Abstract
This work demonstrates the capability of biosynthesized silver nanoparticles as a simple platform for copper(II) sensing together with the assessment of their antimicrobial activity. Butterfly pea aqueous extract was used to produce spherical AgNPs with sizes ranging from 10 to 30 nm showing the surface plasmon peak at 420 nm as a characteristic. The sensing capability of copper(II) was examined by observing an abrupt color change in colloidal AgNPs from a yellowish-brown to a light-violet color that was visible to the naked eye and occurred without any prior surface alteration. The synthesized AgNPs were found to be highly selective and sensitive in their application for identifying copper(II), with a detection limit of 6 mg L−1. The overall expression of copper(II) content in water and pharmaceutical samples was consistent with results obtained using the conventional AAS method. Furthermore, the antibacterial activity of the obtained AgNPs was also determined by the broth microdilution method. The minimum inhibitory concentration and minimum bactericidal concentration values against Methicillin-resistant Staphylococcus aureus, Staphylococcus aureus, Acinetobacter baumannii clinical, and Acinetobacter baumannii were 0.16, 0.16, 0.32, 0.64 and 5.10, 5.10, 1.27, 1.27 μg mL−1, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Green metallic nanoparticles, particularly those synthesized from plant-based resources, have recently attracted significant interest due to their simplicity, versatility, low cost, easy scalability, low energy usage, eco-friendliness, and low biohazard rating [1, 2]. Biomedicine, chemistry, and pharmaceuticals, as well as catalysis and environmental implementations, can all benefit from plant-mediated nanoparticles [3,4,5]. Their distinct properties, which are determined by their size, distribution, and morphology, are crucial for their application in multiple capacities [6].
Pollution in aquatic ecosystems is one of the world's most significant environmental issues. Heavy metals, among other toxins, are a major source of worry as they penetrate and persist in the food chain [7]. A comprehensive analysis of a heavy metals determination in aqueous media employing nanomaterial-based sensors from 2013 to 2019 is detailed in [8]. One heavy metal that appears in the environment as a by-product of various human and natural activities is copper(II). Though it is essential for human survival, an excess of 1.3 µg mL−1 can instigate health problems such as nausea, blood cell destruction, and kidney failure [9]. Effective strategies that can enable rapid copper(II) detection are therefore necessary. Several analytical approaches have been reported for the monitoring of copper(II), including atomic absorption spectroscopy (AAS) [10,11,12], inductively coupled plasma optical emission spectrometry (ICP-OES) [13, 14], and inductively coupled plasma mass spectrometry (ICP-MS) [15, 16]. These techniques are highly reliable, but they are expensive and require complicated sample treatments, rendering them unsuitable for on-site testing. Other techniques such as fluorescence [17,18,19,20] and spectroscopy [21,22,23,24] have also been reported to have the same shortcomings of these methods. Unfortunately, these methods are still deemed inappropriate and have limited practical use for on-site monitoring owing to their slow response time and the need of specialized analytical instrumentations. Therefore, the implementation of rapid, inexpensive, and real-time detection is needed as an alternative method. In this regard, a selective “naked-eye” detection of copper(II), which can be performed without accessing any advanced instrumental techniques, is highly desirable. Reports of naked-eye detection of various heavy metals using metallic nanoparticles, which is usually carried out together with spectrophotometry, have now been published [25,26,27,28,29,30,31,32,33,34]. Nevertheless, some of them required an additional step involving nanoparticle surface modification, which is time-consuming, vulnerable to contamination, and usually involves specialized instruments as well as skilled personnel, making on-site detection problematic [35].
In the present study, silver nanoparticles (AgNPs) greenly synthesized by butterfly pea extract were exhibited as a naked-eye sensing probe for semiquantitative analysis of copper(II), as well as antimicrobial agents. The aqueous extract of butterfly pea flower, which is rich of flavonoid, phenolics, and anthocyanins, was utilized as a source of reducing and stabilizing agents for AgNPs formation. The colloidal solution of AgNPs changed color from yellowish brown to light violet in the presence of copper(II), which could easily be distinguished by the naked eye. The advantages of the proposed method in relation to copper(II) detection are listed as follows: (i) Since plant-based material is used in the production of AgNPs, it is considered to be less harmful, more cost-effective, and environmentally friendly; (ii) there was no need for the additional step of functionalization onto the AgNPs surface to apply as copper(II) sensing probe; and (iii) this method is suitable for on-site analysis as the detection principle is based on a visual observation of a color change of AgNPs and can be conducted without the use of other analysis instruments. For antimicrobial properties, the AgNPs were tested against Gram-positive and Gram-negative bacteria of two standard bacterial strains, namely Staphylococcus aureus and Acinetobacter baumannii, together with their isolates to determine the minimal inhibitory concentration and minimal bactericidal concentration values.
