Abstract
Background
Sleep deprivation (SD) impairs pre-stimulus inhibition, but the effect of quetiapine (QET) remains largely unknown.
Objective
This study aimed to investigate the behavioral and cognitive effects of QET in both naïve and sleep-deprived rats.
Materials and methods
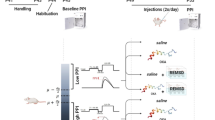
Seven groups (n = 49) of male Wistar Albino rats were used in this study. SD was performed using the modified multiple platform technique in a water tank for 72 h. Our study consists of two experiments investigating the effect of QET on pre-pulse inhibition (PPI) of the acoustic startle reflex. The first experiment tested the effect of short- and long-term administration of QET on PPI response in non-sleeping (NSD) rats. The second experiment used 72 h REM sleep deprivation as a model for SD-induced impairment of the PPI response. Here, we tested the effect of QET on the % PPI of SD rats by short- and long-term intraperitoneal injection at the last 90 min of sleep SD and immediately subsequently tested for PPI.
Results
72 h SD impaired PPI, reduced startle amplitude, and attenuated the PPI% at + 4 dB, + 8 dB, and + 16 dB prepulse intensities. 10 mg/kg short and long-term QET administration completely improved sensorimotor gating deficit, increased startle amplitude, and restored the impaired PPI% at + 4 dB, + 8 dB, and + 16 dB after 72 h SD in rats.
Conclusion
Our results showed short- and long-term administration of QET improved sensorimotor gating deficit in 72 h SD. Further research is required for the etiology of insomnia and the dose-related behavioral effects of QET.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia has common points in neurobehavioral, pathophysiological, and neurochemical aspects associated with sleep deprivation (SD). Schizophrenia was one of the first disorders to be studied using polysomnography, based on the marked similarities between sleep patterns and positive symptoms of schizophrenia (hallucinations, delusions) [1, 2]. SD has the strength to learn more about the pathophysiology of psychosis, given the complexity and heterogeneity of schizophrenia [3].
Similar aspects of schizophrenia related to SD are neurochemical connectivity abnormalities, especially in the brain. The ascending activating reticular system (ARAS), which extends to the thalamus, hypothalamus, basal forebrain, and neocortex in the brain, are critical region in maintaining wakefulness and is responsible for cortical activation and include major brain neuromodulatory systems; all neurotransmitters such as acetylcholine (ACh), dopamine (DA), norepinephrine (NE) and 5-HT take an active role in various parts of the brain during the wake and sleep cycle [4,5,6]. Dysfunction of DA D2, DA D1 [7], 5-hydroxytryptamine (5-HT) 2A, and 5-HT1A receptors have all been associated with schizophrenia [8]. SD also shows abnormal functionality on serotonin receptors [9]. Receptor expression studies have shown that REM SD reduces muscarinic M2 cholinergic receptors in the pons and hippocampus [10]. Increased tissue concentration of 5-HT metabolite, 5-hydroxyindoleacetic acid, in the dorsal raphe nucleus (DRN) and thalamus of rats deprived of sleep for 24 h. SD also increases 5-HT turnover and decreases 5-HT transporter binding in certain brain regions. 5-HT1A of SD causes a gradual desensitization in experimental animals [11]. Wang et al. reported higher GABA levels in the cortex, hypothalamus, and brain stem after 72 h of sleep deprivation in mice [12]. Downregulation of D2/D3R in the ventral striatum in total SD may influence schizophrenia-like behaviors as well as induce decreased alertness. Specifically, DA stimulation of the D2/D3R in the ventral striatum is associated with attention, and thus D2/D3R downregulation may contribute to the inattention observed with SD [13]. Chronic widespread stress-induced serotonergic overload in the cerebral cortex, particularly the anterior cingulate cortex and the dorsolateral frontal lobe in schizophrenia is the main cause of the disease. Excessive serotonergic impulses from the DRN in response to stress may impair the function of cortical neurons in schizophrenia [14]. The abnormality in the DAergic system also affects the serotonergic system. For example, mutant mice that overexpress D2 receptors in the striatum, an animal model of schizophrenia, exhibit both reduced desires to work for reward and upregulation of 5-HT2C receptors. Taken together, genetic predisposition to 5-HT receptors may mediate the diversity of incentive motivation that is impaired in patients receiving biological and/or psychosocial treatments [15].
QET is FDA-approved for the treatment of schizophrenia, bipolar disorder, and major depression [16, 17]. QET is a second-generation atypical antipsychotic drug, that is widely used in the treatment of schizophrenia and there is evidence that it is effective in cognitive impairment as well as positive and negative symptoms in patients with schizophrenia [18, 19]. QET has the ability to bind to many receptors, including DA receptors (D1, D2, D3, D4, D5), 5-HT receptors (5-HT1A, 5-HT2A, 5-HT2C), alpha1-adrenergic receptor, H1-histaminergic receptor, M1-muscarinic receptor, and NMDA receptor [20]. QET also inhibits the NE transporter, mainly due to norquetiapine action. Blockade of D2 in the mesocortical and mesolimbic pathways has been proposed as the interaction responsible for the treatment of schizophrenia, in which increased DA levels are responsible for negative and positive symptoms, respectively. 5-HT2 and α2 receptor antagonism is associated with the antidepressant activity of QET as well as noradrenaline transporter blockade by norquetiapine [21]. On the other hand, norquetiapine has a moderate to high affinity for several muscarinic receptors, which may explain its anticholinergic effects [22].
