Abstract
Purpose
Obstructive sleep apnea (OSA) and metabolic syndrome (MetS) are common chronic disorders associated with cardiovascular morbidity and mortality. The goal of our research is to identify the associations between OSA and MetS, including different components of MetS, in adults.
Methods
We used data from the 2007–2008 National Health and Nutrition Examination Survey, which included 5909 eligible subjects (2898 men, 3011 women), aged 20 and over, who had undergone a complete medical examination and had self-reported three OSA symptom items. The primary study outcome was possible obstructive sleep apnea (pOSA) and MetS components.
Results
Participants in the pOSA group had significantly more MetS components (p < 0.001). In the group aged ≥ 60 years, there was a stronger relationship between pOSA and MetS components. After additional adjustment, the odds ratios for pOSA among those with 2, 4, and 5 MetS components were 3.11, 3.19, and 4.89, respectively (p < 0.05).
Conclusions
In conclusion, our study indicates that the risk of pOSA is higher in association with increased MetS factors, particularly among the elderly. Leading a healthy lifestyle may help reduce OSA risk in elderly patients with MetS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is characterized by intermittent collapse of the upper airway during sleep, resulting in recurrent partial or complete breathing cessation; the primary symptom of OSA is heavy snoring, and its primary consequence is daytime sleepiness [1]. OSA is a common, chronic disorder linked to hypertension and cardiovascular events [2,3,4]. Moreover, it is also linked with cerebrovascular morbidity and mortality [5]. Metabolic syndrome (MetS) is a cluster of metabolic abnormalities, including obesity, dyslipidemia, hypertension, and impaired glucose tolerance, which, like OSA, is associated with excessive cardiovascular risk [6]. Rates of both OSA and MetS have increased over the past few decades [7]. Previous research has indicated that globally, approximately 2–5% of women and 3–7% of men have OSA [8]. Approximately 40% of U.S. adults are recognized as having MetS [9].
The term ‘syndrome Z’ is used to refer to MetS that is comorbid with OSA [10]. H However, the underlying mechanisms that mediate the association between OSA and MetS are not well understood, particularly across different age groups, and the most important factors influencing MetS remain unclear. Because both OSA and MetS are associated with cardiovascular disease, our study goal was to identify the degree of correlation between OSA and the components of MetS in adults.
Furthermore, a previous study has indicated that OSA causes morbidity in the elderly population and is associated with higher healthcare utilization in elderly patients [11]. Importantly, the high prevalence of OSA in the elderly is underestimated due to a lack of clinical symptoms in that population [12]. Because the economic burden of sleep disorders is greater than other pulmonary diseases and is similar in scope to diabetes [13], more attention should be focused on OSA, especially in the elderly. Therefore, for further analysis, we divided our participants into two subgroups: those aged 20–59 years and those aged ≥ 60 years.
Materials and methods
Study population
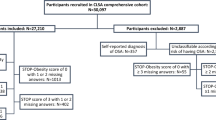
Our data were from the 2007–2008 National Health and Nutrition Examination Survey (NHANES). The National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) conduct the NHANES, a health survey and assessment of non-institutionalized U.S. citizens. The NHANES uses a stratified multistage design with intentional oversampling of minority groups and certain age groups. Clinical examinations are performed at mobile centers by study staff who have formal training to collect: weight, height, waist circumference, blood pressure, serum glucose concentration, and serum lipid profiles. Because our analyses focused on adults, we used only data from participants aged ≥ 20 years who had completed the medical examination (4214 participants < 20 years old were excluded). We also excluded participants with missing sleep questionnaire data (n = 7) and those who refused to answer the key questions or who responded, “Don’t know” (n = 19). Our final sample included 5909 eligible subjects (3011 women, 2898 men). The 2007–2008 NHANES was approved by the NCHS Institutional Review Board, and each subject underwent a basic informed consent process prior to participation.
