Abstract
Commonly used hypnotics are not very effective for the management of chronic insomnia as they produce several unpleasant side effects. According to a recent report, short-term administration of 10 mg/kg α-Asarone, an active principle of Acorus species, used in traditional Indian and Chinese medicine for a prolonged period without any ill-effects, promoted sleep in rats. However, the efficacy of α-Asarone in promoting sleep on long-term use has not been studied. Study was conducted on four groups of rats, with electrodes implanted for recording sleep. Thermocouple and radio-transmitter were implanted for recording temperatures from hypothalamus and peritoneum. Of these, three groups of rats were sleep deprived for 5 h (9:00–14:00 h) for 21 days after drug (α-Asarone or midozolam) or vehicle administration. The anxiety levels were also studied in these rats. Another group, that received α-Asarone for 21 days, was not subjected to sleep deprivation. Long-term administration of α-Asarone improved both the quantity and quality of NREM sleep, not only in comparison to the vehicle, but also in contrast to midazolam. Moreover, there was no withdrawal effect after stopping the daily administration of α-Asarone for 3 weeks. Anxiety alleviation produced by α-Asarone was better than midazolam. α-Asarone-mediated anxiolysis, mild hypothermia, and NREM sleep-related alterations in temperatures, and its known antioxidant property, might have contributed towards the improvement in NREM sleep and maintenance of REM sleep. The study provides strong pre-clinical evidences for further research on α-Asarone as a possible treatment option for chronic insomnia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insomnia, experienced by approximately one-third of the general population [1], is associated with various mental and physical health disorders [2,3,4,5]. For insomnia and its co-morbid medical conditions, the clinically accepted pharmacotherapy, which includes benzodiazepines and non-benzodiazepines, has short-term treatment outcomes and long-term side effects [6]. These hypnotics are not very useful for chronic insomnia as they may produce unpleasant side effects like drug dependence, tolerance, rebound insomnia, amnesia, muscle relaxation, and depression [7,8,9]. In addition, these drugs increase the risk of accidents, poisoning, and falls [10, 11]. Moreover, to identify a drug that addresses the psychiatric illness, mainly the anxiety disorders, co-morbid with insomnia is also important [12, 13].
In view of these concerns, focus needs to be directed to traditional medicines, which are apparently used for a prolonged period without any ill-effects [14, 15]. α-Asarone, an active principle from Acorus species, is one such product which is found to have hypnotic property at a low dose in an acute sleep deprivation (SD) model [16]. It was suggested that the administration of 10 mg/kg α-Asarone improved sleep quality by modulation of the hypothalamic (Thy) and body (Tbody) temperatures. It was also shown that this hypnotic is an antioxidant and has anxiolytic and memory restoring properties when administered for a shorter duration [17]. However, before any clinical trials, it is important to address the efficacy and safety of α-Asarone after long-term usage. Henceforth, this study was aimed to investigate the effect of long-term administration (21 days) of α-Asarone on sleep–wakefulness (S–W), Thy and Tbody in normal and sleep-deprived rats. The long-term changes in anxiety levels were also measured.
Materials and methods
Animals
The study was conducted on 20 adult male Wistar rats (250–350 g) housed in polystyrene cages, kept in controlled temperature (26 ± 1 °C) and light–dark schedule of 12 h (lights on at 6:00 h), with food and water provided ad libitum. In this investigation, the efficacy of α-Asarone in promoting sleep on long-term use was studied not only on normal rats, but also on rats where repeated and limited period sleep deprivation was used as a model of insomnia. All the surgeries and procedures employed in this study were approved by the Institutional Animal Ethics Committee of the Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, Kerala (SCT/TAEC-019/June/2012/77).
Drugs
α-Asarone and Tween80 were obtained from Sigma-Aldrich Co. LLC, while midazolam (positive control) was obtained from Neon Laboratories Ltd. α-Asarone was freshly prepared in the base containing 5% Tween80 and normal saline. The base was taken as vehicle. The route of administration was intra-peritoneum.
Experimental design
All the animals were chronically implanted under anesthesia (Ketamine: 60 mg/kg and Xylaxine: 5 mg/kg, bw) to record S–W, Thy, and Tbody as reported previously [16]. In brief, bipolar EEG (AP: − 3 mm and ML: 2 mm [18]) and EMG electrodes were implanted to assess S–W. For Thy measurement, a pre-calibrated K-type thermocouple was implanted 1 mm above the hypothalamus (AP: − 0.26 mm, ML: 3 mm, DV: 6 mm [18], at an angle of 25°). Radio-transmitter (TA10TA-F40, Data Sciences International, USA) implanted in the peritoneum measured Tbody telemetrically. After 2 weeks recovery, the rats were given overnight habituation in the recording chamber, before being subjected to various experimental schedules.