Dysfunctions between the prefrontal cortex and the mediodorsal thalamus draw attention to one of the patterns of schizophrenia [23]. SD impairs neuronal connectivity functions at the thalamocortical level [24]. In schizophrenia, information processing in the thalamocortical pathway is known as the "filtering hypothesis" and the "gate function" of the thalamus is impaired due to dysfunction of some thalamic nuclei [25]. The Prepulse inhibition (PPI) provides a simple operational measure of thalamo-cortical-controlled sensory-motor gating that serves to avoid disruption of ongoing perceptual and early sensory analysis and the corticostriato-pallido-thalamic circuit modulates PPI in the rat [26]. Numerous studies have been reported on the reduction of PPI in patients with schizophrenia. PPI is also important as a translational marker in the development and research of antipsychotic drugs [27]. PPI is used to measure the effect of atypical antipsychotics, and pharmacological (e.g. dopamine-serotonin agonists or antagonists such as NMDA) agents [28]. For example, QET can increase basal PPI levels in rats and mice [Gayer et al., 2001] and SD reduces PPI in rats and this reduction can be selectively treated by antipsychotic drugs [29]. Therefore, the use of SD as an animal model for schizophrenia can be recommended [30] and QET may ameliorate these adverse effects.
To the best of our knowledge, there are no studies on the effects of QET on PPI in SD rat model. Therefore, the main aim of this study is to investigate the effect of QET on sensorimotor gating in both naïve and sleep-deprived rats. Here, 72 h SD was used to mimic the sleep pattern in schizophrenia by disrupting PPI, and the effects of QET, an adjunct therapy in psychotic disorders, on this model were examined using the PPI test.
Materials and methods
A total of 53 adult (14–18 weeks old) male Wistar albino rats were used for this study. Four animals were excluded from the experiment because their response was below 20 after baseline PPI measurements.
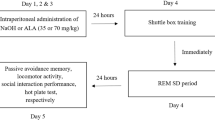
For the first experiment, 28 adult male Wistar albino rats were assigned into three non-sleep-deprived (NSD) groups, short-term control group (n = 7) received a single intraperitoneal (ip) injection of 1 ml saline (4 days), long-term control group (n = 7) received single intraperitoneal (ip) injection of 1 ml saline (30 days) whereas experimental groups; Control AQET 10 mg/kg group received short term ip injection of either (n = 7) and Control CQET 10 mg/kg group received long term ip injection of either (n = 7) QET (Cayman Chemical, USA) dissolved in saline.
In the second experiment, 21 rats were exposed to SD and assigned to three SD groups; one positive control group (n = 7) received a single intraperitoneal (ip) injection of 1 ml saline. SD groups consisted of short and long-term QET administered groups at a dose of 10 mg/kg/day dissolved in saline. Short-term injections were made intraperitoneally once a day to the SD/AQET 10 mg/kg group, in the morning for 4 days, and the experiments were started in the morning for 90 min after the last injection. Long-term injections were administered intraperitoneally once a day to SD/CQET 10 mg/kg group, in the morning for 30 days, and the experiments were started in the morning for 90 min after the last injection. PPI measurement was taken from the NSD and SD groups after 4 days of saline injection to prevent further rat loss. Then, saline injection was continued until the 30th day, and PPI measurement was taken again. Since there was no difference when the results were compared, they were left as a single group and the results were compared with other groups.
All experiments were performed at Üsküdar University Neuropsychopharmacology Application and Research Center (NPARC). Animals were produced and raised in the same place in accordance with international ethical and accreditation rules. Animals were housed under controlled conditions (temperature, 22 ± 2 °C; humidity, 50 ± 5%; and 12 h light/dark cycle, lights on from 07:00 to 19:00). Animals had free access to tap water and standard pellet food throughout the procedures. All experiments were performed at the same time of day and light period (09:00–12:00).
Drug (quetiapine)
Pure Quetiapine (hemifumarate) was used in this study (Cayman Chemical, USA; CAS Number:111974-72-2). The pure powder quetiapine was injected via intraperitoneal in 10 mg/kg/day dose dissolved in 1 ml saline.”
Modified multi-platform tank for sleep deprivation
Rats were tested in socially stable groups of seven in a water aquarium (125 × 43x445 cm) containing 14 round platforms (small platforms) approximately 6.5–7 cm in diameter. The tanks were filled with water to 1 cm below the surface of the platforms or grates that is, at a height of 15 cm. After 72 h of total SD, the mice were returned to their home cage for PPI measurements. Seven deprivation studies were performed with different animals to obtain a final N of 7 registered animals per group. The tank was covered with grates that allowed access to water and food, as was the case with the cages. The animals of a whole group were put into the tank at the same time. The technique was modified from [31].
Prepulse inhibition measurement protocol
The SR-LAB Startle Response System (San Diego Instruments, San Diego, California, USA) was used for the measurement of PPI describing the acoustic startle reflex. From day 51 onwards, rats are accustomed to the injection grip and the worker by touching (handling) for three days. On the fourth day, practice is done by holding the device for 15 min. The familiarization phase is accomplished by introducing a few minutes of background noise (70 dB) without a stimulus. On the fifth day, basal prepulse measurements are taken from the rats without giving any drug and without injection, in the same way as the experimental procedure in which the actual measurements were made. Rats with a startle response of less than 20 units to these stimuli are excluded. The actual measurements are made 24 h after this measurement. The procedure to be applied begins with 5 min of practice. No warning is given at this time. Audible warnings begin with five 120 dB startle warnings. Then the stimulus blocks are repeated ten times in a row. In each of these blocks, five different audible stimuli, the sequence of which will change randomly with each trial, are applied at random intervals (ranging between 10 and 30 s). These:
I. An audible warning with a duration of 40 ms (ms) with an intensity of 120 dB,
II. 100 ms after a basal + 4 dB pre-stimulus of 20 ms duration, a 40 ms duration of 120 dB audible warning,
III. 100 ms after a basal + 8 dB initial stimulus of 20 ms duration, a 40 ms duration of 120 dB audible warning,
IV. 100 ms after a baseline + 16 dB pre-stimulus of 20 ms duration, a 120 dB sound for 40 ms duration.
V. Background-field sound only (this stimulus is to control the response of the rat's movements in the cage).
Finally, five startle warnings given at the beginning of the measurement are applied at random intervals (10–30 s) and the startle response is evaluated. This protocol takes approximately 25 min. At the end of the trials, the percent reduction in startle intensity for each of the three different pre-stimulus intensities is called “pre-stimulus-mediated inhibition due to pre-stimulus intensity” and is calculated for each prestimulus intensity (basal + 4, + 8 and + 16 dB) with the following formula: %PPI = (startle response for pulse alone—startle response for pulse with pre-pulse)/(startle response for pulse alone) × 100. Prior to testing, it was held by the experimenters for three days and acclimated in startle chambers for 15 min without background noise or pulses on the fourth day.
On the fifth day, the PPI protocol was applied, and the percentage reduction (PPI%) in the startle response to each frontal impact severity was estimated in detail in [32, 33]. After each measurement, the chambers were cleaned with ethanol. Measurement intervals were arranged to be at least 10 days to prevent the animal from getting used to the sound [34].
Data analysis
Study variables were sleeping status (SD and NSD) and QET (including 10 mg/kg/day short- and long-term administration). Baseline PPI measurements were analyzed by one-way ANOVA for each prepulse intensity (+ 4, + 8, and + 16 dB) and mean percentage of prepulse, showing that lower and higher baseline PPI values were evenly distributed among each group. For the PPI measurements after the QET administration, the differences between the pulse intensities and groups were primarily assessed by repeated measures ANOVA using Greenhouse–Geisser correction. This step was required to identify any factor-group correlation if existed. The results were then compared amongst groups for each prepulse intensity (+ 4, + 8, and + 16 dB) using one-way ANOVA (p = 0.05 in all cases). Analyses were followed by multiple comparison tests using Tukey’s method or simple effect calculations as needed. The statistical measures are given as p and F or T scores from one-way ANOVA. The error bars in the plots represent standard errors. The level of statistical significance was set at p < 0.05 for all tests.
Results
The distribution among the experimental groups, which naturally exhibited low, medium, and high mean PPI %, was analyzed by one-way ANOVA. None of the groups differed significantly from the baseline mean PPI % at baseline (p > 0.05, F (5.36) = 0.6836). This result shows that the PPI differences within the group are valid and evenly distributed.
Long-term QET administration enhances PPI and startle responses of non-sleep-deprived rats
We first tested the effect of QET on the PPI response of NSD rats (Figs. 1A and B). One-way ANOVA for group average % PPI (p = 0.0437, F (2, 18) = 3.742) and group startle revealed significance (p = 0.0422, F (2.18) = 3.793). Group averages and standard deviations for PPI% at + 4 dB, + 8 dB, + 16 dB prepulse intensities, average PPI% and startle are given for the non-sleep-deprivated rats in Table 1. LT-QET group significantly altered the average mean % PPI response compared to the CLT-Vehicle group (p = 0.0135, T = 4.728) but the ST-QET group not significantly altered the average mean % PPI response compared to the CST-Vehicle group (p > 0.05, T = 0.4627) (Fig. 1A). LT-QET group significantly altered the startle compared to the CLT-Vehicle group (p = 0.0296, T = 4.242) but ST-QET not significantly altered the startle response compared to the ST-Vehicle group (p > 0.05, T = 0.1004). Similarly, there is no significant difference between the ST-QET group and the LT-QET group (p > 0.05, T = 2.861) for startle (Fig. 1B).
Average PPI% and startle values for non-sleep-deprived groups. The average PPI% (A) and startle (B) are presented in the figure. Startle responses are in arbitrary units (AU). Statistical analysis was performed by one-way ANOVA, followed by Tukey’s post-hoc test. *p < 0.05. Abbreviations: Short term 10 mg/kg quetiapine (ST-QET), Long term 10 mg/kg quetiapine (LT-QET), Control Short term vehicle group (CST-Vehicle), Control long term vehicle group (CLT-Vehicle)
For the average PPI %, there was only significant improvement in the LT-QET group compared to the LT-Vehicle group (p = 0.0021, T = 5.436) for + 4 dB (Fig. 2).
PPI% of non-sleep-deprived groups at + 4 dB, + 8 dB, + 16 dB prepulse intensities. Statistical analysis was performed by one-way ANOVA, followed by followed by Tukey’s post-hoc test. **p < 0.005. Abbreviations: Short term 10 mg/kg quetiapine (ST-QET), Long term 10 mg/kg quetiapine (LT-QET), Control Short term vehicle group (CST-Vehicle), Control Long term vehicle group (CLT-Vehicle)
Short- and long-term administration of 10 mg/kg QET ameliorates the impaired PPI response after sleep deprivation
We secondly tested the effect of QET on the PPI response of sleep-deprived rats. One-way ANOVA for group average % PPI (p < 0.0001, F (4, 30) = 10.08) and group startle revealed significance (p = 0.0002, F (4, 30) = 7.672). Group averages and standard deviations for PPI% at + 4 dB, + 8 dB, + 16 dB prepulse intensities, average PPI% and startle are given for the sleep-deprivated rats in Table 2.
The average mean % PPI response in the SD group was significantly impaired compared to the CST-Vehicle group (p = 0.0082, T = 5.159) group and CLT-Vehicle group (p = 0.0381, T = 4.271). Also, the average mean % PPI response significantly improved in the SD/ST-QET group (p = 0.0454, T = 4.163) and SD/LT-QET group (p < 0.0001, T = 8.911) compared to the SD group (Fig. 3A). The average mean % PPI response significantly improved in the SD/LT-QET group compared to the CLT-Vehicle group (0.0170, T = 4.748) (Fig. 3A).
