Abstract
Not much evidence exists concerning the effects on sleep and mood of transcranial bright light. In this study, 50 students, all with wake-up time 9:00 a.m. or later in weekends/free days, participated in a double-blind placebo-controlled experiment comparing the effects of bright light (n = 27; 8.0 lm) and placebo (n = 23; 0.1 lm). Data collection consisted of sleep assessment, with both sleep diaries and actigraphy. In addition, the Fatigue Severity Scale and the Profile of Mood States (POMS) were administered. Following 1 week of baseline recording, the therapy was initiated, aiming to phase advance the sleep–wake period. The therapy lasted for 2 weeks and consisted of gradually advancing daily light exposure of 12 min’ transcranial bright light. Subjects in the two conditions did not change differently from baseline to post-treatment on any sleep parameters. A significant condition × time interaction was found for one of six subscales (vigor–activity) of the POMS, suggesting a more favorable development from baseline to post-treatment in the placebo compared to the bright light condition. No differences in terms of side-effects were reported between conditions. It is concluded that transcranial bright light, at times where conventional light therapy has phase-advancing properties, did not influence any sleep parameters differently than placebo. Transcranial bright light was associated with a less favorable development from baseline to post-treatment on one mood parameter compared to placebo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep and wakefulness and several other bodily processes oscillate according to a 24-h rhythm (circadian rhythm). These rhythms are governed by the master biological clock, the suprachiasmatic nuclei (SCN), which normally has an endogenous period that is somewhat longer than 24 h [1]. The SCN is normally entrained to a 24-h rhythm by external cues (time givers), such as daylight, physical activities, meals, etc. [2]. The circadian rhythm controls the core body temperature, which typically falls in the evening and continues to drop to its lowest point (nadir) in the early morning hours, after which it rises [3]. Melatonin, which is synthesized and secreted by the pineal gland, shows an inverse rhythm compared to the core body temperature rhythm [4].
In adults, sleep normally and most easily occurs from about 6 h before nadir of the core body temperature to about 2 h following nadir of the core body temperature [5]. The circadian rhythm strongly affects human performance, which is poorest during the circadian trough [6].
There exist several major input fiber systems to the SCN. The most important stem from photoreceptors in the retina in the eye, which convey signals to the suprachiasmatic nucleus via a monosynaptic pathway, the retinohypothalamic tract. There exists a subset of retinal cells (2,500 of a total of 100,000 cells) containing a light-sensing pigment, melanopsin, which is assumed to be involved in circadian photoentrainment [7]. Light has been shown to affect the circadian rhythm according to a phase response curve (PRC). According to the PRC, light exposure prior to the nadir for the core body temperature (which typically occurs about 2 h prior to the habitual wake-up time) delays the circadian rhythm, whereas light exposure after the nadir phase advances the circadian rhythm [3].
The phase-changing properties of light have been extensively used in the treatment of circadian rhythm sleep–wake disorders, normally by exposing patients to light in the visible part of the spectrum with intensities up to 10,000 lux [8]. Studies indicate that light in the blue spectrum of the visible light (~480 nm) is especially efficient at phase resetting the circadian clock [9]. Several studies have attested to the phase-advancing effects of bright light when administered after nadir of the core body temperature [10–12]. Bright light for phase entrainment has been administered in several ways. The most common way is by exposure to light from stationary light boxes. The exposure time varies according to the light intensity and normally lasts between 30 min and 2 h. Dawn simulators have also been used, in which a microprocessor-controlled lighting device delivers a mimic of gradual twilight transitions found outdoors [13]. Head-mounted light visors have also been employed, where the light source is attached to a visor hat and where the light beam directly targets the eye area [14]. A potential problem with several of the devices described above is low compliance, as exposure time is relatively long and since the ability to engage in other activities during light exposure is normally very limited. In addition, light from the aforementioned sources has been associated with several, although mild, side effects such as hypomania, irritability, headache, nausea, eyestrain, and blurred vision [15].