Materials and methods
Chemical and reagents
All chemicals in the present study were of analytical grade and used without purification. Silver nitrate (AgNO3, ≥99.8%) and sodium hydroxide (NaOH, ≥97%) were purchased from Fischer (China) and Loba Chemie (India), respectively. Deionised (DI) water was employed to prepare all solutions. Fresh butterfly pea petal was collected from Songkhla Province, Thailand, in 2020. Copper(II) standard solution for AAS (1000 mg L−1 Cu in nitric acid) was purchased from Merck (Germany). The pathogenic bacteria (Staphylococcus aureus ATCC25923, Methicillin-resistant Staphylococcus aureus PW001, Acinetobacter baumannii ATCC19606, Acinetobacter baumannii PW001) and Mueller–Hinton Broth/Agar (MHB and MHA) were received from the Department of Biology, School of Science, Walailak University, Nakhon Si Thammarat, Thailand.
Green synthesis of AgNPs
The procedure and conditions for producing biogenic AgNPs in the present study were adapted with slight modification from those mentioned in our previous paper [36]. Briefly, the butterfly pea extract was prepared by mixing 0.01 g of dried butterfly pea powder with 20 mL of DI water and then stirred for 5 min before filtering. Silver nanoparticles were synthesized by slowly adding 3 mM AgNO3 solution to the extract solution (1:5 volume ratio), adjusting pH to 10 with 0.1 M NaOH; the modified mixture was subsequently heated at 50 °C for 15 min while being stirred. The appearance of a yellowish-brown color indicates the formation of AgNPs.
Characterizations
UV–Vis spectroscopy was carried out to record the spectra of the AgNPs using a JASCO V630 UV–Vis spectrophotometer (Japan). Transmission electron microscope (TEM) images were recorded on a JEOL JEM-2010 (USA) to obtain the morphological characteristics of the AgNPs, including the size, shape, and other physical properties. A surface analytical technique confirming the elemental compositions of the AgNPs was conducted using a field emission scanning electron microscope (FE-SEM), MERLIN compact, Zeiss, with energy-dispersive X-ray spectrometer (SEM/EDX), from Aztec, Oxford, UK.
Antimicrobial assay
Silver nanoparticles greenly synthesized with butter pea flower extract were tested to determine their minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC). Gram-positive and Gram-negative standard bacteria strains, namely Staphylococcus aureus ATCC25923 (S. aureus) and Acinetobacter baumannii ATCC19606 (A. baumannii), and their clinical isolates, i.e., Methicillin-resistant Staphylococcus aureus PW001 isolated from tissue (MRSA), and Acinetobacter baumannii clinical PW001 isolated from sputum (A. baumannii clinical isolate), were tested. The MIC test was performed on a 96-well plate using standard broth microdilution methods, while the MBC test was performed on the MHA plates. The MIC and MBC tests of green synthesized AgNPs were performed using the method described in [37] with slight modification. Briefly, five colonies were transferred to 3 mL MHB and cultured for 6 h at 37 °C. The bacterial suspension was adjusted to a final OD600 of 1, which corresponded to approximately 1 × 108 CFU/mL. To determine the MIC values, 100 µL of bacterial suspension was pipetted to the plate containing 100 μL of serial twofold dilutions in MHB of the synthesized AgNPs. The plates were then incubated at 37 °C for 16 h. The last row of the plate contained only medium (no adding of the AgNPs) to serve as a control. The MIC was determined by visual observation of growth and adding resazurin. The minimum concentration of the AgNPs that showed no detectable growth or the lowest concentration prior to color change was taken as the MIC. For the MBC evaluation, 10 μL of the broth with concentrations higher than or equal to the MIC value was dropped onto Mueller–Hinton Agar (MHA) plates and then incubated at 37 °C for 12 h, using the agar-streaked method. The MBC was determined where no colony growth occurred.
Copper(II) sensing
To evaluate the sensing performance of the synthesized AgNPs with respect to the recognition of heavy metal ions, tests on various ions such as iron(II), lead(II), cobalt(II), zinc(II), cadmium(II), copper(II), nickel(II), magnesium(II), and aluminum(III) were performed. 1 mL of the AgNPs colloidal solution was added to 1 mL of each metal ion (10 mg L−1). The mixtures were maintained at room temperature for 10 min, and the absorbance of each solution was then recorded in the range 300–700 nm against a blank solution. For the naked-eye detection of copper(II), different concentrations of copper(II) between 2 and 10 mg L−1 were mixed with the AgNPs colloidal solution at a 1:1 volume ratio, following the same procedure as mentioned above. The lowest copper(II) concentration that causes the most significant change in the color of the AgNPs is considered as the visual eye detection limit.