Average PPI% and startle values of Control (-/-), Control (SD/-), and (SD/QET) groups. The average PPI% (A) and startle (B) values are shown in the figure. Startle responses are in arbitrary units (AU). Statistical analysis was performed by one-way ANOVA, followed by Tukey’s post-hoc test. Error bars represent standard errors. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.0001. Abbreviations: Control Short term vehicle group (CST-Vehicle), Control long term vehicle group (CLT-Vehicle), sleep deprivation group (SD), Sleep deprivation and short-term 10 mg/kg quetiapine (SD/ST-QET), Sleep deprivation and long-term 10 mg/kg quetiapine (SD/LT-QET)
The startle responses were significantly impaired in the SD group compared to the CST-Vehicle group (p = 0.0003, T = 6.831) and the CLT-Vehicle group (p = 0.0201, T = 4.650). Startles were significantly increased in the SD/LT-QET group (p = 0.0024, T = 5.827) compared to the control SD group (Fig. 3B). Startles were decreased in SD/ST-QET (p = 0.0240, T = 4.546) compared to the CST-Vehicle group. However, no significant change in the SD/ST-QET group compared to the control SD group (p = 0.4994, T = 2.285) (Fig. 3B).
The PPI responses were significantly impaired in the SD group compared to the CST-Vehicle group (p = 0.0063, T = 5.305) and CLT-Vehicle (p = 0.063, T = 5.305) group for + 8 dB prepulse intensities (in Fig. 4). The PPI responses were significantly impaired in SD group compared to the CST-Vehicle group (p < 0.0001, T = 8.037) and CLT-Vehicle (p < 0.0001, T = 8.037) group for + 16 dB prepulse intensities (in Fig. 4). 10 mg LT-QET administration in SD group significantly increased PPI response compared to SD group for + 4 dB (p = 0.0169, T = 4.750). The PPI responses were significantly increased in the SD/ST-QET group (p = 0.05, T = 4.096) and the SD/LT-QET group (p = 0.0064, T = 5.291) at + 8 dB. The PPI responses were significantly increased after 10 mg LT-QET administration (p = 0.0005, T = 6.639) and 10 mg ST-QET SD/ST-QET group (p = 0.0008, T = 6.363) administration compared to the SD group for + 16 dB. (Fig. 4).
PPI% of sleep-deprived groups at + 4 dB, + 8 dB, + 16 dB prepulse intensities. Statistical analysis was performed by one-way ANOVA, followed by Tukey’s post-hoc test. Error bars represent standard errors. *p < 0.05, **p < 0.005, ***p < 0.0005. Abbreviations: Control Short term vehicle group (CST-Vehicle), Control long term vehicle group (CLT-Vehicle), sleep deprivation group (SD), Sleep deprivation and short-term 10 mg/kg quetiapine (SD/ST-QET), Sleep deprivation and long-term 10 mg/kg quetiapine (SD/LT-QET)
Discussion
Sleep is a circadian process that lasts 7.5–8.5 h on average in adults and consists of 90-min cycles of “non-rapid eye movement (NREM)” and “rapid eye movement (REM)”. The NREM phase consists of sub-stages 1 to 4, and a normal sleep process occurs by experiencing the cycle 4–5 times in a row, in which the person completes the NREM phases 1–4 and the REM phase, respectively. While the proportion of REM in total sleep in newborns is around 50%, it gradually decreases with age and drops to 25% in an adult [52]. Claverie et al. allowed a more direct comparison with human sleep stages to subclassify NREM sleep in rats into three stages. Although NREM sleep is typically viewed as a homogeneous state in rodents, it encompasses many electrophysiological features, including delta and theta waves, sleep spindles, hippocampal oscillations, slow oscillations, and staggered rhythms [53]. This suggests that NREM sleep in rodents can be classified into distinct substages that likely have distinct neural correlates and are potentially involved in various functions of sleep. The second stage has the highest rate of sleep spindles in rats compared to all three stages. It corresponds to the third stage in humans and is called slow-wave sleep and is the deepest NREM sleep stage. The 72 h SD in rats by using the modified multiple platform technique is the REM sleep deprivation model and is standard for each rat [31].
This study investigated the effects of short- and long-term QET administration on PPI% and startle amplitude in groups with and without SD for the first time in rats. Here, we used a modified multi-platform technique of the sleep deprivation model to establish, in part, the behavioral aspects of psychosis. Our results showed that in comparison to NSD, 72 h SD induced via the modified multiple platform technique significantly disrupted sensorimotor gating, SD group rats had significantly reduced PPI% compared to NSD group rats. This shows that the 72 h SD itself was responsible for disruption of sensorimotor gating in rats.
Clinical doses of QET for adults in the treatment of schizophrenia are 150–750 mg/day for the immediate-release formulation. According to Nair and Jacob, the formula used to determine the human equivalents of drug doses used in preclinical studies is: human dose (mg/kg) = Animal dose (mg/kg) x [Animal Km / Human Km] [49]. The Km value for a 250 g rat is 7, and the Km value for a 60 kg human being is considered 37. Thus, 10 mg/kg of QET selected in the study is the corresponding dose in a 60 kg human. For this reason, rational drug dosage was preferred in this study.