Probable obstructive sleep apnea
The probable obstructive sleep apnea (pOSA) score was based on three self-reported questionnaire items designed to identify OSA risk [14]. The first question was whether the participant had been previously diagnosed with sleep apnea. The second question was whether they had ever experienced gasping, snorting or cessation of breathing during sleep. The third question was about the occurrence of snoring during sleep. All subjects were asked “How often did you snort, gasp, snore or stop breathing while asleep in the past 12 months?”, with the following response options: “never”; “1–2 nights/week”; “3–4 nights/week”; and “5 or more nights/week.” Participants who responded that they had these symptoms more than 3–4 nights per week or who had been previously diagnosed with OSA were identified as pOSA.
The criteria of metabolic syndrome
Using the revised National Cholesterol Education Program’s Adult Treatment Panel III (NCEP: ATP III), MetS was identified based on the presence of three or more of the following components: central obesity; waist circumference (WC) ≥ 88 cm in females and ≥ 102 cm in males; elevated blood pressure (diastolic blood pressure ≥ 85 mmHg or systolic blood pressure ≥ 130 mmHg); currently taking antihypertensive drugs; high plasma triglyceride level (≥ 150 mg/dL in individuals who do not take lipid-lowering medicines); low high density lipoprotein cholesterol (HDL-C) (< 50 mg/dL in women and < 40 mg/dL in men); receiving medical treatment for a cardiovascular or related condition; high fasting glucose (≥ 100 mg/dL); and currently using an oral hypoglycemic agent or insulin [15].
Associated clinical variables
Clinical NHANES variables, including biochemistry profiles, demographics and medical conditions, have been previously described [16]. Briefly, participant information included age, sex, race/ethnicity, anthropometry (weight, height, and WC), blood pressure, and medical conditions. Body mass index (BMI) was calculated according using the formula kg/m2. Moderate recreational activities were defined based on participants’ answers to the question about engagement in recreational activities, fitness or moderate-intensity sports that induce little increase in heart rate or breathing, such as bicycling, swimming or running for at least 10 min without interruption. Details about data processing and specimen collection are available on the NHANES website.
Statistical analysis
Predictive Analytics Suite Workstation Statistics, version 18.0 for Windows (SPSS Inc., Chicago, IL, USA) was used to perform statistical analyses. Participants were divided into age groups: aged 20–59 years or aged ≥ 60 years. These groups were further divided into pOSA or non-pOSA. Chi-square test was used to analyze discrete variables and Mann–Whitney U test was used to analyze continuous variables. The correlation between pOSA and the number of MetS components was calculated. Based on previous studies, important medical and demographic factors were used as covariates in adjusted multivariable logistic regression models. Model 1 was adjusted for gender, age and race/ethnicity. Model 2 was extended to also adjust for body mass index, smoking status, alcohol consumption, stroke, coronary heart disease, moderate recreational activities, non-restorative sleep, daytime sleepiness, and sleep duration. Model 3 was adjusted for serum uric acid, total bilirubin, homeostatic model assessment for insulin resistance (HOMA-IR) and C-reactive protein (CRP). Additionally, p values were evaluated for trends to determine the relationship between the number of MetS components (as a continuous variable) and pOSA status.
Results
Demographics and clinical characteristics
Table 1 shows the characteristics of the 5909 subjects (2898 men, 3011 women) categorized by age group and pOSA status. Participants with pOSA status were significantly more likely than those with non-pOSA status to quality as having MetS (p < 0.001).
Those aged ≥ 60 years had notably higher age, BMI, waist circumference, HOMA-IR, prevalence of non-restorative sleep, and prevalence of daytime sleepiness compared with those aged 20–59 years. In addition, those aged ≥ 60 years in the pOSA group had significantly lower HDL-C compared with those in the same-age non-pOSA group (all p < 0.001); they also had a higher number of MetS components, with the exception blood pressure, and a higher prevalence of MetS.
In the group aged 20–60 years, BMI, waist circumference, blood pressure, low density lipoprotein cholesterol, serum glucose levels, triglycerides, uric acid concentration, HOMA-IR, CRP status, and prevalence of daytime sleepiness were significantly higher than those aged ≥ 60 years; this group also had notably lower HDL-C and shorter sleep duration in the pOSA group compared with the non-pOSA group (all p < 0.001). Furthermore, patients in the pOSA group had a higher prevalence of MetS and higher number of MetS components.