Rats were randomly distributed into four groups of five animals each. All four groups were repeatedly administered with the drug for 3 weeks, wherein group 1 was kept under normal conditions and the other three groups were sleep deprived. Drug injections and recordings were done during the day time which is the peak time of sleep for rats.
Group 1 (normal rats)
Three control recordings of S–W, Thy, and Tbody were taken simultaneously for 7 h (10:00–17:00 h) from the implanted rats after administering vehicle at 10:00 h, to obtain the pre-drug injection control readings. The rats were then subjected to intra-peritoneal injections of α-Asarone (10 mg/kg) at 10:00 h for 21 consecutive days and S–W, Thy, and Tbody were recorded simultaneously for 7 h (10:00–17:00 h) on days 7 and 21. A one-hour pre-injection baseline recording was also taken from 9:00 to 10:00 h on these days.
Groups 2, 3, and 4 (sleep- deprived rats)
Three control recordings of S–W, Thy, and Tbody were taken simultaneously for 8 h (9:00–17:00 h) from the implanted rats. The first group received vehicle, the second received 10 mg/kg α-Asarone and the third group received 2 mg/kg midazolam intra-peritoneally at 9:00 h followed by SD in rotating wheel for 5 h (9:00–14:00 h) for 21 consecutive days. On days 7 and 21, S–W, Thy, and Tbody were recorded simultaneously from the rats after SD for 3 h (14:00–17:00 h). The data obtained during this “recovery period” of 3 h (14:00–17:00 h) was analyzed as described below.
Baseline anxiety level of the rats was taken in the elevated plus maze (EPM) before starting the entire SD procedure. On day 21, the sleep-deprived rats were tested for their anxiety levels in EPM at 13:55 h immediately after the SD procedure followed by S–W recording starting at 14:00 h.
After 21 days of SD, S–W was recorded on day 22 for 8 h (9:00–17:00 h) without any treatment to check the withdrawal effects of α-Asarone and midazolam (“withdrawal period”).
Anxiety measurement
Anxiety of rats was measured using EPM having two open arms (50 × 10 cm) and two closed arms (50 × 10 × 50 cm), arranged in plus shape, kept at a height of 45 cm. The behavior of rats on the maze was tested for 5 min.
Sleep deprivation procedure
Custom-made rotating wheel consisting of a motor unit and a wheel (diameter: 365 mm × length: 150 mm) moving at 2.5 rotations per minute was used for SD. Water and food was made available inside the wheel during SD.
Acquisition and analysis of signals
S–W data was acquired using data acquisition system MP 150 (BIOPAC, Inc.) simultaneously (time-matched) with Thy and Tbody measurements. The acquired S–W signals were amplified, filtered and digitized. Thy was acquired using a digital multimeter (FLUKE Technologies Pvt. Ltd.) while Tbody was acquired using DSI telemetric system (Data Sciences International, USA). S–W data were visually staged in the Acknowledge software, taking 10 s epochs, and were classified into three stages, W, NREM sleep, and REM sleep [16].
Group 1 (normal rats)
Changes in percentage time, bout duration and frequency of NREM and REM sleep and W for 7 h after the administration of vehicle and 10 mg/kg α-Asarone were calculated and compared. Arousal index was calculated as the number of arousals (wake bouts of 3–10 s duration) per hour of sleep. After administration of vehicle and 10 mg/kg α-Asarone, filtered (0.5–30 Hz) NREM and REM sleep EEG signals were subjected to power spectral density analysis to measure the relative power at delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz) and beta (12–30 Hz) frequency bands [16]. Changes in Thy and Tbody were compared with the vehicle and the pre-injection baseline values. The correlation of sleep bout durations with Thy and Tbody for 7 h on days 7 and 21 after the administration of 10 mg/kg α-Asarone was assessed as described before [16].
Group 2, 3, and 4 rats (sleep-deprived rats)
Assessment of sleep quantity, i.e., the percentage time in S–W and sleep quality which included the duration and frequency of S–W bouts, arousal index, latency to sleep (time for the onset of first NREM sleep bout after the termination of SD) and the spectral analysis were done as reported previously [16] for the 3-h recovery period on days 7 and 21 of SD.