It has been speculated that also extraocular light might have entrainment effects on the circadian rhythm. However, so far evidence has failed to support such notions [16]. Recently, a new artificial light source was developed (Valkee©), providing transcranial brain-targeted bright light treatment via the ear canal. Valkee is based on the fact that in mammals, light penetrates the skull bone and reaches the brain, representing extraocular transcranial phototransduction. In one study based on functional magnetic resonance imagery, it was found that the Valkee light source produced an increased functional connectivity in lateral visual and sensorimotor networks in a resting state [17]. In an uncontrolled study, Valkee was associated with improvement in symptoms of seasonal affective disorder (SAD) over a 4-week study period [18]. In addition, in a randomized double-blind dose response study light administered via Valkee seemed to have antidepressant and anxiolytic effect in SAD patients, however, the effect was unrelated to the light dosage [19].
In terms of mechanisms involved it has been suggested that the opsin panopsin (OPN3) which exists in significant amounts in GABAergic Purkinje cells in the cerebellum might be the mediator of the effect [17]. It has further been suggested that phototransduction induces excitatory signaling with broad effects via neurotransmitters like dopamine, serotonin or noradrenaline [17]. Still, it has to be acknowledged that so far neither any theoretical explanation nor any firm empirical findings exist that convincingly elucidate any specific mechanisms of action for transcranial light. In addition, a recent study showed that melatonin levels were not suppressed by transcranial light, in contrast to bright light administered through the eyes [20].
To measure the circadian phase of a person in an objective manner, either core body temperature or melatonin levels (in saliva, urine or blood) are normally assessed. In ordinary clinical practice, however, such approaches are normally not viable. In such settings we have instead advocated for the estimation of nadir of the core body temperature based on subjective data [5]. As the nadir of the core body temperature is located about 2 h before the habitual time of awakening, nadir can as a rule of thumb be estimated at 2 h before the final awakening taking place on free days, when a person can sleep uninterrupted until he or she awakens spontaneously [5]. Further support for this rule of thumb stems from studies that have shown that subjective sleep data from uninterrupted sleep can be used as a reasonably good estimate of circadian phase [21, 22], and that sleep offset correlates more highly with different phase markers than sleep onset [21, 23].
So far no study has investigated whether Valkee can alter sleep–wake parameters. There is also limited evidence from independent research groups investigating whether Valkee can influence feelings of fatigue and mood. Many students have delayed sleep–wake rhythms [24], and impaired mood and fatigue also seem to be prevalent among students [25]. Hence, this group may profit from interventions aiming at advancing their sleep–wake rhythm and combating fatigue and impaired mood. Against this background, we therefore designed a placebo-controlled 2-week intervention study addressing whether daily morning exposure (12 min) of transcranial brain-targeted bright light compared to a placebo condition will: (a) change the sleep–wake rhythm assessed by sleep diaries and actigraphy, (b) improve mood and levels of fatigue, and (c) yield different side-effects profiles in students with habitual (free time) spontaneous waking at 9:00 a.m. or later.
Methods
Sample
In all, 54 students (13 males and 41 females, mean age 21.24, SD 2.27) at the University of Bergen were recruited. There were two inclusion criteria: (1) the subjects needed to have a habitual spontaneous wake-up time on free days at 9:00 a.m. or later verified by the Munich Chronotype Questionnaire (MCTQ) [26]. We did not use wake-up time on weekdays as a criterion as wake-up time on such days typically reflects external demands (such as university classes) and to a little extent reflect the endogenous sleep–wake rhythm of the individual. (2) Further, they needed to aim for advancement of their sleep–wake rhythm. One exclusion criterion, lifetime history of bipolar disorder assessed with the Mood Disorder Questionnaire (MDQ) [27], was employed.
Procedure
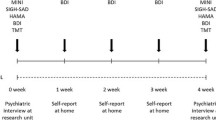
Information about the study was given in lectures, and those who were interested were invited to information meetings about the project. In the meetings, details about the procedures involved were given. Those who agreed to participate signed an informed consent form. Upon signing the informed consent, the participants were instructed to wear an actigraph on the non-dominant wrist throughout the study period. For baseline recordings, the participants were instructed to keep a sleep diary for 1 week. They also completed the Fatigue Severity Scale (FSS) [28] and the Profile of Mood States (POMS) [29] at baseline. Following the baseline recordings, the sleep diary and the questionnaires were handed in. The participants were then allocated to one of two conditions (bright light or placebo) by a consecutive randomization process (the first who read and signed the form was allocated to the bright light condition, the next was allocated to the placebo condition, the third person was allocated to the bright light condition, etc.). All participants were then handed a Valkee unit with two light-emitting diodes (LEDs) attached to an earplug. Both the research assistant who administered the unit and the participants were blind to the condition (double blind). In the bright light condition, the light emitted from each diode equaled 8.0 lm/5.762 mW/cm [2] (which is 2.28 times than that used in the commercial product), whereas the placebo condition had 0.1 lm/0.072 mW/cm [2]. Apart from the light intensity, the placebo device looked and operated in exactly the same manner as the bright light device. The light emitted from the placebo condition was also clearly visible, which would reduce demand characteristics from influencing the results [30].
