3 by UV–Vis spectral analysis.
HPTLC analysis
Spectrophotometric analysis at 530 nm of inoculated medium, containing Reactive Red 3, showed a simultaneous decrease in the peak of samples taken at various time intervals. Evidence of dye removal can be seen with virtually zero absorbance at λmax, after 48 h inoculation with the SPB-4 consortium, and shifting of the peak to the UV region. Compared to the control dye, the HPTLC results, showed the development of new peaks. The results received to confirm that the chromophoric groups, have been completely removed from the intact colouring structure. The multiple bands obtained in the metabolite pathway, confirmed the biodegradation of the azo dye, compared to a single band of the control dye. The difference in the Rf values of the control dye and the metabolites supports the FTIR data, which suggests biodegradation of Reactive Red 3. Figure 4 shows the HPTLC analysis for Reactive Red 3 (a) 3D plot of Rf values versus absorbance (b) TLC fluorescent plate under UV chamber.
FTIR analysis
The results of the FTIR analysis of the control and the sample, obtained after decolourization, showed various peaks. In the case of Reactive Red 3, the FTIR spectrum for the control, showed a peak at 3441.1 cm−1 for the intra-molecular stretch of aromatic hydrogen bond –OH and –OH, a peak at 2865.3 cm−1 and 1596.69 cm−1 for -C=C stretch of aromatic rings, stretch 1456.08 cm−1 and 1438.73 cm−1—CH of alkyl acetals, peak at 1248.32 cm−1 and 1111.96 cm−1 for stretch -CN due to amines, peak at 853.93 cm−1, 746.18 cm−1 for stretch -CH, 621.39 cm−1 -C- Cl stretch. Reactive Red 3 degradation metabolites, using the SPB-4 consortium, showed a peak at 3318.65 cm−1 for –OH- stretch, a peak at 2903.01 cm−1 –C=C l 'alkenes stretch, 1607.85 cm−1 for CH stretch, due to -CH3, peak at 1021.62 cm−1, 611.3 cm−1 for –C–Cl stretch, indicating the presence of chloride alkyl. Here, a peak at 1248.32 cm−1 is missing, which proves the disappearance of the amines, and the formation of new peaks, indicates the biotransformation of the Reactive Red 3 dye. The appearance of peaks before and after the bacterial treatment, has been shown in Figure 5. Similar results have been reported by McMullan et al. [24].
Conclusion
The composition of textile effluent varies often in a wide range, depending upon the textile process, employed in the unit. The wastewater generated from cotton textile dyeing industry, is enormously contaminated, due to the presence of reactive dyes, which is extremely difficult for treatment. The bacterial strain, when endowed with optimal environmental situation and nutritional source, accelerates the process of bioremediation. In this present research study, the efficacy of a potential bacterial SPB-4 consortium, was investigated for the decolourization and subsequent degradation of Reactive Red 3. The maximum decolourization (94%) of the culture, was observed at a concentration initial 30 mg L−1 dye after 6 hours of the incubation period. Optimal conditions for decolourization were pH 6, temperature 32 °C, 10% inoculum (v/v), glucose (0.1% w/v), and (0.3% w/v) yeast extract concentration. The isolates in the consortium developed best under static conditions, where oxygen can be easily depleted, thus creating favourable conditions for the fading of azo dyes. A reduction in the COD (greater than 80%) of the dye, after treatment with SPB-4, indicates successful degradation of the dye. The degradation of the dye, was confirmed by the results of HPTLC and FTIR analyzes. The consortium SPB-4, showed decolorizing activity through a degradation mechanism. This observation has established that the bacterial culture is adaptive in nature, and can degrade contaminants. The ability of the strain to tolerate, decolorize azo dyes at high concentration, gives it an advantage for treatment of textile industry waste waters. The potential of this bacterial consortium can be estimated by its high capacity for decolourization and degradation, thus, serving as a potential consortium that can be successfully employed to the treatment of industrial effluent. The potential of this bacterial consortium can be estimated by its high capacity for decolourization and degradation, thus, serving as a potential consortium to be used in the treatment of industrial effluent.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Shah MP, Kavita AP (2014) Microbial degradation of Reactive Red 195 by three bacterial isolates in anaerobic-aerobic bioprocess. Int J Environ Bioremediation Biodegrad 2(1):5–11. https://doi.org/10.12691/ijebb-2-1-2
Forss J, Markus VL, Jarone P, Ulrika W (2017) Microbial biotreatment of actual textile wastewater in a continuous sequential rice husk biofilter and the microbial community involved. PLoS ONE 12(1):1–16. https://doi.org/10.1371/journal.pone.0170562