Changes in Thy and Tbody during recovery period were noted and compared with the control values obtained during the same time bin. The correlation of sleep bout durations with Thy and Tbody during the 3 h recovery period on days 7 and 21 of SD was also assessed as reported previously [16]. For anxiety measurement, the number of open and closed arm entries and the time spent in the open and closed arms in EPM were assessed. Body weight was monitored across the SD period.
To check the withdrawal effect of α-Asarone and midazolam, the spectral profiles of NREM and REM sleep EEG on day 22 (withdrawal period) were analyzed. Correlation of sleep bout durations with Thy and Tbody during this period was also noted.
Statistical analysis
All values expressed as mean ± SEM. p ≤ 0.05 were considered as statistically significant. All statistical analyses were done in SPSS (version 16.0). For group 1 (normal rats) repeated measures ANOVA, followed by Bonferroni’s correction, was used to compare the effects of the drug on different days. For groups 2, 3, and 4 rats (sleep-deprived rats) S–W, Thy, and Tbody data, two-way ANOVA with repeated measures on one factor was done to compare across the time bin and among three groups. One-way ANOVA was used to compare the withdrawal effect of the three groups. Linear regression analysis was done to assess the relationship between sleep duration and temperature after drug administration. For anxiety data, the Kruskal–Wallis test was done to compare the findings among the groups and the Wilcoxon signed-rank test was used for comparison with the baseline.
Results
Effects of long-term administration of α-Asarone on S–W in normal rats
The values of the three control recordings (after vehicle administration) in the various S–W quality parameters and their changes on long-term administration of α-Asarone 10 mg/kg is given in Table 1. There was an increase in the NREM sleep bout duration [F(1.513, 6.051) = 20.616, p = 0.003] and a decrease in the NREM sleep bout frequency [F(1.419, 5.677) = 12.429, p = 0.011] and arousal index [F(1.497, 5.989) = 10.337, p = 0.014] on both days 7 and 21 after α-Asarone administration (Table 1). No alterations were observed in the quantity of S–W.
In comparison to the vehicle treatment, a significant increase in the relative delta power [F(1.436, 5.744) = 8.741, p = 0.022] was observed during the NREM sleep on both days 7 and 21 of α-Asarone administration (Fig. 1). EEG power spectrum remained unaltered during the REM sleep.
EEG power spectra of NREM sleep after long-term administration of 10 mg/kg α-Asarone in normal rats. The relative power in % at delta, theta, alpha, and beta frequency ranges during NREM sleep in Group 1 (N = 5) rats administered with 10 mg/kg α-Asarone on days 7 and 21. The data points represent the mean ± SEM. *Indicates the difference from the vehicle group. *p ≤ 0.05
Effects of long-term administration of α-Asarone on T hy, and T body and their relationship with S–W in normal rats
In comparison to the vehicle, a significant decrease in Thy [F(1.700, 6.799) = 7.186, p = 0.023] and Tbody [F(1.090, 4.359) = 12.756, p = 0.019] was observed on day 7 of α-Asarone administration (Table 2). However, only a marginal reduction was observed in Thy and Tbody on day 21 of drug administration (Table 2).
A significant negative correlation was observed between the NREM sleep bout duration and Thy after the administration of 10 mg/kg α-Asarone and vehicle (Fig. 2 left panel). The strength of association of NREM sleep bout duration with Thy was found to be higher on days 7 (37%) and 21 (40%) after administration of α-Asarone, in comparison to the vehicle (Fig. 2 left panel b, c). Similarly, the correlation between the Tbody and NREM sleep bout duration also improved marginally after α-Asarone administration (Fig. 2 right panel). Moreover, the number of longer NREM bouts (≥ 20 min) was increased 4.5–5-folds after α-Asarone administration. There was no change in the correlation between REM bout duration and Thy and other REM parameters throughout the study after α-Asarone administration in normal rats.
Scatter plot showing correlation between the NREM sleep bout duration and the Thy and Tbody after long-term administration of 10 mg/kg α-Asarone in normal rats. Correlation between the NREM sleep bout duration in min and the Thy (left panel) and Tbody (right panel) during NREM sleep after administration of a vehicle and α-Asarone for b 7 and c 21 days in Group 1 rats (N = 5). Thy and Tbody are plotted as the percentage change from the preceding stage (taken as 100%) of the transition to NREM sleep. R2 represents the coefficient of determination, #represents the significance of the regression model and *indicates the difference from the vehicle group. *,#p ≤ 0.05
Effects of long-term administration of α-Asarone on S–W in sleep-deprived rats
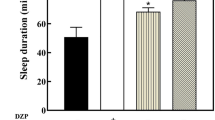
α-Asarone administration produced a significant increase in the NREM sleep after 21 days [F(2,12) = 3.367, p = 0.030] in comparison to the vehicle group (Fig. 3a). By the end of 3rd week, the NREM sleep in α-Asarone group was significantly higher than the midazolam group (Fig. 3a). No change was observed in the quantity of REM sleep in any group.