During the first 5 days of light exposure, the participants were instructed to use the device about 2 h after they normally woke up spontaneously on free days. During the following 5 days of light exposure, the participants were instructed to use the device 1 h earlier (advancing light exposure by 1 h). On the last 4 days, the participants were told to use the Valkee unit about the time they normally wake (advancing light exposure further by 1 h). The participants were instructed to keep a log registering times for use of the Valkee unit. Each day the light exposure lasted for 12 min. When the 12-min exposure time had elapsed, a sound signal announced that the light exposure had come to an end and the unit turned itself off. During the last week of light exposure the participants kept a sleep diary and again completed the FSS [28] and the POMS [29]. When handing in the Valkee unit following the 14-day bright light exposure, the participants completed the Light Visor Side Effects Questionnaire (LVSEQ) [31]. All participants were paid 500 NOK (~70 US$) for participating. None of the 54 included participants withdrew from the study. However, four participants (all from the placebo condition) were excluded as according to the compliance log, they had used the light therapy <9 of 14 days within 30 min of the prescribed light exposure time. Thus, a total of 50 participants were included in the data analyses. A flow-chart of the study is presented in Fig. 1. The study was approved by the Regional Committee for Medical and Health Research Ethics, Eastern/Southern Norway (2013/2124) and conformed to the provision of the Declaration of Helsinki. It was conducted between January 2014 and May 2014.
Instruments
Demography
Questions about age, gender, living arrangement, study program, and part-time work were asked. These questions were only administered at baseline.
Mood Disorder Questionnaire
MDQ is a validated self-report instrument that screens for the presence of a lifetime history of bipolar disorder. It contains 13 yes/no items that cover topics such as mood, self-confidence, energy, sociability, interest in sex, loquaciousness, distractibility, and other behaviors. There are in addition two questions assessing whether the symptoms ever co-occurred and to which degree the symptoms caused functional impairment. A positive case entails endorsement of seven or more of the 13 symptoms, endorsement of the co-occurrence item and reporting moderate or serious degree of functional impairment [27]. The MDQ was administered for screening purposes at baseline only.
Munich Chronotype Questionnaire
The MCTQ asks participants to report bedtime, time for lights out, sleep onset latency, and final wake-up time. These parameters are reported for both work days and days off. The most commonly used index from the MCTQ is the days off midpoint of sleep [26]. The MCTQ was administered at baseline only and was used to verify that the inclusion criterion (final wake-up time at 9:00 a.m. or later on free days) was fulfilled.
Sleep diary
A sleep diary comprising daily estimates of the following sleep variables was kept for 1 week at baseline and during the last week of the light treatment: (a) the time at which the individual attempted to fall asleep (lights out); (b) sleep onset latency; (c) waking time after sleep onset, (d) time of final awakening, and (e) perceived sleep quality [rated via a Likert scale ranging from 1 (worst) to 5 (best)].
Fatigue Severity Scale
The FSS is a nine-item questionnaire that assesses the effect of fatigue on daily living. Each item is a statement on fatigue that the subject rates from 1 (“completely disagree”) to 7 (“completely agree”). The scale is brief, easy to administer, and has demonstrated high reliability and internal consistency [28]. The FSS was administered at baseline and at post-treatment. The Cronbach alpha for the FSS was 0.88 and 0.92 at baseline and post-treatment, respectively.