This study firstly showed that 10 mg/day QET had a statistically significant effect on the average PPI% in the rats at the long-term administration while short-term administration did not, this result shows that QET has a time-dependent impact on PPI response which also differs for non-sleep-deprived and sleep-deprived rats. Secondly, 72 h SD significantly impaired average PPI% and reduced startle in rats. Subsequent short and long-term administration of 10 mg/kg QET has a statistically significant effect on SD-impaired PPI. On the other hand, only long-term QET administration significantly increases the startle amplitude in SD rats. However, it should be emphasized that these results are associated with different administration times of 10 mg/kg dose QET. Our results also suggest that a more detailed investigation of the different dose–response relations between QET and PPI response is definitely required, where other models of PPI impairment together with the SD model are preferably utilized.
Our results revealed that 72 h SD disrupted sensorimotor gating in higher PPI intensities (especially in + 8 dB and + 16 dB). The long-term QET administration impact on PPI response differs for non-sleep deprived. When this intensity was only 4 dB higher than 70 dB background noise, it was detected by control subjects receiving long-term QET treatment, but no significant difference was observed at + 8 and + 16 dB. More intense noises such as + 8 dB and + 16 dB may still caught by the subjects with short- and long-term drug administration. For non-sleep-deprived rats, the short and long-term QET administration did not significantly impair PPI% at 78 dB and 86 dB prepulse intensities with a pulse intensity of 120 dB (in Fig. 2). In this setting, the result suggests that short and long-term QET administration does not have a disruptive effect on non-sleep-deprived animals, and probably this result can be carried on to humans, considering the evolutionarily preserved nature of PPI response. Further research with different doses of QET injection could enlighten whether this biphasic effect was coincidental or not. The 72 h SD distorted the PPI response at + 4 dB, + 8 dB and + 16 dB more intense noises than 70 dB. However, short- and long-term QET application has been shown to increase the startle response in more intense noises such as + 8 dB and + 16 dB. Also, only long-term QET administration can restore PPI response + 4 dB significantly (in Fig. 4).
In addition, long-term administration of QET may increase the mean PPI percentage in healthy subjects but would be an indication that some doses could impair or benefit sensorimotor gating and thus trigger psychotic symptoms. Similar future studies should use higher or slightly different doses of QET in NSD rats. Previous studies have shown that 72 h of sleep deprivation (REM sleep deprivation) also significantly reduced PPI response in rats compared to control groups; this suggests the critical role of appropriate REM sleep in the modulation of the PPI response [29, 30, 33, 35, 36]. The results of our study were similar to previous studies, and it was found that 72 h SD significantly decreased PPI and even startle response compared to control vehicle groups. SD was proposed as a schizophrenia-like animal model because it has similar features including the partial and symptom-oriented endophenotype of schizophrenia and the PPI disorder schizophrenia. This deterioration caused by the 72 h SD effect was reversed with the use of antipsychotics such as haloperidol, clozapine, and risperidone [30]. Similarly, in our study, rational doses of short-term and long-term QET 72 h reversed this deterioration by increasing the decrease in PPI response caused by 72 h SD. This, together with the clinical studies pointing out a similar direction, guided our attention to the possible role of QET in the mediation/modulation of PPI response in rats. Gründer et al. reported that second-generation antipsychotics (SGAs) have begun to be used more widely instead of first-generation antipsychotics (FGAs) in the clinical treatment of schizophrenia [37]. Unlike typical antipsychotics that preferentially block DA D2 receptors, SGAs not only reduce DA neurotransmission but also act on multiple receptors, including 5-HT2A receptors. QET is previously shown to have varying impacts on the receptor activity in the brain. QET can bind to many receptors such as DA receptors D1, D2, D3, D4, D5, 5-HT receptors 5-HT1A, 5-HT2A, 5-HT2C, α1 adrenergic, H1 histaminergic, M1 muscarinic, NMDA [20, 37]. PPI could be impaired in different animal models associated with many different receptor dysfunctions, which can be the results of the 72 h SD, but QET could reverse these adverse effects. In our study, we utilized a 72 d SD model on rats to understand the impact of intraperitoneal application of short and long-term QET administration on the prepulse inhibition response. The startle responses of control and experimental groups both in first and second experimental settings remained statistically different, implying that the effect of long-term QET administration is more likely on the regulatory network of PPI and it can relate with acoustic startle reflex itself. While previous studies evaluated data from a single application time, It should also be noted that our results not only indicate the effect of acute applications of QET but also the impact on PPI response in chronic applications. For instance, Powel et al. reported that QET (2.5 mg/kg), both selective D2 receptor and 5-HT2A receptor antagonists, attenuated PPI deficits in male DAT knockout mice [38]. He et al. reported that (10 mg/kg/day, intraperitoneally) QET attenuated for 28 days attenuated sensorimotor gating deficit in the MK-801 mice [39]. Results of Chamera et al.'s research revealed the beneficial effect of 14-day administration of QET on deficits in sensorimotor gating observed in prenatally lipopolysaccharide-exposed offspring [40]. Tanibuchi et al.'s findings suggest that QET ameliorates dizocilpine-induced PPI deficits in mice viaα1-adrenoceptor antagonism, and hence, α1-adrenoceptor antagonism may play a prominent role in QET’s psychopharmacological effects [41]. Duncan et al. demonstrated that 20 mg/kg QET reduced startle magnitude and increased PPI in a mouse model of chronic NMDA receptor hypofunction [42]. Conversely, 10 mg/kg short-term QET administration failed to ameliorate PPI disruption in animal models of phencyclidine compared with most antipsychotics, but short-term clozapine administration did [43]. Shoemaker et al. also showed basolateral amygdala (BLA) lesions significantly disrupted PPI 1-week post-surgery and QET (0 vs 7.5 mg/kg) in a within-subject design 2–3 weeks post-surgery revealed a normalization of PPI in rats [44]. There are many animal models that can be used for the schizophrenia pattern. In our results, 72 h SD disrupts sensorimotor gating, which is the endophenotype of schizophrenia. Short- and long-term administration of 10 mg/kg of QET reverses this effect on ppi and startle responses Short and long-term administration of 10 mg/kg of QET reverses this effect on ppi and startle responses. In conclusion, although the effects of Short- and long-term administration of QET on sensorimotor gating and startle response have been demonstrated in many different psychotic animal models, further investigation is required at different doses and durations of administration. Results from animal research are generally not directly applicable to humans. However, if the results of this study are interpreted for humans, it can be stated that the use of QET in clinical practice may have time-dependent effects on sensorimotor gating, which is a physiological parameter accepted as an endophenotype in schizophrenia and may have an effect against insomnia in the schizophrenia pattern. In rats, and to a lesser extent, in humans, PPI can be diminished by DA D2/D3 and 5-HT(1A) receptor agonists [45, 48]. However, longitudinal clinical trials are few and their results are inconsistent: some results show a reduction in PPI deficits with treatment with atypical antipsychotics, while others do not. 21 days QET treatment may not modify PPI measures in schizophrenia patients [46]. Interestingly, Aggernaes et al. showed treatment with QET for 6 months increased male schizophrenia patients of PPI to a level where it was no longer statistically different from the controls [47]. Although the clinical results of the short and long-term effects of QET appear to be negative, the studies are significantly limited and insufficient.