S–W parameters after long-term administration of vehicle, α-Asarone and midazolam in sleep-deprived rats. a NREM sleep duration in %, b NREM bout duration in minutes, c arousal index, d latency to sleep in minutes and the relative power in % at e delta, f theta, g alpha and h beta frequency ranges during NREM sleep after repeated SD of 5 h for 7 and 21 days in rats treated with vehicle (Group 2), α-Asarone (Group 3) and midazolam (Group 4). Data is represented as mean ± SEM. *Represents the difference from the Group 2, #represents the difference from the respective controls and $represents the difference between Group 3 and 4. *,#,$p ≤ 0.05. N = 5 for each group
In terms of sleep quantity, a significant increase in the NREM bout duration [F(2,12) = 13.476, p = 0.001] was also observed in the α-Asarone-treated group in comparison to both the vehicle and midazolam groups for 21 days (Fig. 3b). Moreover, the bout frequencies of NREM sleep [F(2,12) = 7.805, p = 0.005] and W [F(2,12) = 10.879, p = 0.002] were reduced in the α-Asarone group. Arousal index was considerably lowered [F(2,12) = 23.099, p = 0.000] in α-Asarone group in comparison to the vehicle and midazolam treatments (Fig. 3c). On day 21, the latency to sleep also lowered in the α-Asarone group (p < 0.05), as compared to the vehicle group (Fig. 3d).
Repeated SD for 7 and 21 days increased the relative delta power during NREM sleep in the vehicle-treated group (Fig. 3e). However, a moderate increase in the relative beta power [F(2,12) = 4.065, p = 0.04] was also observed in this group on day 21 (Fig. 3h). On the other hand, in the α-Asarone group, the relative delta power was significantly increased [F(2,12) = 4.930, p = 0.03] with simultaneous decrease in the theta [F(2,12) = 6.422, p = 0.01], alpha [F(2,12) = 4.20, p = 0.04] and beta [F(2,12) = 4.065, p = 0.04] power during NREM sleep in comparison to the midazolam group (Fig. 3 right panel). REM sleep power spectrum remained unaltered.
On day 22, which is taken as the period of withdrawal effect, relative delta power was significantly higher and relative beta power was significantly lower in the α-Asarone SD group in comparison to the vehicle and midazolam groups (p ≤ 0.001), and in comparison to its control during NREM sleep (Fig. 4 left panel). During REM sleep, α-Asarone-treated group showed significantly higher theta power and lower delta, alpha, and beta power during withdrawal period in comparison to the vehicle and midazolam groups (p ≤ 0.001) and to its own control (Fig. 4 right panel).
EEG power spectrum of NREM and REM sleep during withdrawal period on day 22, 24 h after long-term administration of α-Asarone and midazolam in sleep-deprived rats. The relative power in % at a delta, b theta, c alpha and d beta frequency ranges during NREM (left panel) and REM (right panel) sleep on day 22, 24 h after repeated SD in vehicle- (Group 2), α-Asarone- (Group 3) and midazolam- (Group 4) administered rats. Data is represented as mean ± SEM. *Represents the difference from Group 2, #represents the difference from the respective control and $represents the difference between Group 3 and 4. *,#,$p ≤ 0.05
Effects of long-term administration of α-Asarone on T hy and T body and their relationship with S–W in sleep-deprived rats
SD produced an increase in Tbody and Thy in all the groups (Fig. 5b). However, Thy was significantly lower in the α-Asarone group in comparison to both the vehicle and midazolam groups on all days (Fig. 5a). Tbody was also observed to be slightly lower [F(2,12) = 9.084, p = 0.02] in the α-Asarone group (Fig. 5b).
Thy and Tbody profile after long-term administration of vehicle, α-Asarone and midazolam in sleep-deprived rats. aThy and bTbody in °C after repeated SD of 5 h for 7 and 21 days in vehicle- (Group 2), α-Asarone- (Group 3), and midazolam- (Group 4) administered rats. Data is represented as mean ± SEM. *Represents the difference from Group 2, #represents the difference from the respective control and $represents the difference between Group 3 and 4. *,#,$p ≤ 0.05. N = 5 for each group
Here also a significantly higher negative correlation between NREM bout durations and Thy was observed in the α-Asarone-treated group during the recovery period on all the days in comparison to the vehicle (p ≤ 0.01) and midazolam (p ≤ 0.001) groups (Fig. 6). Midazolam treatment significantly reduced the association between NREM bout durations and Thy during the recovery period (Fig. 6c). Furthermore, during the withdrawal period, the negative correlation of NREM bout durations and Thy remained significantly lower (p ≤ 0.001) in the midazolam-treated group (Fig. 6c).
