Abstract
Disruption of the circadian rhythm and sleep–wake cycles is a consequence of aging and is associated with the cognitive decline and many neurodegenerative conditions. We investigated the bedtime, wake-up time, sleep timing (midpoint between bedtime and wake-up time), and sleep timing standard deviation (SD) using the actigraphy among 80 consecutive volunteers aged ≥ 60 years. Global cognitive function and executive function of detailed cognitive domains were evaluated using the mini-mental state examination (MMSE) and Wisconsin card sorting test (WCST) and subjective daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS). The category achievement (CA), total errors (TE), perseverative errors of Nelson (PEN), non-perseverative errors (NPE), and difficulties in maintaining set (DMS) on the WCST were significantly correlated with sleep timing SD (CA: r = − 0.276, p = 0.013, TE: r = 0.311, p = 0.005, PEN: r = 0.241, p = 0.032, NPE: r = 0.250, p = 0.025, DMS: r = 0.235, p = 0.036), but not with the MMSE score. Multiple regression analyses with the stepwise forward selection method including age, ESS score, bedtime, sleep timing, and sleep timing SD, revealed that the ESS score, and sleep timing SD were significant factors related to CA on the WCST (ESS score: β = − 0.322, p = 0.004; sleep timing SD: β = − 0.250, p = 0.022). Assessment of sleep–wake rhythms, daytime sleepiness, and cognitive function using the MMSE and WCST is valuable for the prediction of cognitive decline in the geriatric population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of sleep disturbances and cognitive decline increases with aging [1]. The age-related functional changes in circadian organization in humans may be associated with subtle degenerative alterations in the suprachiasmatic nucleus and the surrounding clock, leading to disruption of the circadian system [2]. Sleep disruption and circadian dysfunction could precipitate neurodegeneration in the brain by promoting inflammation and synaptic damage [3]. The circadian rhythm affects the cortical and subcortical regions of the thalamus, anterior hypothalamus, and locus coeruleus in the brainstem, all of which are related to the processes of cognition [4]. Therefore, clarifying the effects of sleep–wake rhythms on the cognitive function in the geriatric population may be clinically helpful in detecting mild cognitive impairment [5, 6].
The Mini-Mental State Examination (MMSE) has been used widely to assess global cognitive function [7]. On the other hand, the Wisconsin Card Sorting Test (WCST) for assessing the higher brain functions, especially the more detailed cognitive domain to set maintenance and conversion, has been commonly used in the executive function tests [8]. Executive function comprises the complicated cognitive processes that facilitate one's behavior to optimize their approach to unfamiliar circumstances [9], and executive dysfunction emerges in the initial phase of cognitive domain impairment [10]. Hence, a combination of the MMSE and WCST can provide an early and subtle sign of cognitive decline with aging.
Accordingly, we investigated the effects of the sleep–wake rhythms on cognitive function using the MMSE and WCST to assess a broad spectrum of cognitive domains in community-dwelling older adults.
Methods
Participants
Eighty consecutive volunteers aged ≥ 60 years (51 men, 29 women; mean age, 70.8 ± 4.5 years) were enrolled in this study. The following data were collected from the participants: age, body mass index (BMI), smoking status, alcohol intake, presence of hypertension, and diabetes mellitus and hyperlipidemia, and current medications.
An active smoker was defined as a participant who was either currently smoking or had quit it within the last 4 years [11]. Alcohol intake was defined as a regular intake of alcoholic drinks [12]. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using plethysmography (BP-203RPEIII, OMRON COLIN Co., Ltd., Tokyo, Japan). The participants with SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg, or those receiving antihypertensive therapy were considered to have hypertension [13]. Diabetes mellitus and hyperlipidemia were defined as the use of oral hypoglycemic and lipid-lowering drugs, respectively. None of participants were diagnosed with dementia. The participants with habitual naps were excluded. The participants had no history of myocardial infarction, angina pectoris, heart failure, cerebral infarction, cerebral hemorrhage, chronic obstructive pulmonary disease, psychiatric disorders, or the use of antidepressants, benzodiazepines, or current sleep medications. This study was approved by the Ethics Committee of Chubu University (number 270098). After explaining the nature of the study and the procedures involved, the written informed consent was obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki and Japan's Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Questionnaires
Epworth Sleepiness Scale (ESS)
Subjective daytime sleepiness was evaluated using the ESS [14]. In this questionnaire, the participants used a four-point scale to rate their chances of dozing off in eight different situations, all of which were often encountered in daily life. ESS scores included the sum of all responses, ranging from 0 to 24.