There are some limitations with the animal model used in this study. In the multiple modified water tank used in the study, the animals were kept on small platforms for 72 h. As they enter REM sleep, they wake up with their tails touching the water. The platform, which causes a high level of stress, triggers acute stress due to environmental conditions, rather than just the effect of SD. This may limit the measurement of effects due to the SD pattern alone. Also, while investigating the effects of SD, EEG and EMG results [50, 51] especially, the rat-psychomotor vigilance task (rPVT) is also recommended in future studies to answer in more detail to what extent the rats are sleep deprived and whether this is constant for all rats. In this sense, it is clear that EEG and EMG can be included in SD studies as a supportive method to reveal changes in brain activities in this process. It is also recommended to investigate whether these data are effective in the same way that long- and short-term QET effects are effective on sensorimotor loss at 72 h SD.
Conclusion
Our results show that QET reversibly modulates the PPI response in rats with short- and long-term effects, for SD rats. A more detailed investigation should be done for the etiology and dose-related behavior of this effect.
References
Sprecher KE, Ferrarelli F, Benca RM. Sleep and plasticity in schizophrenia. Curr Top Behav Neurosci. 2015;25:433–58. https://doi.org/10.1007/7854_2014_366.
Waters F, Chiu V, Atkinson A, Blom JD. Severe sleep deprivation causes hallucinations and a gradual progression toward psychosis with increasing time awake. Front Psychiatry. 2018;10(9):303. https://doi.org/10.3389/fpsyt.2018.00303.
Davies G, Haddock G, Yung AR, Mulligan LD, Kyle SD. A systematic review of the nature and correlates of sleep disturbance in early psychosis. Sleep Med Rev. 2017;31:25–38. https://doi.org/10.1016/j.smrv.2016.01.001.
Malik V, Parthasarathy S. Sleep in intensive care units. Curr Respir Care Reports. 2014;3(2):35–41. https://doi.org/10.1007/s13665-014-0077-1.
Owens J, Gruber R, Brown T, Corkum P, Cortese S, O’Brien L, Stein M, Weiss M. Future research directions in sleep and ADHD: report of a consensus working group. J Atten Disord. 2013;17(7):550–64. https://doi.org/10.1177/1087054712457992.
Pisani MA, Friese RS, Gehlbach BK, Schwab RJ, Weinhouse GL, Jones SF. Sleep in the intensive care unit. Am J Respir Crit Care Med. 2015;191(7):731–8. https://doi.org/10.1164/rccm.201411-2099CI.
Brisch R, Saniotis A, Wolf R, Bielau H, Bernstein HG, Steiner J, Bogerts B, Braun K, Jankowski Z, Kumaratilake J, Henneberg M, Gos T. The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: old fashioned, but still in vogue. Front Psychiatry. 2014;19(5):47. https://doi.org/10.3389/fpsyt.2014.00047.
Kim SA. 5-HT1A and 5-HT2A signaling, desensitization, and downregulation: serotonergic dysfunction and abnormal receptor density in schizophrenia and the prodrome. Cureus. 2021;13(6): e15811. https://doi.org/10.7759/cureus.15811.
Elmenhorst D, Kroll T, Matusch A, Bauer A. Sleep deprivation increases cerebral serotonin 2A receptor binding in humans. Sleep. 2012;35(12):1615–23. https://doi.org/10.5665/sleep.2230.
Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454(7208):1110–4. https://doi.org/10.1038/nature07141.
Zant JC, Leenaars CHC, Kostin A, Van Someren EJW, Porkka-Heiskanen T. Increases in extracellular serotonin and dopamine metabolite levels in the basal forebrain during sleep deprivation. Brain Res. 2011;1399:40–8. https://doi.org/10.1016/j.brainres.2011.05.008.
Wang X, Wang Z, Cao J, Dong Y, Chen Y. Melatonin alleviates acute sleep deprivation-induced memory loss in mice by suppressing hippocampal ferroptosis. Front Pharmacol. 2021;16(12): 708645. https://doi.org/10.3389/fphar.2021.708645.
Benedict C, Brooks SJ, O’Daly OG, Almèn MS, Morell A, Åberg K, Gingnell M, Schultes B, Hallschmid M, Broman JE, Larsson EM, Schiöth HB. Acute sleep deprivation enhances the brain’s response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97(3):E443–7. https://doi.org/10.1210/jc.2011-2759.
Eggers AE. A serotonin hypothesis of schizophrenia. Med Hypotheses. 2013;80(6):791–4. https://doi.org/10.1016/j.mehy.2013.03.013.