Scatter plot showing correlation of NREM sleep bout duration and the Thy during recovery period after long-term administration of vehicle, α-Asarone and midazolam in sleep-deprived rats. Correlation between the NREM sleep bout duration in minutes and the Thy during recovery period in sleep-deprived rats administered with a vehicle (Group 2), b α-Asarone (Group 3) and c midazolam (Group 4) on days 7 and 21 and during the withdrawal period. Thy and Tbody are plotted as the percentage change from the preceding stage (taken as 100%) of the transition to NREM sleep. R2 represents the coefficient of determination and #represents the significance of the regression model. *Indicates the difference from Group 2 and $indicates the difference between Group 3 and 4. *,#,$p ≤ 0.05. N = 5 for each group
Positive correlation of REM sleep bout duration with Thy was higher (p ≤ 0.05) in the α-Asarone-treated group during the recovery period on all the days in comparison to the vehicle and midazolam groups (Fig. 7). However, in the withdrawal period, all three groups showed significant association between REM sleep bout duration and Thy (Fig. 7). Correlation of sleep bout durations with Tbody during the recovery and withdrawal period was low in all groups.
Scatter plot showing correlation of REM sleep bout duration and the Thy during recovery period after long-term administration of vehicle, α-Asarone and midazolam in sleep-deprived rats. Correlation between the REM sleep bout duration in minutes and the Thy during recovery period in sleep-deprived rats administered with a vehicle (Group 2), b α-Asarone (Group 3) and c midazolam (Group 4) on days 7 and 21 and during the withdrawal period. Thy and Tbody are plotted as the percentage change from the preceding stage (taken as 100%) of the transition to NREM sleep. R2 represents the coefficient of determination and #represents the significance of the regression model. *Indicates the difference from Group 2 and $indicates the difference between Group 3 and 4. *,#,$p ≤ 0.05. N = 5 for each group
Effects of long-term administration of α-Asarone on anxiety levels in sleep-deprived rats
In the EPM test, the entry into the open arm and the time spent in that arm was lower in the vehicle-treated group after 21 days of SD (Fig. 8). α-Asarone-treated group showed significantly higher entry in the open arm (p = 0.05) and had spent more time (p = 0.001) in that arm, as compared to the vehicle group (Fig. 8). Moreover, the time spent in the open arm was significantly higher (p = 0.003) in the α-Asarone group than in the midazolam group (Fig. 8a).
Changes in EPM parameters after long-term administration of vehicle, α-Asarone and midazolam in sleep-deprived rats. Change in percentage a time spent and b entries into the open arm after repeated SD of 5 h for 21 days in rats treated with vehicle (Group 2), α-Asarone (Group 3) and midazolam (Group 4). Data is represented as mean ± SEM. *Represents difference from Group 2, #represents difference from the respective control and $represents difference between Group 3 and 4. *,$,#p ≤ 0.05. N = 5 for each group
Though there was increase in the body weights of all the sleep-deprived rats after 21 days, compared to their own control readings before SD procedure, it was significant (p = 0.03) only for the vehicle-administered rats. But there was no significant difference in the body weights of these rats when compared to the α-Asarone and midazolam groups. There was significant (p = 0.03) increase in the body weights of vehicle-administered, sleep-deprived, rats during the course of the experiment. At the end of the experiment, there was no significant difference in the body weights of all the three groups of sleep-deprived rats.
Discussion
Effect of long-term administration of α-Asarone on sleep
Long-term administration of α-Asarone for 3 weeks shortened the latency to sleep in comparison to the hypnotic midazolam, suggesting α-Asarone’s role in sleep initiation. This property of α-Asarone might be helpful in addressing the increased sleep latency in chronic insomniacs [19]. The increase in NREM bout duration, the decrease in W bout frequency and the arousal index suggest the role of α-Asarone in the maintenance of sleep without fragmentation. This may benefit the poor quality sleep and frequent arousal observed in chronic insomnia [19]. The present study also showed that the drug α-Asarone improved the quantity of NREM sleep without affecting the REM sleep under SD condition. Moreover, no tolerance was developed towards α-Asarone after long-term usage unlike other hypnotics including midazolam [20,21,22].