Pittsburgh Sleep Quality Index (PSQI)
Subjective sleep quality over the past month was assessed using the PSQI [15]. The PSQI contained 19 items in 7 component domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction. The 19 self-rated items were combined to form seven “component” scores, each of which had a range of 0–3 points. In all cases, a score of “0” indicated no difficulty, while a score of “3” indicated severe difficulty. The seven component scores were then added up to yield the PSQI scores, which ranged from 0 to 21, with higher scores indicating worse sleep quality.
Sleep diary
A sleep diary was obtained concurrently with actigraphy to assess subjective sleep. The participants reported in their sleep diary what time they went to bed to sleep and what time they got out of bed.
Actigraphy
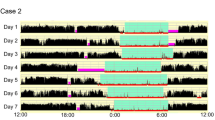
Actigraphy (Ambulatory Monitoring Inc., New York, NY, USA) was performed for at least five consecutive days, and the device of which was worn around the wrist on the non-dominant side to store data in 1-min increments. The results with actigraphy were compared with those using sleep diary to validate recordings. Bedtime and wake-up time were determined, with bedtime being the time the participant went to bed to sleep and wake-up time being the time the participant awoke using actigraphy. We analyzed the data using the algorithm supplied by the Action W-2 software package for Windows (Ambulatory Monitoring Inc., New York, NY, USA), in which sleep and activity were scored according to the Cole-Kripke formula [16]. We evaluated the total sleep time (TST), sleep efficiency (calculated as TST/time spent in bed × 100), and wake after sleep onset (WASO), each of which was averaged per night during the actigraphy recordings. Moreover, the bedtime, wake-up time, sleep timing (midpoint between bedtime and wake-up time) [17], and sleep timing standard deviation (SD) were used to assess sleep–wake rhythms.
Home sleep apnea test (HSAT)
The participants underwent the screening for sleep apnea using a portable device (SAS-2100, NIHON KOHDEN, Tokyo, Japan), in which a nasal pressure sensor and a pulse oximeter were used to record airflow, pulse waves, and oxygen saturation (SpO2), respectively. The participants were instructed how to wear and use the device. We evaluated the respiratory event index (REI) as the number of apnea and hypopnea events per hour during the recording time, along with the minimum SpO2.
Cognitive function tests
Mini-mental state examination (MMSE)
The MMSE, a measure of global cognitive function, was the standard screening test for dementia. It measured orientation in time and place, attention and calculation, language, and memory [7]. MMSE consisted of 11 questionnaires to obtain the MMSE scores ranging from 0 to 30, with scores < 27 indicating the lower global cognitive ability [18].
Wisconsin card sorting test (WCST)
The WCST (WCST-Keio F-S version, Japanese Stroke Data Bank, Japan) was a measure of executive functions, including the ability to reason the abstract and then to shift cognitive strategies in response to the changing environmental contingencies [8]. The main outcomes in the WCST were category achievement (CA), total errors (TE), perseverative errors of Milner (PEM), perseverative errors of Nelson (PEN), non-perseverative errors (NPE), and difficulties in maintaining set (DMS).
In this study, we particularly measured the CA, TE, PEM, PEN, NPE, and DMS. The CA was the number of categories for which six consecutive correct responses were achieved (eight was the maximum number of categories). The TE was the total number of incorrect responses. The PEM referred to the number of incorrect responses in the same category as the immediately preceding correct response after the category changed. The DMS referred to the number of times an incorrect response occurred after two to five consecutive correct responses [19].
Statistical analyses
All data are expressed as the mean ± SD. We compared the data on the smoking status, alcohol intake, hypertension, diabetes mellitus, hyperlipidemia, ESS score, PSQI score, sleep–wake rhythm parameters, HSAT outcomes, and cognitive function between the groups aged < 75 years versus ≥ 75 years and MMSE scores < 27 versus ≥ 27 [18] using the Chi-square test or non-paired t test.
Pearson’s correlation analysis was performed to evaluate the relationship between the baseline/sleep characteristics and cognitive function. Additionally, the multiple regression analyses including the stepwise forward selection method were performed to determine the independent parameters, that were correlated with cognitive function (as assessed by the WCST and MMSE), in relation to age, ESS score, bedtime, sleep timing, and sleep timing SD. Statistical significance was set at p < 0.05.
All statistical analyses were performed using the SPSS Statistics version 25.0 (IBM Corporation, Armonk, New York, USA).
Results
Baseline/sleep characteristics and cognitive function by age and gender
Regarding age, the PEM on the WCST was significantly lower in participants aged ≥ 75 years of age than in those aged < 75 years (0.6 ± 0.7 versus 1.2 ± 1.5, p = 0.042). There was no significant difference in the MMSE scores and WCST parameters between participants aged ≥ 75 years and those aged < 75 years (Table 1).