Sumiyoshi T, Kunugi H, Nakagome K. Serotonin and dopamine receptors in motivational and cognitive disturbances of schizophrenia. Front Neurosci. 2014;4(8):395. https://doi.org/10.3389/fnins.2014.00395.
World Health Organization. International classification of diseases 10th revision (ICD-10). Geneva: World Health Organization; 1994.
American Psychiatric Association (APA). Diagnostic and statistical manual of mental disorder. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
Iqbal Y, Connell C, Worthington M, Elrafei H, Mulvaney CA, Kaewchaluay C. Quetiapine dose for people with schizophrenia. Cochrane Database Syst Rev. 2019;2019(7):CD013372. https://doi.org/10.1002/14651858.CD013372.
KivircikAkdede BB, Alptekin K, Kitiş A, Arkar H, Akvardar Y. Effects of quetiapine on cognitive functions in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):233–8. https://doi.org/10.1016/j.pnpbp.2004.11.005.
Li P, Snyder GL, Vanover KE. Dopamine targeting drugs for the treatment of schizophrenia: past, present and future. Curr Top Med Chem. 2016;16(29):3385–403. https://doi.org/10.2174/1568026616666160608084834.
Björkholm C, Jardemark K, Marcus MM, Malmerfelt A, Nyberg S, Schilström B, Svensson TH. Role of concomitant inhibition of the norepinephrine transporter for the antipsychotic effect of quetiapine. Eur Neuropsychopharmacol. 2013;23(7):709–20. https://doi.org/10.1016/j.euroneuro.2012.05.012.
López-Muñoz F, Alamo C. Active metabolites as antidepressant drugs: the role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders. Front Psychiatry. 2013;12(4):102. https://doi.org/10.3389/fpsyt.2013.00102.
Pergola G, Selvaggi P, Trizio S, Bertolino A, Blasi G. The role of the thalamus in schizophrenia from a neuroimaging perspective. Neurosci Biobehav Rev. 2015;54:57–75. https://doi.org/10.1016/j.neubiorev.2015.01.013.
Khan MA, Al-Jahdali H. The consequences of sleep deprivation on cognitive performance. Neurosciences (Riyadh). 2023;28(2):91–9. https://doi.org/10.17712/nsj.2023.2.20220108.
Sherman SM, Guillery RW. Distinct functions for direct and transthalamic corticocortical connections. J Neurophysiol. 2011;106(3):1068–77. https://doi.org/10.1152/jn.00429.2011.
Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199(3):331–88. https://doi.org/10.1007/s00213-008-1072-4.
Mena A, Ruiz-Salas JC, Puentes A, Dorado I, Ruiz-Veguilla M, De la Casa LG. Reduced prepulse inhibition as a biomarker of schizophrenia. Front Behav Neurosci. 2016;18(10):202. https://doi.org/10.3389/fnbeh.2016.00202.
Carli M, Invernizzi RW. Serotoninergic and dopaminergic modulation of cortico-striatal circuit in executive and attention deficits induced by NMDA receptor hypofunction in the 5-choice serial reaction time task. Front Neural Circuits. 2014;11(8):58. https://doi.org/10.3389/fncir.2014.00058.
Liu YP, Tung CS, Chuang CH, Lo SM, Ku YC. Tail-pinch stress and REM sleep deprivation differentially affect sensorimotor gating function in modafinil-treated rats. Behav Brain Res. 2011;219:98–104. https://doi.org/10.1016/j.bbr.2010.12.012.
Frau R, Orrù M, Puligheddu M, Gessa GL, Mereu G, Marrosu F, Bortolato M. Sleep deprivation disrupts prepulse inhibition of the startle reflex: reversal by antipsychotic drugs. Int J Neuropsychopharmacol. 2008;11(7):947–55. https://doi.org/10.1017/S1461145708008900.
Machado RB, Hipólide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004(1–2):45–51. https://doi.org/10.1016/j.brainres.2004.01.019.
Uzbay T, Kayir H, Goktalay G, Yildirim M. Agmatine disrupts prepulse inhibition of acoustic startle reflex in rats. J Psychopharmacol. 2010;24(6):923–9. https://doi.org/10.1177/0269881109102533.
Öz P, Gökalp HK, Göver T, Uzbay T. Dose-dependent and opposite effects of orexin A on prepulse inhibition response in sleep-deprived and non-sleep-deprived rats. Behav Brain Res. 2018;2(346):73–9. https://doi.org/10.1016/j.bbr.2017.12.002.
Kaya-Yertutanol FD, Uzbay İT, Çevreli B, et al. Effect of gabapentin on sleep-deprivation-induced disruption of prepulse inhibition. Psychopharmacology. 2020;237:2993–3006. https://doi.org/10.1007/s00213-020-05587-9.
Tekin M, Kaya-Yertutanol FD, Çevreli B, Özdoğru AA, Kulaksız H, Uzbay İT. Sodium valproate improves sensorimotor gating deficit induced by sleep deprivation at low doses. Turk J Med Sci. 2021;51(3):1521–30. https://doi.org/10.3906/sag-2011-229.
Zubedat S, Freed Y, Eshed Y, Cymerblit-Sabba A, Ritter A, Nachmani M, Harush R, Aga-Mizrachi S, Avital A. Plant-derived nanoparticle treatment with cocc 30c ameliorates attention and motor abilities in sleep-deprived rats. Neuroscience. 2013;3(253):1–8. https://doi.org/10.1016/j.neuroscience.2013.08.021.
Gründer G, Heinze M, Cordes J, Mühlbauer B, Juckel G, Schulz C, Rüther E, Timm J, NeSSy Study Group. Effects of first-generation antipsychotics versus second-generation antipsychotics on quality of life in schizophrenia: a double-blind, randomised study. Lancet Psychiatry. 2016;3(8):717–29. https://doi.org/10.1016/S2215-0366(16)00085-7.