Patients suffering from chronic insomnia have increased high-frequency activity in EEG during their NREM sleep [23, 24] which was evident even in the present SD model after vehicle administration. Moreover, the decreased delta power and increased high-frequency power during the NREM sleep is one of the side effects associated with long-term usage of midazolam and other hypnotics [25,26,27]. Enhanced delta power and reduced high-frequency activity during NREM sleep in sleep-deprived rats further confirm the efficacy of α-Asarone over midazolam. Long-term usage of midazolam produces withdrawal effects, 24 h after its discontinuation [28,29,30]. This is characterized by reduced delta power and increased high-frequency activity during NREM sleep which is evident in the present study also. The effect of midazolam on majority of parameters studied was similar to the vehicle group. This may point towards the tolerance or diminished response developed by the rats towards midazolam after its repeated administration.
Correlation between drug-induced sleep and temperature changes
The decrease in Thy and minimal difference between the Thy and Tbody after administration of an optimal dose of α-Asarone (10 mg/kg) helped in the maintenance of NREM sleep bout duration and also improved the sleep quality [16]. Linear regression analysis between sleep bout duration and temperature after drug administration showed a good negative correlation between the NREM sleep bout duration and Thy after the administration of α-Asarone. Similar association is maintained even after 21 days of administration in both normal and sleep-deprived rats.
NREM sleep is associated with the lowering of Thy [31]. Lowering of both Thy and Tbody would have contributed to improved NREM sleep quality in normal rats. Notably, even under SD condition, where Thy and Tbody were increased, α-Asarone was effective in improving NREM sleep, by relatively reducing the increase in the temperature. Thus it is possible that the initiation of physiological mechanisms that are responsible for lowering the temperatures by α-Asarone is sufficient to improve NREM sleep quality. Lowering of both Thy and Tbody temperatures by α-Asarone would have resulted from cutaneous vasodilation, which in itself is a trigger for sleep initiation [32]. At the same time, it cannot be ruled out that the improved NREM sleep may also have contributed to the lowering of the temperatures. Even under the elevated temperature after SD, the linear regression analysis showed a good negative correlation between the NREM sleep bout duration and Thy as well as Tbody. Decrease in core body temperature before sleep is found to increase sleep propensity and reduces sleep onset latency [33]. Increased NREM sleep further lowers the core body temperature. This may thereby reinforce the drive to sleep and consolidate the sleep bouts [33]. α-Asarone-mediated lowering of Thy and Tbody would have thus shortened the sleep latency and improved sleep.
Midazolam treatment, on the other hand, produced poor quality sleep, probably as a result of higher Thy and lowered association between NREM sleep bout duration and Thy. Moreover, the improved positive correlation of REM sleep bout durations with Thy and absence of changes in the REM sleep parameters further confirm the role of α-Asarone in preserving the REM sleep. This study further strengthens the concept of inter-relationship between sleep and thermoregulation [33,34,35].
The association between NREM sleep bout duration with Thy during the withdrawal period was still high in the α-Asarone group (unlike midazolam) which further suggests that the α-Asarone improved the quality of sleep without producing withdrawal effect, probably by modulating Thy.
α-Asarone and anxiolysis
In the present study, the increase in anxiety associated with repeated SD substantiated the association of anxiety with insomnia [36, 37]. α-Asarone treatment was found to alleviate anxiety produced by the repeated SD. Moreover, this effect was found to be better than that produced by midazolam, which is a well-known anxiolytic drug [38, 39]. α-Asarone-mediated improvement in sleep may be also be attributed to anxiety alleviation. However, it is reported that the oxidative stress associated with hyperthermia may lead to anxiety [40, 41] and in the present study, SD produced both hyperthermia and anxiety. It has been recently shown that α-Asarone is not only an anxiolytic, but also has antioxidant property [17]. Nevertheless, it is difficult to conclude whether the hypnotic effects of α-Asarone lead to anxiolysis, or anxiolysis leads to hypnosis. However, it may be emphasized that both hypnosis and anxiolysis were superior in the α-Asarone group in comparison to the clinically used benzodiazepine midazolam.