Metabolic components and probable obstructive sleep apnea
Multivariable logistic regression analyses examining the three models of relationship between number of metabolic components and pOSA status, classified by age, are shown in Table 2. In the group aged ≥ 60 years, there was a trend toward higher rates of pOSA with an increased number of MetS components. After full adjustment, the odds ratios of pOSA predicted by 2, 4, and 5 MetS components were 3.11, 3.19, and 4.89, respectively (p < 0.05). Participants with more MetS components showed an increased tendency to have pOSA, but this was not statistically significant.
Table 3 shows the correlations between each MetS component and pOSA status in participants aged ≥ 60 years. Chi-square analyses showed that each component of MetS was significantly associated with pOSA status (p < 0.05), except blood pressure, and remained significant in unadjusted logistic regression analysis. However, the fully adjusted model did not reach statistical significance.
Discussion
In this cross-sectional study, we investigated the correlations between pOSA status and MetS components. The primary finding was that rate of pOSA increased with the number of MetS components in elderly participants.
Obstructive sleep apnea syndrome is associated with an increased incidence of hypertension, cerebrovascular accident and cardiovascular disease [17]. Many risk factors may contribute to developing OSA, including age, gender, anatomic abnormalities and body habits [18]. Previous research has suggested that airway anatomy and collapsibility play a relatively greater pathogenic role in older adults with OSA [19]. Our study indicates that MetS may also be a major factor affecting OSA in older adults. Parish and colleagues reported a significantly higher proportion of OSA with MetS in men older than 50 years than in women or those younger than 50 years [20]. Indeed, a bi-directional association exists between OSA and MetS, meaning that they are two sides of the same coin [21]. One article published in the Journal of Internal Medicine in 2003 noted that visceral obesity and insulin resistance play significant roles in the development of OSA [22]. In 2008, a review article reported that OSA and MetS are characterized by the same theoretical pathophysiologic link to development of cardiovascular disease, including their respective relationships with insulin resistance and visceral fat accumulation [23]. Other studies have also demonstrated that inflammation, insulin resistance and abdominal obesity contribute to the development of OSA [24, 25].
Our study shows a close connection between pOSA and MetS in older but not younger adults, which raises interesting questions. A 2009 review article indicated that aging is associated with increased adiposity and redistribution of body fat to the abdominal or visceral region [26]. Another study revealed that visceral fat is a major factor in insulin resistance and MetS [27]. The pathophysiology of visceral obesity includes release of proinflammatory cytokines that lead to insulin resistance and inflammation [28]. In fact, a 2014 review article demonstrated that chronic inflammation induces impairment of normal lipid accumulation and dysfunction of adipose tissue, and leads to endoplasmic reticulum stress which cause insulin resistance [29]. A theory of aging-induced insulin resistance and metabolic dysfunction has also been proposed [30]. As is well-known, the components of MetS include visceral obesity, insulin resistance and the inflammatory process, which account for the pathophysiological mechanism of cardiovascular disease [31]. The mechanism common to insulin resistance, visceral obesity, aging-induced inflammation, and MetS might be explained by the results of our study: the close association between pOSA and MetS specifically in the elderly.
Our findings also raise the question of which MetS component is most strongly associated with OSA. The conclusions based on previous research findings have been inconsistent. In 2015, Bakker JP [32] proposed that impaired fasting glucose was significantly associated with OSA in specific ethnic groups in the Multi-Ethnic Study of Atherosclerosis. Another study indicated that BMI was the major contributing factor to OSA [33]. However, BMI was not the major criteria of MetS according to NCEP: ATP III. A recent article, published in 2016, demonstrated that mesenteric fat, a specific type of visceral adipose, is the primary factor affecting OSA [34]. Another 2016 paper showed that visceral adiposity is significantly associated with OSA [35]. On the basis of these studies, we can hypothesize that visceral adiposity was the most likely and important MetS component contributing to OSA in our study. This hypothesis is consistent with the mechanism we have proposed herein to explain the association between pOSA and MetS in elderly participants. However, the fully adjusted model in our study to examine the associations between pOSA and each components of MetS did not reach statistical significance. We speculate that the cumulative metabolic variables may predict OSA, but that such prediction is not specific to any single MetS component.