Sample analysis
The practical applicability of the butterfly pea-mediated AgNPs solution was examined in water samples and pharmaceutical preparations. Tap water and groundwater samples were collected from Walailak University, Nakhon Si Thammarat, Thailand. The samples were filtered to remove some suspended particles and acidified with concentrated HNO3. Pharmaceutical samples of Natures Aid and Natures Plus were purchased from Nakhon Si Thammarat, Thailand. In each case, 10 tablets of each of the preparations were precisely weighed and ground into a fine powder so that the required amount of the powder could be ignited in a muffle furnace at 400 °C for 2 h. The ash was dissolved in 5 mL of conc. HCl; then, the final solution was filtered and diluted to 100 mL with distilled water.
Results and discussion
Green AgNPs production and characterization
The use of an aqueous extract of butterfly pea petal as both a bio-reductant and stabilizer to produce AgNPs, suggested herein, is considered as one of the greener synthesis methods as it avoids the use of hazardous chemicals that are commonly applied in the conventional chemical approach to synthesize nanoparticles. Results reflecting the achievement of the green AgNPs generation using the extract are shown in Fig. 1. The SPR spectrum was located at 420 nm (Fig. 1a), and visual observation of the color change in the reaction mixture from light blue to a yellowish brown (Fig. 1b) confirmed the characteristic properties of AgNPs. SEM and TEM micrographs of the green synthesized AgNPs showed a well-dispersed spherical morphology with an average size of 30 nm (Fig. 1c–d). Figure 1e indicates the characteristic peak of Ag at about 3 keV. The presence of C, O, Na, and Cl demonstrated the presence of surface functional groups that could have been affected by biomolecules of butterfly pea extract functioning as stabilizing and reducing agents.
The flower of the butterfly pea (Clitoria ternatea), which belongs to the Fabaceae family, has long been used as a natural food colorant and antioxidant [38]. The existence of phenolics, flavonoids, anthocyanins, ternatins, triterpenoids, flavanol glycoside, and sterols has been credited to bioactive compounds found in butterfly pea petals [39, 40]. The major constituents of the butterfly pea, however, differed depending on type and location of the flower and type of extraction solvent and process used [41,42,43]. According to [43], flavonoids were found to be the major phenolic compositions in an aqueous extract of butterfly pea flower determined by the HPLC technique, followed by polyphenolic acids and anthocyanins, respectively. The study reported that myricetin, or 3,5,7,3’,4’,5’-hexahydroxyflavone, which has a chemical structure presented in Fig. 2, was the major flavonoid presence in butterfly pea flower. Therefore, myricetin might be involved in the reduction of silver ions to form AgNPs.
Metal nanoparticle formation is typically broken down into three stages: metal ion reduction, clustering, and further nanoparticle growth. The type of reducing agent, pH, and AgNO3 precursor, as well as the reducing agent used, determines the characteristics of each stage [44]. The tentative mechanism for butterfly pea-mediated AgNPs formation is depicted in Fig. 2. Two hydroxyl groups of myricetin, particularly at position 4' and 5' in the B ring, are responsible for reducing Ag+ ions to form AgNPs by becoming carbonyl groups [45, 46]. Since the bond dissociation energy of the –OH groups at positions 4' and 5' is lower than that of the normal phenolic –OH group, they have the potential to be the key reducing groups for metal ions [47]. This step releases two electrons that would otherwise be transferred to the free orbital of 2Ag+ ions, converting them to Ag0. Ag0 undergoes further aggregation to larger clusters as the reaction progresses, eventually forming AgNPs. The oxidized hydroxyl groups also serve as a stabilizer in the formation of AgNPs, preventing the particles from aggregating. Aside from myricetin, it is worth noting that other oxygen-containing compounds in the extract, including phenolics, anthocyanins, and other flavonoids as well as their oxidized forms, have reducing and capping roles for the formation and stabilization of nanoparticles. The mechanism proposed in the present work correlated with the findings obtained by other studies where flavonoid molecules such as quercetin [35], rutin [39], myricetin [40, 41], and kaempferol [48] have previously been reported to reduce metal ions to nanoparticles.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The values of MIC and MBC, in general, provide quantitative information on the antimicrobial efficacy of the synthesized nanoparticles. The MIC was described as the lowest AgNPs concentration that inhibited detectable bacterial growth by serial dilution, while the MBC was defined as the lowest AgNPs concentration that killed the bacteria, showing no growth on the agar plate. To investigate antibacterial activity of butterfly pea-mediated AgNPs, four bacteria strains were tested. As shown in Table 1, the MIC values ranged from 0.16 to 0.64 μg mL−1. MRSA and S. aureus had an MIC value of 0.16 μg mL−1, while A. baumannii clinical isolate and A. baumannii exhibited MIC values of 0.32 and 0.64 μg mL−1, respectively. The MBC value for MRSA and S. aureus was 5.10 μg mL−1, while standard and clinical isolates of A. baumannii were found to be 1.27 μg mL−1. In addition, the results revealed that the aqueous extract of butterfly pea had no antimicrobial activity, while butterfly pea-mediated AgNPs showed more potential antibacterial activity. This is possibly due to the AgNPs' role in killing bacteria by destroying the cell membrane which follows by the cell rupture [49], as their large surface area allows for more contact with microorganisms [50, 51].