Repeated sleep deprivation model for studying chronic insomnia
It is extremely difficult to have an ideal animal model for chronic insomnia. Though this is the first report in which hypnotic α-Asarone was repeatedly administered in the rats that were sleep-deprived for 3 weeks, usefulness of the procedure was shown in an earlier report in which rats were sleep-deprived for 5 days [16]. The results from the SD vehicle injection group showed that the repeated SD produced increased latency to sleep (difficulty in falling asleep), sleep fragmentation/increased arousal index (frequent awakenings) and reduced sleep quality. All these SD-induced changes in S–W satisfy the clinical guidelines laid down as criteria for chronic insomnia [19]. So this SD procedure in rats may be a good animal model for studying the various aspects of chronic insomnia [19]. No loss of body weight after 5 h of SD for 3 weeks suggests that the method employed for depriving sleep or repeated SD per se was not very stressful for the rats in the present study.
Increase in the Tbody in all the rats of the SD groups may be attributed to the method employed for this procedure. Rotating wheel produces total SD by forcing specific patterns of locomotion in rats per se may be stressful. However, according to one report, the forced locomotion is a minor factor and may not contribute significantly in the studies on SD [42]. Also, this method may be more efficient for repeated SD procedures as it provides standardized equal stimulation to all the experimental rats on all days unlike other methods like gentle handling [43]. In the current study, this procedure produced an increase in Tbody as reported previously [44]. However, Tbody was relatively lower in the α-Asarone group due to the hypothermic property of this drug.
Conclusion
The present study provides strong evidences on the efficacy and safety of long-term administration of α-Asarone. α-Asarone, unlike midazolam, improved NREM sleep for prolonged period without producing tolerance and withdrawal effect. α-Asarone also alleviated anxiety associated with SD. Mild hypothermia, improved association of Thy with sleep bout durations and anxiolysis may have facilitated improvement in sleep. Hence, this drug may be considered for clinical trials as a potential and safe anxiolytic-hypnotic for the treatment of chronic insomnia.
Abbreviations
- S–W:
-
Sleep–wakefulness
- T hy :
-
Hypothalamic temperature
- T body :
-
Body temperature
- SD:
-
Sleep deprivation
- NREM:
-
Non-rapid eye movement
- REM:
-
Rapid eye movement
References
Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111.
Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? JAMA. 1989;262:1479–84.
Shapiro CM, Devins GM, Hussain M. ABC of sleep disorders. Sleep problems in patients with medical illness. BMJ. 1993;306:1532.
Taylor DJ, Mallory LJ, Lichstein KL, et al. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213.
Vgontzas AN, Kales A. Sleep and its disorders. Annu Rev Med. 1999;50:387–400.
Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5–11.
Ashton H. Guidelines for the rational use of benzodiazepines. Drugs. 1994;48:25–40.
Chouinard G. Issues in the clinical use of benzodiazepines: potency, withdrawal, and rebound. J Clin Psychiatry. 2004;65:7–12.
Stone JR, Zorick TS, Tsuang J. Dose-related illusions and hallucinations with zaleplon. Clin Toxicol. 2008;46:344–5.
Herings RM, Stricker BHC, de Boer A, et al. Benzodiazepines and the risk of falling leading to femur fractures: dosage more important than elimination half-life. Arch Intern Med. 1995;155:1801–7.
Serfaty M, Masterton G. Fatal poisonings attributed to benzodiazepines in Britain during the 1980s. Br J Psychiatry. 1993;163:386–93.
Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–68.
Taylor DJ, Lichstein KL, Durrence HH, et al. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–64.
Gulia KK, Radhakrishnan A, Kumar VM. Approach to sleep disorders in the traditional school of Indian medicine. Complementary and alternative medicine. In: Chokroverty S, editor. Sleep disorders medicine part 2, 4th ed. New York: Springer Science + Business Media, LLC; 2017.
Kumar VM, Gulia KK. Sleep medicine in Ayurveda. Sleep Med Rev. 2016;25:131.
Radhakrishnan A, Jayakumari N, Kumar VM, et al. Sleep promoting potential of low dose α-Asarone in rat model. Neuropharmacology. 2017;125:13–29.
Radhakrishnan A, Jayakumari N, Kumar VM, et al. α-Asarone in management of sleep deprivation induced memory deficits and anxiety in rat model. Sleep Biol Rhythm. 2018. https://doi.org/10.1007/s41105-018-0181-7
Paxino G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. San Diego: Academic Press, Inc.; 1997.
Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. JCSM. 2008;4:487.
Michelini S, Cassano GB, Frare F, et al. Long-term use of benzodiazepines: tolerance, dependence and clinical problems in anxiety and mood disorders. Pharmacopsychiatry. 1996;29(4):127–34.
Sanger DJ, Zivkovic B. Investigation of the development of tolerance to the actions of zolpidem and midazolam. Neuropharmacology. 1987;26(10):1513–8.