There were several limitations to this study. First, since it was cross-sectional and we do not have long-term follow-up data, we are unable to draw causal inferences between pOSA and MetS. Second, the definition of pOSA used here was based on self-report rather than polysomnography (PSG), the standard diagnostic test for OSA [36]. However, use of PSG in clinical practice is limited due to its expense, and subjective screening tools are often used clinically both to estimate of the likelihood that patients have OSA and to identify those who should be referred for confirming examination [37]. Future studies may design a screening questionnaire with high sensitivity and acceptable specificity. Third, our study did not consider anatomic abnormality such as neck circumference, which has direct causal effects on obstructive sleep apnea [38, 39]. As it reflects parapharyngeal fat that contributes to upper airway narrowing, if we were able to add neck circumference as a covariate in our regression analysis, they would be more convincing. Fourth, we did not confirm whether participants previously diagnosed with OSA had been treated with medication or continuous positive airway pressure. Excluding those with severe OSA but no clinical symptoms (i.e., because they had received treatment) may have biased our results. Finally, we were unable to determine the severity of OSA based on the limited questionnaire information available; such information may have revealed different influences of MetS based on severity.
In conclusion, our study indicates that in the elderly, the rate of pOSA status increases with increased MetS components. Based on these findings, those who met MetS criteria have a higher likelihood of developing OSA due to visceral obesity, insulin resistance and inflammatory reaction, especially if they are elderly. These results suggest that older patients with MetS should be asked about their sleep quality and patterns, as well as whether they have excessive daytime sleepiness, and they should be referred as appropriate for further evaluation to rule out or treat OSA to decrease the rates of mortality due to cardiovascular disease.
References
Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717.
Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med: JCSM. 2009; 5: 263 – 76.
Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. Jama. 2012;307:2169–76.
Kuniyoshi FH, Garcia-Touchard A, Gami AS, Romero-Corral A, van der Walt C, Pusalavidyasagar S, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–6.
Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Critical Care Med. 2010;182 269 – 77.
Grassi G, Quarti-Trevano F, Seravalle G, Dell’Oro R. Cardiovascular risk and adrenergic overdrive in the metabolic syndrome. Nutr, Metab Cardiovasc Dis: NMCD. 2007; 17: 473–81.
Trombetta IC, Somers VK, Maki-Nunes C, Drager LF, Toschi-Dias E, Alves MJ, et al. Consequences of comorbid sleep apnea in the metabolic syndrome—implications for cardiovascular risk. Sleep. 2010;33:1193–9.
Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society. 2008;5:136–43.
Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–9.
Nock NL, Li L, Larkin EK, Patel SR, Redline S. Empirical evidence for “syndrome Z”: a hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. Sleep. 2009;32:615–22.
Diaz K, Faverio P, Hospenthal A, Restrepo MI, Amuan ME, Pugh MJ. Obstructive sleep apnea is associated with higher healthcare utilization in elderly patients. Ann Thoracic Med. 2014;9:92–8.
Kinugawa K, Nguyen-Michel VH, Mariani J. [Obstructive sleep apnea syndrome: a cause of cognitive disorders in the elderly?] La Revue de Medecine Interne. 2014;35:664–9.
AlGhanim N, Comondore VR, Fleetham J, Marra CA, Ayas NT. The economic impact of obstructive sleep apnea. Lung. 2008;186:7–12.
Sands-Lincoln M, Grandner M, Whinnery J, Keenan BT, Jackson N, Gurubhagavatula I. The association between obstructive sleep apnea and hypertension by race/ethnicity in a nationally representative sample. J Clin Hypertens (Greenwich Conn). 2013;15:593–9.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52.
Liaw FY, Kao TW, Hsueh JT, Chan YH, Chang YW, Chen WL. Exploring the Link between the Components of Metabolic Syndrome and the Risk of Depression. BioMed Res Int. 2015;586251.
Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25.
Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. Jama. 2004;291:2013–6.
Edwards BA, Wellman A, Sands SA, Owens RL, Eckert DJ, White DP, et al. Obstructive sleep apnea in older adults is a distinctly different physiological phenotype. Sleep. 2014;37:1227–36.
Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med: JCSM. 2007;3:467–72.
Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9:211–24.
Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Internal Med. 2003;254:32–44.