Regarding gender, there were no differences between male and female participants in the MMSE score and WCST parameters (MMSE score: 28.8 ± 1.7 versus 29.0 ± 1.6, p = 0.687; CA: 5.1 ± 0.9 versus 4.9 ± 1.3, p = 0.475; TE: 13.6 ± 3.2 versus 14.4 ± 5.5, p = 0.399; PEM: 0.9 ± 1.3 versus 1.3 ± 1.5, p = 0.297; PEN: 2.3 ± 2.3 versus 2.8 ± 3.1, p = 0.418; NPE: 10.4 ± 2.6 versus 10.4 ± 3.4, p = 0.979; DMS: 0.5 ± 0.8 versus 0.6 ± 1.2, p = 0.787).
Baseline/sleep characteristics and cognitive function according to the MMSE score
Bedtimes, wake-up times, and sleep timing were significantly earlier in participants with the MMSE scores < 27 than in those with the MMSE scores ≥ 27 (bedtime: 21:59 ± 0:59 versus 23:04 ± 1:09, p = 0.002; wake-up time: 5:25 ± 1:31 versus 6:21 ± 1:01, p = 0.006; sleep timing: 1:42 ± 0:58 versus 2:43 ± 0:56, p = 0.001), respectively. There were no significant differences in the characteristics and WCST parameters between participants with the MMSE scores < 27 and those with the MMSE scores ≥ 27 (Table 2).
Relationships between cognitive function and baseline/sleep characteristics
The MMSE score was significantly correlated with the ESS score, bedtime, and sleep timing (ESS score: r = 0.239, p = 0.033; bedtime: r = 0.340, p = 0.002; sleep timing: r = 0.324, p = 0.003), but not with sleep timing SD (r = − 0.195, p = 0.082) (Table 3).
Regarding WCST outcomes, the CA was significantly correlated with the ESS score and sleep timing SD (ESS score: r = − 0.297, p = 0.008; sleep timing SD: r = − 0.276, p = 0.013). The TE was significantly correlated with the ESS score and sleep timing SD (ESS score: r = 0.299, p = 0.007; sleep timing SD: r = 0.311, p = 0.005). The PEM was significantly correlated with age (r = − 0.258, p = 0.021). The PEN was significantly correlated with the ESS score and sleep timing SD (ESS score: r = 0.262, p = 0.019; sleep timing SD: r = 0.241, p = 0.032). The NPE was significantly correlated with sleep timing SD (r = 0.250, p = 0.025). The DMS was significantly correlated with the ESS score, bedtime, sleep timing and sleep timing SD (ESS score: r = 0.298, p = 0.007; bedtime: r = − 0.242, p = 0.031; sleep timing: r = − 0.267, p = 0.017; sleep timing SD: r = 0.235, p = 0.036) (Table 3).
Multiple regression analysis among cognitive function and baseline/sleep characteristics
The ESS score and sleep timing SD were the significant factors related to CA on the WCST (ESS score: β = − 0.322, p = 0.004; sleep timing SD: β = − 0.250, p = 0.022) and the ESS score was the most significant factor (β = − 0.323, p = 0.003). The ESS score and sleep timing SD were the significant factors related to TE on the WCST (ESS score: β = 0.327, p = 0.003; sleep timing SD: β = 0.295, p = 0.007) and the sleep timing SD was the most significant factor (β = 0.290, p = 0.007). The age was a significant factor related to PEM on the WCST (β = − 0.229, p = 0.044). The ESS score was a significant factor related to PEN on the WCST (β = 0.278, p = 0.015). The sleep timing SD was a significant factor related to NPE on the WCST (β = 0.276, p = 0.016). The ESS score was a significant factor related to DMS on the WCST (β = 0.330, p = 0.003). The ESS score was a significant factor related to MMSE score (β = 0.217, p = 0.049) (Table 4).
Discussion
We found that the sleep timing SD was associated with the CA, TE, PEN, NPE, and DMS on the WCST, but not with the MMSE score of global cognitive function. Our findings suggest that an irregular sleep–wake rhythm may be an early sign of cognitive decline in the geriatric population.
Circadian clock disruption is a consequence of aging and it promotes oxidative stress, inflammation, and a loss of synaptic homeostasis, while wakefulness increases sympathetic output and suppresses the functioning of the glymphatic system, which may be an early sign of the neurodegeneration [3]. We showed that the sleep timing SD was a significant factor related to the CA, TE, PEN, and NPE, but not to the MMSE in the geriatric population. A prospective observational study of 1287 older women demonstrated that executive function alone was positively associated with circadian rhythm measures, which was independent of the baseline MMSE score [20]. In 1282 older women, the alterations in the peak and phase in the circadian rhythm of activity using actigraphy increased the risk of developing dementia and mild cognitive impairment [21]. Taken together, an evaluation of the sleep–wake rhythm could help detect cognitive decline in older adults.