Bharagava RN, Pankaj C (2018) Emerging and eco-friendly approaches for waste management. Emerg Eco-Friendly Approaches Waste Manag. https://doi.org/10.1007/978-981-10-8669-4
Bera SP, Tank SK (2020) Screening and identification of newly isolated Pseudomonas Spp. for biodegrading the textile Azo Dye C.I. Procion Red H-3B. J Appl Microbiol. https://doi.org/10.1111/jam.14920
Ozdemir S, Kevser C, Dilek A, Erkan S, Ozer C (2013) Treatment of azo dye-containing synthetic textile dye effluent using sulfidogenic anaerobic baffled reactor. Biores Technol. https://doi.org/10.1016/j.biortech.2013.07.066
Dzionek A, Danuta W, Urszula G (2016) Natural carriers in bioremediation: a review. Electron J Biotechnol. https://doi.org/10.1016/j.ejbt.2016.07.003
Arora S (2014) Textile dyes: it’s impact on environment and its treatment. J Biorem Biodegrad. https://doi.org/10.4172/2155-6199.1000e146
dos Santos RF, Heloisa R, Neseli D, Ricardo AFM, de Aguiar CRL, Cintia M (2018) Influence of different textile fibers on characterization of dyeing wastewater and final effluent. Environ Monit Assess. https://doi.org/10.1007/s10661-018-7068-6
Gitis V, Nicholas H (2018) Water treatment chemicals: trends and challenges. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2018.06.003
Jiang S, Yuening L, Bradley PL (2017) A review of reverse osmosis membrane fouling and control strategies. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.03.235
Paz A, Julia C, María JP, José Manuel D (2017) Biological treatment of model dyes and textile wastewaters. Chemosphere. https://doi.org/10.1016/j.chemosphere.2017.04.046
Bera SP, Tank SK (2021) Microbial degradation of Procion Red by Pseudomonas Stutzeri. Sci Rep. https://doi.org/10.1038/s41598-021-82494-9
Kaushik G (2015) Bioremediation of industrial effluents: distillery effluent. In: Applied environmental biotechnology: present scenario and future trends. https://doi.org/10.1007/978-81-322-2123-4-2.
Sriram N, Reetha D (2015) Isolation and characterization of dye degrading bacteria from textile dye effluents. Central Eur J Exp Biol
Parisi ML, Enrico F, Daniele S, Rebecca P, Riccardo B (2015) Environmental impact assessment of an eco-efficient production for coloured textiles. J Clean Prod. https://doi.org/10.1016/j.jclepro.2015.06.032
Lade, H, Avinash K, Diby P, and Sanjay G (2015) Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J https://doi.org/10.17179/excli2014-642.
Karunya A, ValliNachiyar C, Ananth PB, Swetha S, AnuradhaJabasingh S (2014) Development of microbial consortium CN-1 for the degradation of mordant black 17. J Environ Chem Eng 2(2):832–840. https://doi.org/10.1016/j.jece.2014.02.012
Islam I, Md Elias M, Sudipta C, Pobittro S, Hamida JS, and Mrityunjoy B (2015) Physicochemical analysis of textile dye effluent and screening the textile dye degrading microbial species. IOSR J Environ Sci Ver. II
Singh RL, Singh RP, Singh PK (2014) Bacterial decolorization of textile azo dye acid orange by Staphylococcus Hominis RMLRT03. Toxicol Int 21(2). https://doi.org/10.4103/0971-6580.139797
Collivignarelli MC, Alessandro A, Marco CM, Silvestro D (2019) Treatments for color removal from wastewater: state of the art. J Environ Manage. https://doi.org/10.1016/j.jenvman.2018.11.094
Pereira L, Madalena A (2012) Dyes-environmental impact and remediation. In: Environmental protection strategies for sustainable developmenthttps://doi.org/10.1007/978-94-007-1591-2_4
Nigam P, Ibrahim MB, Dalel S, Roger M (1996) Microbial process for the decolorization of textile effluent containing azo, diazo and reactive dyes. Process Biochem. https://doi.org/10.1016/0032-9592(95)00085-2
Shah MP (2015) Quantification of genes of activated sludge through real time PCR 3(1):161–169
McMullan G, Meehan C, Conneely A, Kirby N, Robinson T, Nigam P, Banat IM, Marchant R, Smyth WF (2001) Microbial decolourisation and degradation of textile dyes. Appl Microbiol Biotechnol. https://doi.org/10.1007/s002530000587
Acknowledgements
We are thankful to the Department of Biosciences, VNSGU for providing us with the required instrumentation facilities and guidance by the faculty members.
Funding
We didn’t receive any funding.
Author information
Authors and Affiliations
Contributions
MPS Final approval of the version to be submitted for any revised version. SPB Designing, acquisition, analysis and interpretation of data, drafting the article, reviewing it critically for significant intellectual content.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, M.P., Bera, S.P. Microbial treatment of textile dye Reactive Red 3 by a newly developed bacterial consortium. Nanotechnol. Environ. Eng. 6, 62 (2021). https://doi.org/10.1007/s41204-021-00156-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41204-021-00156-7