Naked-eye detection of copper(II)
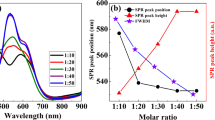
To investigate the potential application of AgNPs greenly synthesized by butterfly pea extract in heavy metal detection, several cations such as iron(II), copper(II), zinc(II), lead(II), cadmium(II), cobalt(II), nickel(II), aluminum(III), and magnesium(II) have been examined. The metal ions were selected because they were potentially toxic and could be detected in real water samples. After adding 1 mL of 10 mg L−1 of each metal ion to 1 mL of the AgNPs solution only in the case of copper(II) was there an immediate change of color from yellow to light violet, while the other metal ions had negligible effects on the color of the AgNPs solution (Fig. 3a). This may be due to a higher binding affinity caused between copper(II) and biomolecules on the surface of the AgNPs, resulting in faster aggregation. It was evidently observed from the absorption spectra as presented in Fig. 3b where the absorption peak of the AgNPs at 420 nm dramatically decreased, which is accompanied by a simultaneous color change of the AgNPs from yellow to light violet for copper(II) ions. This finding suggests there is potential for this qualitative measurement being used for copper(II) detection.
In the presence of copper(II), the hydroxyl and carbonyl groups (at the position 3–OH and 4–C=O or 4–C=O and 5–OH) of myricetin, representative of flavonoid compounds, adsorbing the AgNPs are able to form coordination bonding with metal cations through Cu–O and Cu=O interaction. The preferred binding site highly depends on the type of flavonoids, metal ions, and experimental conditions. The properties and application of flavonoid metal complexes are fully described in [52]. The interaction of copper(II) with myricetin resulted in reduced interparticle distance to induce aggregation of AgNPs, which is observed as the changes of color. Figure 4 illustrates a potential mechanism for the naked-eye detection of copper(II) with butterfly pea-mediated AgNPs. It should be noted that the selectivity of the green synthesized metallic nanoparticles toward metal ions depends on various parameters, especially phytochemical compositions found in the extract, the quantitative amount of the active molecules present in the extract, and the type of experimental method or synthesis condition used. These reasons were proven by [53] where different plant extracts, i.e., neem leaves and bark, mango leaves, tea and pepper seeds, have been reported for AgNPs formation. The study clearly demonstrated that using different types of extract, different parts and different forms, fresh and dried variations, as well as different pH conditions, exhibited significant metal ion sensor characteristics. In this experiment, it may be found that copper(II) does have a slightly higher binding affinity with myricetin, in agreement with the ESI–MS analysis [54] whereby the stability of the complex between copper(II) and quercetin was reported to be the highest when compared with nickel(II), cobalt(II), iron(II), and zinc(II).