Soldatos CR, Dikeos DG, Whitehead A. Tolerance and rebound insomnia with rapidly eliminated hypnotics: a meta-analysis of sleep laboratory studies. Int Clin Psychopharmacol. 1999;14(5):287–304.
Buysse DJ. Chronic insomnia. Am J Psychiatry. 2008;165(6):678–86.
Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10(5):1826–34.
Aeschbach D, Dijk DJ, Trachel L, et al. Dynamics of slow wave activity and spindle frequency activity in the human sleep EEG: effect of midazolam and zopiclone. Neuropsychopharmacology. 1994;11:237–44.
Bastian CH, LeBlanc M, Carrier J, et al. Sleep EEG power spectra, insomnia, and chronic use of benzodiazepines. Sleep. 2003;26:313–7.
Parrino L, Terzano MG. Polysomnographic effects of hypnotic drugs. Psychopharmacology. 1996;126:1–16.
Borbely AA, Achermann P. Ultradian dynamics of sleep after a single dose of benzodiazepine hypnotics. Eur J Pharmacol. 1991;195:11–8.
Feige B, Voderholzer U, Riemann D, et al. Independent sleep EEG slow-wave and spindle band dynamics associated with 4 weeks of continuous application of short-half-life hypnotics in healthy subjects. Clin Neurophysiol. 1999;110:1965–74.
Kales A, Scharf MB, Kales JD. Rebound insomnia. A potential hazard following withdrawal of certain benzodiazepines. JAMA. 1979;241:1692–5.
Deboer T, Franken P, Tobler L. Sleep and cortical temperature in the Djungarian hamster under baseline conditions and after sleep deprivation. J Comp Physiol. 1994;174:145–55.
Parmeggiani PL, Velluti RA. The physiologic nature of sleep. World Sci. 2005;18:391.
Gilbert SS, van den Heuvel CJ, Ferguson SA, et al. Thermoregulation as a sleep signalling system. Sleep Med Rev. 2004;8:81–93.
Amici R, Bastianini S, Berteotti C, et al. Sleep and bodily functions: the physiological interplay between body homeostasis and sleep homeostasis. Arch Ital Biol. 2014;152:66–78.
Mallick HN, Kumar VM. Basal forebrain thermoregulatory mechanism modulates auto-regulated sleep. Front Neurol. 2012;3:102.
Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30(7):873–80.
Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9–15.
Anseloni VZ, Brandao ML. Ethopharmacological analysis of behaviour of rats using variations of the elevated plus-maze. Behav Pharmacol. 1997;8:533–40.
Zangrossi H, Viana MB, Graeff FG. Anxiolytic effect of intra-amygdala injection of midazolam and 8-hydroxy-2-(di-n-propylamino) tetralin in the elevated T-maze. Eur J Pharmacol. 1999;369:267–70.
Groenink L, Vinkers C, Oorschot R, et al. Models of anxiety: stress-induced hyperthermia (SIH) in singly housed mice. Curr Protoc Pharmacol. 2009;5:16.
Olivier B, Zethof T, Pattij T, et al. Stress-induced hyperthermia and anxiety: pharmacological validation. Eur J Pharmacol. 2003;463:117–32.
Borbely AA, Neuhaus HU. Sleep-deprivation: effects on sleep and EEG in the rat. J Comp Physiol. 1979;133(1):71–87.
Colavito V, Fabene PF, Grassi Zucconi G, et al. Experimental sleep deprivation as a tool to test memory deficits in rodents. Front Syst Neurosci. 2013;7:106.
Shido O, Sugimoto N, Sakurada S, et al. Body core temperature of rats subjected to daily exercise limited to a fixed time. Int J Biometeorol. 1997;40(3):135–40.
Acknowledgements
The work was supported by the research grant from the Council of Scientific and Industrial Research, New Delhi, India (CSIR Sanction No: 37(1543)/12-EMR II). AR was supported by CSIR Junior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any financial interest or conflicts of interest related to this work.
Ethical approval
All the surgeries and procedures employed in this study were approved by the Institutional Animal Ethics Committee of the Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, Kerala (SCT/TAEC-019/June/2012/77).
Animals
Wistar rats were obtained from the Division of Laboratory Animal Sciences, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum, Kerala.
Rights and permissions
About this article
Cite this article
Radhakrishnan, A., Jayakumari, N., Kumar, V.M. et al. α-Asarone: a hypnotic with a potential for long-term use. Sleep Biol. Rhythms 17, 49–61 (2019). https://doi.org/10.1007/s41105-018-0190-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-018-0190-6