Kostoglou-Athanassiou I, Athanassiou P. Metabolic syndrome and sleep apnea. Hippokratia. 2008;12:81–6.
Bonsignore MR, Eckel J. ERS Meeting Report. Metabolic aspects of obstructive sleep apnoea syndrome. Eur Respir Rev Off J Eur Respir Soc. 2009;18:113–24.
Peled N, Kassirer M, Shitrit D, Kogan Y, Shlomi D, Berliner AS, et al. The association of OSA with insulin resistance, inflammation and metabolic syndrome. Respir Med. 2007;101:1696–701.
Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8:339–48.
Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochimica et biophysica acta. 2009;1790:1117–23.
Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiological reviews. 2013;93:359–404.
Park MH, Kim DH, Lee EK, Kim ND, Im DS, Lee J, et al. Age-related inflammation and insulin resistance: a review of their intricate interdependency. Archives of Pharmacal Res. 2014;37:1507–14.
Lipina C, Vaanholt LM, Davidova A, Mitchell SE, Storey-Gordon E, Hambly C, et al. CB1 receptor blockade counters age-induced insulin resistance and metabolic dysfunction. Aging cell. 2016;15:325–35.
Han TS, Lean ME. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis. 2016;5:2048004016633371.
Bakker JP, Weng J, Wang R, Redline S, Punjabi NM, Patel SR. Associations between obstructive sleep apnea, sleep duration, and abnormal fasting glucose. The multi-ethnic study of atherosclerosis. Am J Respir Crit Care Med. 2015;192:745–53.
Wu WT, Tsai SS, Shih TS, Lin MH, Chou TC, Ting H, et al. The association between obstructive sleep apnea and Metabolic markers and lipid profiles. PloS One. 2015;10:e0130279.
Liu KH, Chu WC, To KW, Ko FW, Ng SS, Ngai JC, et al. Mesenteric fat thickness is associated with metabolic syndrome independently of Apnoea-Hypopnoea Index in subjects with obstructive sleep apnoea. Respirology. 2016;21:533–40.
Chen GP, Qi JC, Wang BY, Lin X, Zhang XB, Zhao JM, et al. Applicability of visceral adiposity index in predicting metabolic syndrome in adults with obstructive sleep apnea: a cross-sectional study. BMC Pulmonary Med. 2016;16:37.
Indications. and standards for use of nasal continuous positive airway pressure (CPAP) in sleep apnea syndromes. American Thoracic Society. Official statement adopted March 1944. Am J Respir Crit Care Med. 1994;150:1738–45.
Ward Flemons W, McNicholas WT. Clinical prediction of the sleep apnea syndrome. Sleep Med Rev. 1997;1:19–32.
Yuan H, Schwab RJ, Kim C, He J, Shults J, Bradford R, et al. Relationship between body fat distribution and upper airway dynamic function during sleep in adolescents. Sleep. 2013;36:1199–207.
Cizza G, de Jonge L, Piaggi P, Mattingly M, Zhao X, Lucassen E, et al. Neck circumference is a predictor of metabolic syndrome and obstructive sleep apnea in short-sleeping obese men and women. Metabolic Syndrome Relat Disord. 2014;12:231–41.
Acknowledgements
There was no acknowledgement directly for this manuscript.
Funding
The authors received no funding for this work.
Author information
Authors and Affiliations
Contributions
Chen-Jung Wu contributed to the design of the study, was responsible for the management and retrieval of data, contributed to initial data analysis and interpretation, drafted the initial manuscript. Chen-Jung Wu, Tung-Wei Kao, Yaw-Wen Chang, and Wei-Liang Chen decided upon the data collection methods. Chen-Jung Wu and Wei-Liang Chen were also responsible for the data analysis decisions. Wei-Liang Chen conceptualized and designed the study, supervised all aspects of the study, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. All authors meet the ICMJE criteria for authorship.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they had no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Wu, CJ., Kao, TW., Chang, YW. et al. Examining the association between obstructive sleep apnea and cardiometabolic risk factors in the elderly. Sleep Biol. Rhythms 16, 231–237 (2018). https://doi.org/10.1007/s41105-018-0145-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-018-0145-y