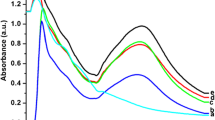
Furthermore, FTIR and TEM analyses were carried out to investigate any detectable interaction and morphological changes when copper(II) was incorporated with the synthesized AgNPs. Considering the FTIR spectra as shown in Fig. 5a, several characteristic bands related to organic functional groups were prominently observed. For IR spectrum of the butterfly pea extract, the band of O–H stretching was detectable at 3383 cm−1, whereas O–H groups bending of the phenol function was observed at around 1400 cm−1. At 1636 cm−1, the C=O stretching peak was evident, while the C=C stretching of aromatic ring absorption was observable at 1513 cm−1. The in-plane bending band of C–H in aromatic hydrocarbon was observed at 1317 cm−1, and out-of-plane bending bands were found at 628 and 811 cm−1. Bands at a region between 1239 and 1060 cm−1 were attributable to the C–O stretching in the aryl ether ring, the C–O stretching in phenol, and the C–CO–C stretch and bending in ketone [55]. These indicate the presence of biomolecules, such as flavonoids and polyphenols in the extract. The presence of noticeable shifts occurred at 3421 and 1600 cm−1 following the interaction of AgNO3 solution with the butterfly pea extract, confirming the involvement of the hydroxyl group in the reduction process. Peaks at 2926, 1055, and 598 cm−1 decreased after bio-reduction, implying that polyols are the main species responsible for the reduction of Ag ions. Moreover, the appearance of an intense peak in the 1384 cm−1 region attributed to C–O–H deformation confirms the formation of AgNPs [56]. In the presence of copper(II), the shift of carbonyl group at 1600 cm−1 to a lower frequency and the decrease of O–H group at 3400 cm−1 support the binding of copper(II) with AgNPs. The result is similar to those obtained by [56] and [57]. The TEM micrographs shown in Fig. 5b, c further confirmed the aggregation of AgNPs with the addition of copper(II). The synthesized AgNPs were drawn closer to one another and became denser in the presence of copper(II) ions. Based on these outcomes, it can be concluded that the aggregation of AgNPs and the color change response may have been caused by the binding reaction of copper(II) and the biomolecules in the extract.
The testing of the sensitivity of copper(II) detection was performed in the range of 2–10 mg L−1. Figure 6 shows that an apparent change could be distinguished from the original solution when the concentration of copper(II) was 6 mg L−1 (and at higher concentrations). As a result, 6 mg L−1 copper(II) was chosen as the detection limit because it was the lowest concentration that could induce AgNPs aggregation and could be measured with the naked eye.
The precision of the naked-eye probe responding to copper(II) was investigated at different copper(II) concentrations, including 5, 6, and 10 mg L−1 on the same day (intra-day measurements, n = 11) and with the day distances (inter-day measurements, n = 5). Results showed no color change of the yellowish colloidal AgNPs in the case of 5 mg L−1 copper(II), whereas copper(II) concentration at 6 and 10 mgL−1 exhibited a distinctive color change that was noticeable to the naked eye for both intra-day repeatability and inter-day reproducibility experiments. This indicated an excellent precision of this measurement process. The stability of the prepared AgNPs was also examined. The colloidal AgNPs suspension was kept at room temperature, and the absorption spectra were recorded. The results revealed that over 97% of the intensity was maintained after 10 days, with no shifting of the absorption band, indicating that the AgNPs are highly stable.
Applications
The practical application of synthesized AgNPs was evaluated in terms of their potential use for screening copper(II) in pharmaceutical vitamins and water samples (tap water and groundwater). For water samples, different known amounts of copper(II) were added into each of the samples. The semiquantitative results of naked-eye detection are considered acceptable when compared with those obtained by standard AAS, which is evidenced in Table 2. Therefore, the developed method could be applied as naked-eye probes for copper(II) detection which holds great potential in routing analysis and pharmaceutical quality assurance.
The comparison of the methodology of the present study with previously published reports based on green synthesized AgNPs toward copper(II) detection is shown in Table 3. The sensitivity of this method is comparable (in the range of 10–5 M) to some reported methods. As can be seen in the table, most of the other methods that provided higher sensitivity than those obtained by the proposed method, unfortunately, involved an additional step for the AgNPs surface’s functionalization [58], long period for AgNPs formation [59, 60], longer incubation time [61], higher cost or had a significant effect from interfering ions [62]. The LOD in this study refers to copper(II) concentration that caused significant color changes from yellow to light violet which were easily visible to the naked eye. This might be a reason that our work had a higher LOD value (lower sensitivity) than the others who reported the LOD from the spectrophotometric detection with calculations made using 3S/N. In order to develop a simple, convenient, rapid, and cost-effective on-site strategy for copper(II) detection, the developed naked-eye method can be presented as a simple tool for semiquantitative analysis.
Conclusions
Exploration of antimicrobial activity and naked-eye copper(II) sensing of AgNPs greenly synthesized by aqueous butterfly pea extract was described herein. The results from UV–Vis spectrophotometer, FTIR, EDS, SEM, and TEM confirm the synthesis of spherical AgNPs with an average size of 30 nm. The MIC and MBC values against Methicillin-resistant S. aureus, S. aureus, A. baumannii clinical, and A. baumannii were founded to be 0.16, 0.16, 0.32, 0.64 and 5.10, 5.10, 1.27, 1.27 μg mL−1, respectively. The color change of the unmodified AgNPs from yellowish brown to light violet observed by the naked eye in the presence of copper(II), at the lowest concentration of 6 mg L−1, was utilized as a simple and rapid semiquantitative analysis tool of copper(II). The copper(II) concentrations reported from the developed method were acceptable when compared with those obtained by standard AAS technique, applying to the use of real water samples and pharmaceutical vitamins. As a result, the proposed assay may be considered as an alternate method for screening copper(II) in the pharmaceutical quality assurance and water industries.