The DMS on the WCST was significantly correlated with the sleep timing and sleep timing SD. The DMS score reflects the degree to which the subject loses track of the classification category caused by a disorder of immediate and recent memory [22]. The DMS is therefore an index of the ability to retain information and its score also reflects attention maintenance [23]. In a recent study including 39 participants aged 24–85 years, the DMS score was not significantly different between young and elderly groups [20]. In another previous study of the 124 healthy subjects aged 15–30 years, the DMS was less affected by age than CA and PEN [24]. Hence, irregular sleep–wake rhythm may affect memory and attention maintenance independently of age.
The relationship between daytime sleepiness and cognitive function has been examined [25,26,27]. In a study of 479 frail older adults aged ≥ 70 years, those with excessive daytime sleepiness (EDS) had an increased risk of cognitive decline, independent of potential confounders (age, sex, education level, cerebrocardiovascular and metabolic disease, fatigue, and APOEε4 status) [25]. EDS was associated with global cortical thinning in cognitively normal older adults [26]. In another study of 283 community-dwelling residents aged ≥ 70 years without dementia, EDS was associated with longitudinal β-amyloid accumulation, particularly in the cingulate gyrus and parietal lobes [27]. These findings support our observations showing that the ESS score reflecting the severity of daytime sleepiness was associated with the CA, TE, PEN, and DMS on the WCST, and MMSE scores. Thus, daytime sleepiness may be an important symptom of cognitive decline.
We showed that the TST was not significantly correlated with the cognitive function. Cross-sectional studies, which used actigraphy to measure TST, demonstrated no association between sleep duration and declines in MMSE score, or executive function [28]. In contrast, in a Chinese cohort study, an inverted U-shaped association between self-reported sleep duration and cognitive decline in memory, executive function, and orientation was found [29]. In our recent study, a long sleep time adversely affected the cognitive function in the 63 community-dwelling older adults [30]. From these previous findings, aging-related disturbed sleep–wake rhythms resulted in long sleep or poor sleep quality, which may complicate the relationship between TST and cognitive function in the geriatric population. Hence, an objective evaluation of not only TST but also the sleep–wake rhythm is important for understanding the effect of sleep on the cognitive function.
Regarding the effect of sleep-disordered breathing on cognitive function, the REI was not correlated with the MMSE score or WCST parameters in our study. In cross-sectional study of older men aged ≥ 65 year, no association was found between the apnea–hypopnea index and parameters on cognitive function tests, including the MMSE and tests of attention, and executive function [31]. In our previous study, moderate to severe nocturnal hypoxia associated with sleep disordered breathing and hypertension had negative effects on executive function in adults ≥ 60 years of age [32]. Therefore, we suggest that age-dependent sleep-disordered breathing without severe hypoxia and frequent sleep fragmentation might not have a significant impact on the cognitive decline in older adults.
The present study had some methodological limitations. First, the sample size was not large, and 63.8% of the participants was male. Second, this study was a cross-sectional study, and we did not know whether cognitive decline or sleep disturbance had existed prior to the study. Further interventional trials with a larger sample size are needed to elucidate the relationship between the sleep–wake rhythms and cognitive function in older adults.
Conclusions
An irregular sleep–wake rhythm and ESS score were associated with the WCST parameters of executive function, but not with the MMSE scores of global cognitive function, in community-dwelling older adults. Thus, the sleep–wake rhythms and daytime sleepiness may be novel indicators for cognitive decline in the geriatric population.
References
Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–28.
Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5(1):33–51.
Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354(6315):1004–8.
Schmidt C, Peigneux P, Cajochen C. Age-related changes in sleep and circadian rhythms: impact on cognitive performance and underlying neuroanatomical networks. Front Neurol. 2012;3:118.
Keihani A, Mayeli A, Ferrarelli F. Circadian rhythm changes in healthy aging and mild cognitive impairment. Adv Biol (Weinh). 2022. https://doi.org/10.1002/adbi.202200237.
Xiao Q, Shadyab AH, Rapp SR, et al. Rest-activity rhythms and cognitive impairment and dementia in older women: results from the Women’s Health Initiative. J Am Geriatr Soc. 2022;70(10):2925–37.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16(1):17–42.
Gilbert SJ, Burgess PW. Executive function. Curr Biol. 2008;18(3):R110–4.