Powell SB, Young JW, Ong JC, Caron MG, Geyer MA. Atypical antipsychotics clozapine and quetiapine attenuate prepulse inhibition deficits in dopamine transporter knockout mice. Behav Pharmacol. 2008;19(5–6):562–5. https://doi.org/10.1097/FBP.0b013e32830dc110.
He J, Zu Q, Wen C, Liu Q, You P, Li X, Wang W. Quetiapine attenuates schizophrenia-like behaviors and demyelination in a MK-801-induced mouse model of schizophrenia. Front Psychiatry. 2020;19(11):843. https://doi.org/10.3389/fpsyt.2020.00843.
Chamera K, Curzytek K, Kamińska K, Trojan E, Basta-Kaim A. Quetiapine ameliorates MIA-induced impairment of sensorimotor gating: focus on neuron-microglia communication and the inflammatory response in the frontal cortex of adult offspring of Wistar rats. Cells. 2022;11(18):2788. https://doi.org/10.3390/cells11182788.
Tanibuchi Y, Fujita Y, Horio M, Iyo M, Hashimoto K. Effects of quetiapine on dizocilpine-induced prepulse inhibition deficits in mice possible role of the aα1 adrenergic receptor. Clin Psychopharmacol Neurosci. 2010;8(3):133–6.
Duncan GE, Moy SS, Lieberman JA, Koller BH. Effects of haloperidol, clozapine, and quetiapine on sensorimotor gating in a genetic model of reduced NMDA receptor function. Psychopharmacology. 2006;184(2):190–200. https://doi.org/10.1007/s00213-005-0214-1.
Li M, He E, Volf N. Time course of the attenuation effect of repeated antipsychotic treatment on prepulse inhibition disruption induced by repeated phencyclidine treatment. Pharmacol Biochem Behav. 2011;98(4):559–69. https://doi.org/10.1016/j.pbb.2011.03.007.
Shoemaker JM, Pitcher L, Noh HR, Swerdlow NR. Quetiapine produces a prolonged reversal of the sensorimotor gating-disruptive effects of basolateral amygdala lesions in rats. Behav Neurosci. 2003;117(1):136–43. https://doi.org/10.1037//0735-7044.117.1.136.
Auclair AL, Galinier A, Besnard J, Newman-Tancredi A, Depoortère R. Putative antipsychotics with pronounced agonism at serotonin 5-HT1A and partial agonist activity at dopamine D2 receptors disrupt basal PPI of the startle reflex in rats. Psychopharmacology. 2007;193(1):45–54. https://doi.org/10.1007/s00213-007-0762-7.
Molina V, López DE, Villa R, Pérez J, Martín C, Ballesteros A, Cardoso A, Sancho C. Prepulse inhibition of the startle reflex in schizophrenia remains stable with short-term quetiapine. Eur Psychiatry. 2011;26(5):271–5. https://doi.org/10.1016/j.eurpsy.2010.03.002.
Aggernaes B, Glenthoj BY, Ebdrup BH, Rasmussen H, Lublin H, Oranje B. Sensorimotor gating and habituation in antipsychotic-naive, first-episode schizophrenia patients before and after 6 months’ treatment with quetiapine. Int J Neuropsychopharmacol. 2010;13(10):1383–95. https://doi.org/10.1017/S1461145710000787.
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156(2–3):117–54. https://doi.org/10.1007/s002130100811.
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. https://doi.org/10.4103/0976-0105.177703.
Christie MA, McKenna JT, Connolly NP, McCarley RW, Strecker RE. 24 hours of sleep deprivation in the rat increases sleepiness and decreases vigilance: introduction of the rat-psychomotor vigilance task. J Sleep Res. 2008;17(4):376–84. https://doi.org/10.1111/j.1365-2869.2008.00698.x.
Libourel PA, Corneyllie A, Luppi PH, Chouvet G, Gervasoni D. Unsupervised online classifier in sleep scoring for sleep deprivation studies. Sleep. 2015;38(5):815–28. https://doi.org/10.5665/sleep.4682.
Bhopal N, Khatwa U. Sleep deprivation and human development. In: Bianchi MT, editor. Sleep deprivation and disease: effects on the body, brain and behavior. New York: Springer Science Business Media; 2014. p. 91–9.
Claverie D, Becker C, Ghestem A, Coutan M, Camus F, Bernard C, Benoliel JJ, Canini F. Low β2 main peak frequency in the electroencephalogram signs vulnerability to depression. Front Neurosci. 2016;2(10):495. https://doi.org/10.3389/fnins.2016.00495.
Acknowledgements
The authors dedicated this publication to the 100th anniversary of the Republic of Türkiye. As scientists raised by Türkiye, we are proud of to be citizen of this country.
Funding
Funded by Üsküdar University Scientific Research Projects Unit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
All experiments were conducted according to the ethical guidelines in the Guidelines for the Care and Use of Laboratory Animals adopted by the US National Institutes of Health and published in 1996 and OECD guidelines no. 423. All animal experiments were performed with prior permission from the Animal Research Ethics Committee of Üsküdar University, İstanbul, Turkey. This study was approved by the Local Ethic Committee of Uskudar University on February 20.01.2023 the decision number of Ü.Ü-HADYEK 2023–03.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Özcan, Ö.Ö., Çevreli, B., Temizyürek, A. et al. Quetiapine improves sensorimotor gating deficit in a sleep deprivation-induced rat model. Sleep Biol. Rhythms 22, 269–278 (2024). https://doi.org/10.1007/s41105-023-00504-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-023-00504-x