Availability of data and materials
All data supporting the findings of this study are available within the article. The data that support the findings of this study (TEM, SEM images, and EDS analysis) are available from the corresponding author upon request.
References
Jameel MS, Aziz AA, Dheyab MA (2020) Green synthesis: proposed mechanism and factors influencing the synthesis of platinum nanoparticles. Green Process Synth 9(1):386–398
Saim AK, Kumah FN, Oppong MN (2020) Extracellular and intracellular synthesis of gold and silver nanoparticles by living plants: a review. Nanotechnol Environ Eng 6(1):1
Zhang D et al (2020) Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Front Chem 8:799–799
Pankaj Satapathy SA, Rashmi Shetty M, Akshaya Simha N, Dhanapal G, Aishwarya Shree R, Biswas A, Kounaina K, Patil AG, Avinash MG, Devi AT, Gopal S, Nagendra Prasad MN, Veena SM, Hudeda SP, Muthuchelian K, More SS, Melappa G, Zameer F (2020) Phyto-nano-antimicrobials- synthesis, characterization, discovery and advances. In: Atta-ur-Rahman (ed) Frontiers in anti-infective drug discovery. Bentham Science Publishers, Singapore, pp 196–213.
Satheshkumar M et al (2020) Enhanced photocatalytic dye degradation and antibacterial activity of biosynthesized ZnO-NPs using curry leaves extract with coconut water. Nanotechnol Environ Eng 5(3):29
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12(7):908–931
Ahmad K et al (2014) Determination of heavy metal contents in water, sediments, and fish tissues of Shizothorax plagiostomus in river Panjkora at Lower Dir, Khyber Pakhtunkhwa. Pakistan Environ Monit Assess 186(11):7357–7366
Buledi JA et al (2020) A review on detection of heavy metals from aqueous media using nanomaterial-based sensors. Environ Sci Pollut Res
Bagherian G et al (2019) Determination of copper(II) by flame atomic absorption spectrometry after its perconcentration by a highly selective and environmentally friendly dispersive liquid–liquid microextraction technique. J Anal Sci Technol 10(1):3
Neri TS et al (2019) Highly sensitive procedure for determination of Cu(II) by GF AAS using single-drop microextraction. Microchem J 147:894–898
Panda S, Sarkar P (2020) Leaching behavior of copper slag aggregate cement-mortar by atomic absorption spectroscopy (AAS). Mater Today Proc 33:5123–5129
Tomasin GS et al (2021) Highly sensitive determination of Cu(II) ions in hemodialysis water by FAAS after disposable pipette extraction (DPX) using Moringa oleifera as solid phase. Microchem J 161:105749
Yilmaz V et al (2014) Selective solid phase extraction of copper using a new Cu(II)-imprinted polymer and determination by inductively coupled plasma optical emission spectroscopy (ICP-OES). Microchem J 114:65–72
Smirnova SV, Ilin DV, Pletnev IV (2021) Extraction and ICP-OES determination of heavy metals using tetrabutylammonium bromide aqueous biphasic system and oleophilic collector. Talanta 221:121485
Feng L et al (2017) A novel absolute quantitative imaging strategy of iron, copper and zinc in brain tissues by isotope dilution laser ablation ICP-MS. Anal Chim Acta 984:66–75
Cao Y et al (2020) A highly efficient introduction system for single cell- ICP-MS and its application to detection of copper in single human red blood cells. Talanta 206:120174
Xu C et al (2015) Determination of copper (II) in foodstuffs based on its quenching effect on the fluorescence of N, N′-bis(pyridoxal phosphate)-o-phenylenediamine. Spectrochim Acta A Mol Biomol Spectrosc 149:662–666
Chen L et al (2017) Highly selective and sensitive determination of copper ion based on a visual fluorescence method. Sens Actuat B Chem 240:66–75