Binetti G, Magni E, Padovani A, et al. Executive dysfunction in early Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1996;60(1):91–3.
Kondo T, Osugi S, Shimokata K, et al. Smoking and smoking cessation in relation to all-cause mortality and cardiovascular events in 25,464 healthy male Japanese workers. Circ J. 2011;75(12):2885–92.
Cho Y, Shin SY, Won S, et al. Alcohol intake and cardiovascular risk factors: a Mendelian randomisation study. Sci Rep. 2015;5:18422.
Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235–481.
Takegami M, Suzukamo Y, Wakita T, et al. Development of a Japanese version of the Epworth Sleepiness Scale (JESS) based on item response theory. Sleep Med. 2009;10(5):556–65.
Doi Y, Minowa M, Uchiyama M, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000;97(2–3):165–72.
Cole RJ, Kripke DF, Gruen W, et al. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15(5):461–9.
Youngstedt SD, Kripke DF, Elliott JA, Klauber MR. Circadian abnormalities in older adults. J Pineal Res. 2001;31(3):264–72.
O’Bryant SE, Humphreys JD, Smith GE, et al. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008;65(7):963–7.
Banno M, Koide T, Aleksic B, et al. Wisconsin card sorting test scores and clinical and sociodemographic correlates in Schizophrenia: multiple logistic regression analysis. BMJ Open. 2012;2(6): e001340.
Walsh CM, Blackwell T, Tranah GJ, et al. Weaker circadian activity rhythms are associated with poorer executive function in older women. Sleep. 2014;37(12):2009–16.
Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70(5):722–32.
Goshiki T, Miyahara M. Effects of individual differences and irrelevant speech on WCST and STROOP test. Psychologia. 2008;51:28–45.
Kokudai Y, Honma M, Masaoka Y, et al. Cascade process mediated by left hippocampus and left superior frontal gyrus affects relationship between aging and cognitive dysfunction. BMC Neurosci. 2021;22(1):75.
Okazaki T, Saeki S, Hachisuka K. Adolescent normative data on simple neuropsychological tests for cognitive behavioral disorders: mini-mental state examination, trail making test, Wisconsin card sorting test-KFS version and Miyake’s verbal paired-associate learning test. Jpn J Rehabil Med. 2013;50(12):962–70 (In Japanese).
Gabelle A, Gutierrez LA, Jaussent I, et al. Excessive sleepiness and longer nighttime in bed increase the risk of cognitive decline in frail elderly subjects: the MAPT-sleep study. Front Aging Neurosci. 2017;9:312.
Carvalho DZ, St Louis EK, Boeve BF, et al. Excessive daytime sleepiness and fatigue may indicate accelerated brain aging in cognitively normal late middle-aged and older adults. Sleep Med. 2017;32:236–43.
Carvalho DZ, St Louis EK, Knopman DS, et al. Association of excessive daytime sleepiness with longitudinal β-amyloid accumulation in elderly persons without dementia. JAMA Neurol. 2018;75(6):672–80.
Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61(4):405–10.
Ma Y, Liang L, Zheng F, et al. Association between sleep duration and cognitive decline. JAMA Netw Open. 2020;3(9): e2013573.
Okuda M, Noda A, Iwamoto K, et al. Effects of long sleep time and irregular sleep-wake rhythm on cognitive function in older people. Sci Rep. 2021;11(1):7039.
Blackwell T, Yaffe K, Ancoli-Israel S, et al. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2011;59(12):2217–25.
Kato K, Noda A, Yasuma F, et al. Effects of sleep-disordered breathing and hypertension on cognitive function in elderly adults. Clin Exp Hypertens. 2020;42(3):250–6.
Funding
The study was supported by the Japan Society for the Promotion of Science (KAKENHI Grant number 25282210, 21K07563) and Chubu University research Grant (number 19M27A1, 20M25A1).
Author information
Authors and Affiliations
Contributions
Conception or design; AN. Acquisition; AN, MO. Analysis; MO, AN. Interpretation of data; AN, MO, KI, NH, SM. Drafting the work; MO, AN. Revising it critically for important intellectual content; FY, TT, NO, JAS, SM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed were in accordance with the Declaration of Helsinki and Japan's Ethical Guidelines for Medical and Health Research Involving Human Subjects. This study was approved by the Ethics Committee of Chubu University (Approval number: 270098).
Informed consent
Written informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Okuda, M., Noda, A., Iwamoto, K. et al. Assessment of cognitive function and sleep–wake rhythms in community-dwelling older adults. Sleep Biol. Rhythms 22, 137–145 (2024). https://doi.org/10.1007/s41105-023-00491-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-023-00491-z