Wang H et al (2018) Electrochemically prepared oxygen and sulfur co-doped graphitic carbon nitride quantum dots for fluorescence determination of copper and silver ions and biothiols. Anal Chim Acta 1027:121–129
Khairy GM, Duerkop A (2019) Dipsticks and sensor microtiterplate for determination of copper(II) in drinking water using reflectometric RGB readout of digital images, fluorescence or eye-vision. Sens Actuat B Chem 281:878–884
Pourbasheer E et al (2015) Design of a novel optical sensor for determination of trace amounts of copper by UV/vis spectrophotometry in the real samples. J Ind Eng Chem 26:370–374
Almenares-López D et al (2019) Copper-dependent hydrolysis of trichloronate by turkey serum studied with use of new analytical procedure based on application of chiral chromatography and UV/Vis spectrophotometry. J Chromatogr B 1105:203–209
Zhou F et al (2019) A novel method for simultaneous determination of zinc, nickel, cobalt and copper based on UV–vis spectrometry. Optik 182:58–64
Jain A, Soni S, Verma KK (2021) Combined liquid phase microextraction and fiber-optics-based cuvetteless micro-spectrophotometry for sensitive determination of ammonia in water and food samples by the indophenol reaction. Food Chem 340:128156
Demirezen Yilmaz D, Aksu Demirezen D, Mıhçıokur H (2021) Colorimetric detection of mercury ion using chlorophyll functionalized green silver nanoparticles in aqueous medium. Surf Interf 22:100840
Fang Y et al (2018) Highly sensitive naked eye detection of Iron(III) and H2O2 using poly-(tannic acid) (PTA) coated Au nanocomposite. Sens Actuat B Chem 259:155–161
Faghiri F, Ghorbani F (2019) Colorimetric and naked eye detection of trace Hg2+ ions in the environmental water samples based on plasmonic response of sodium alginate impregnated by silver nanoparticles. J Hazard Mater 374:329–340
Zhang Z et al (2020) Colorimetric detection of Cr3+ based on gold nanoparticles functionalized with 4-mercaptobenzoic acid. J Anal Sci Technol 11(1):10
Lei L et al (2019) Preparation of gold nanoparticles using pyridine-formaldehyde as a reducing agent and its application in high sensitivity colorimetric detection of Pb2+. Anal Methods 11(34):4362–4369
Lu C-H et al (2012) Ultrasensitive detection of Cu2+ with the naked eye and application in immunoassays. NPG Asia Mater 4(3):e10–e10
Alizadeh A et al (2014) Naked-eye colorimetric detection of Cu2+ and Ag+ ions based on close-packed aggregation of pyridines-functionalized gold nanoparticles. Sens Actuat B Chem 190:782–791
Bothra S, Solanki JN, Sahoo SK (2013) Functionalized silver nanoparticles as chemosensor for pH, Hg2+ and Fe3+ in aqueous medium. Sens Actuat B Chem 188:937–943
Jarujamrus P et al (2015) Selective colorimetric sensors based on the monitoring of an unmodified silver nanoparticles (AgNPs) reduction for a simple and rapid determination of mercury. Spectrochim Acta A Mol Biomol Spectrosc 142:86–93
Kanokorn Wechakorn PC (2019) Pimfa kamkalong and suranan anantachisilp, rhodamine-triazole functionalized Fe3O4@SiO2 nanoparticles as fluorescent sensors for heavy metal ions. Orient J Chem 35(3):1054–1061
Sangaonkar GM et al (2020) Selective interaction between phytomediated anionic silver nanoparticles and mercury leading to amalgam formation enables highly sensitive, colorimetric and memristor-based detection of mercury. Sci Rep 10(1):2037
Khwannimit D, Maungchang R, Rattanakit P (2020) Green synthesis of silver nanoparticles using Clitoria ternatea flower: an efficient catalyst for removal of methyl orange. Int J Environ Anal Chem 1–17.
Wintachai P et al (2019) Silver nanoparticles synthesized with Eucalyptus critriodora ethanol leaf extract stimulate antibacterial activity against clinically multidrug-resistant Acinetobacter baumannii isolated from pneumonia patients. Microb Pathog 126:245–257
Oguis GK et al (2019) Butterfly pea (Clitoria ternatea), a cyclotide-bearing plant with applications in agriculture and medicine. Front Plant Sci 10(645)
Phrueksanan W, Yibchok-anun S, Adisakwattana S (2014) Protection of Clitoria ternatea flower petal extract against free radical-induced hemolysis and oxidative damage in canine erythrocytes. Res Vet Sci 97(2):357–363
Shen Y et al (2016) Butterfly pea (Clitoria ternatea) seed and petal extracts decreased HEp-2 carcinoma cell viability. Int J Food Sci Technol 51(8):1860–1868
Kazuma K, Noda N, Suzuki M (2003) Flavonoid composition related to petal color in different lines of Clitoria ternatea. Phytochemistry 64(6):1133–1139
Jaafar NF, Ramli ME, Mohd Salleh R (2020) Optimum extraction condition of Clitorea ternatea flower on antioxidant activities, total phenolic, total flavonoid and total anthocyanin contents. Trop Life Sci Res 31(2):1–17
Siti Azima AM, Noriham A, Manshoor N (2017) Phenolics, antioxidants and color properties of aqueous pigmented plant extracts: Ardisia colorata var. elliptica, Clitoria ternatea, Garcinia mangostana and Syzygium cumini. J Funct Foods 38:232–241
Jain S, Mehata MS (2017) Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep 7(1):15867
Landi M, Tattini M, Gould KS (2015) Multiple functional roles of anthocyanins in plant-environment interactions. Environ Exp Bot 119:4–17
Park K-S, Chong Y, Kim MK (2016) Myricetin: biological activity related to human health. Appl Biol Chem 59(2):259–269
Sharma M et al (2019) Biofabrication and characterization of flavonoid-loaded Ag, Au, Au–Ag bimetallic nanoparticles using seed extract of the plant Madhuca longifolia for the enhancement in wound healing bio-efficacy. Prog Biomater 8(1):51–63
Srinivas Raghavan B et al (2015) Kaempferol mediated synthesis of gold nanoparticles and their cytotoxic effects on MCF-7 cancer cell line. Process Biochem 50(11):1966–1976
Abdelhamid HN, Talib A, Wu H-F (2015) Facile synthesis of water soluble silver ferrite (AgFeO2) nanoparticles and their biological application as antibacterial agents. RSC Adv 5(44):34594–34602
Behravan M et al (2019) Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol 124:148–154
Yousef MS et al (2021) Antimicrobial activity of silver-carbon nanoparticles on the bacterial flora of bull semen. Theriogenology 161:219–227
Kasprzak MM, Erxleben A, Ochocki J (2015) Properties and applications of flavonoid metal complexes. RSC Adv 5(57):45853–45877
Karthiga D, Anthony SP (2013) Selective colorimetric sensing of toxic metal cations by green synthesized silver nanoparticles over a wide pH range. RSC Adv 3(37):16765–16774
Liu Y, Guo M (2015) Studies on transition metal-quercetin complexes using electrospray ionization tandem mass spectrometry. Molecules 20(5):8583–8594
Catauro M et al (2015) Silica/quercetin sol–gel hybrids as antioxidant dental implant materials. Sci Technol Adv Mater 16(3):035001
Sharma R, Dhillon A, Kumar D (2018) Mentha-stabilized silver nanoparticles for high-performance colorimetric detection of Al(III) in aqueous systems. Sci Rep 8(1):5189
Maiti S, Barman G, Konar Laha J (2016) Detection of heavy metals (Cu+2, Hg+2) by biosynthesized silver nanoparticles. Appl Nanosci 6(4):529–538
Basiri S, Mehdinia A, Jabbari A (2017) Biologically green synthesized silver nanoparticles as a facile and rapid label-free colorimetric probe for determination of Cu(2+) in water samples. Spectrochim Acta A Mol Biomol Spectrosc 171:297–304
Cheon JY, Park WH (2016) Green synthesis of silver nanoparticles stabilized with mussel-inspired protein and colorimetric sensing of Lead(II) and Copper(II) ions. Int J Mol Sci 17(12):2006
Wang Y et al (2020) Facile and green fabrication of carrageenan-silver nanoparticles for colorimetric determination of Cu(2+) and S(2). Nanomaterials (Basel) 10(1):83
Ghodake GS et al (2018) Colorimetric detection of Cu2+ based on the formation of peptide–copper complexes on silver nanoparticle surfaces. Beilstein J Nanotechnol 9:1414–1422
Ma Y-R et al (2011) Colorimetric detection of copper ions in tap water during the synthesis of silver/dopamine nanoparticles. Chem Commun 47(47):12643–12645
Miao L-J et al (2013) Exploring a new rapid colorimetric detection method of Cu2+ with high sensitivity and selectivity. Sens Actuat B Chem 176:906–912
Bankura GBAK (2016) Colorimetric assays for detection of Cu2+ ion using punica granatum functionalized gold and silver nanoparticles. Int J Nano Chem 2(3):75–81
Acknowledgements
The authors kindly acknowledge the Plasmas and Electromagnetic Wave Science Centre of Excellence and Functional Materials and Nanotechnology Centre of Excellence at Walailak University.
Funding
This research was partially funded by Walailak University, grant number [WU_IRG61_20], and The Development and Promotion of Science and Technology Talented Project (DPST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Buapoon, S., Khwannimit, D., Wintachai, P. et al. Naked-eye copper(II) sensing and antibacterial performance of silver nanoparticles synthesized using butterfly pea aqueous extract. Nanotechnol. Environ. Eng. 6, 38 (2021). https://doi.org/10.1007/s41204-021-00133-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41204-021-00133-0