Profile of Mood States
The POMS consists of 65 different adjectives rated on a 5-point scale ranging from 1 (“not at all”) to 5 (“extremely”). Scores for a total of six subscales are calculated: (1) tension–anxiety (9 items), (2) depression–dejection (15 items), (3) anger–hostility (12 items), (4) vigor–activity (8 items), (5) fatigue–inertia (7 items), and (6) confusion–bewilderment (7 items). The items can be administered according to two different time frames, right now or during the past week, including now [29]. The latter time frame was used in the present study. The POMS was administered at baseline and at post-treatment and was used as an outcome measure. The alphas for the six subscales at baseline were 0.73, 0.91, 0.70, 0.68, 0.87, and 0.70, respectively. The corresponding alphas for post-treatment were 0.75, 0.91, 0.64, 0.83, 0.91, and 0.57.
Actigraphy
Actigraphy consists of small wrist-worn units, of the size of a watch, containing an accelerometer, a clock and a storing device. Accordingly, wrist movements are recorded and stored. Following recording, the data are transferred to a computer. Based on a software algorithm, the data are analyzed in terms of sleep parameters [32]. In the present study we used the Actiwatch Spectrum from Respironics. Epoch time was set to 1 min. Actigraphic data (lights out, sleep onset latency, wake after sleep onset, final wake-up time and total sleep time) were collected at baseline and at post-treatment, and were used as outcome variables.
Light Visor Side Effects Questionnaire
This self-report questionnaire reflects the subjects’ own experiences of possible side effects associated with exposure to bright light. The subjects are asked to rate 11 symptoms (abdominal pain, dizziness, eyestrain, fatigue, feeling “wired,” headache, disturbed sleep, muscle pain, nausea, sweating, or other) on a scale with anchors of 0 (“not experienced”) to 3 (“severe”) reflecting whether they experienced these symptoms during the treatment period, considered them to be due to the light exposure, and how severe they felt the symptoms were. One item asking about irritated/sore ears was added to the existing 11 items. A composite score was calculated by adding the scores of each of the 12 symptoms [31]. The LVSEQ was only administered at post-treatment. The Cronbach’s alpha was 0.72.
Statistics
Data were analyzed with a 2 × 2 ANOVA with one between subject factor (group: bright light vs. placebo) and one within subject factor (time: baseline and post-treatment). G*Power, version 3.1.7, was used for power calculations [33]. Expecting moderate effect sizes (Cohen’s d = 0.50), significance level (alpha) set to 0.05, expected correlation coefficient between repeated measures of 0.50, and power (1 − β) set to 0.80 showed that a total of 34 subjects would be needed to detect significant group × time interactions. To adjust for multiple testing a Bonferroni-correction was used taking into account the number of sleep diary variables (6), actigraphy variables (5) and mood variables (7) analyzed setting new p values for significance at 0.00833, 0.01000 and 0.00714, respectively.
Results
Demographics and waking time free days at baseline
Participants in the bright light (6♂, 21♀) and the placebo condition (7♂, 16♀) neither differed in terms of gender (χ 2 = 0.11, p = 0.74, continuity correction) nor in age (bright light: m = 21.15, SD 2.01 vs. placebo: m = 21.43, SD 2.68; t = 0.43, df = 48, p = 0.67). The mean wake-up time on free days/weekends did not differ between the two conditions at baseline (bright light: m = 11:50, SD 0.49, placebo: m = 11:40, SD 0.58, t = 0.63, df = 48, p = 0.53).
Sleep variables: sleep diary
The results concerning sleep diary data are presented in Table 1. None of the main effects of group were significant, implying that overall across baseline and post-treatment there were not any differences on sleep diary parameters across the two conditions (bright light vs. placebo). There were not any significant main effects of time, suggesting no overall change in self-reported sleep from baseline to post-treatment. None of the group × time interactions were significant, thus development over time between the bright light and the placebo condition did not differ.
Sleep variables: actigraphy
Table 2 shows the results based on analyses of the actigraph data. No significant main effects of group were found for any of the sleep parameters. One main effect of time was detected, reflecting a later wake-up time at post-treatment compared to baseline. None of the group × time interactions were significant, thus changes over time between the bright light and the placebo condition did not differ.
Fatigue and mood
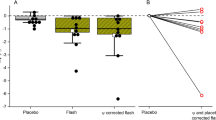
The results concerning fatigue and mood are shown in Table 3. None of the main effects of group were significant. On two of seven parameters, a significant effect of time was detected. This concerned a reduction in scores from baseline to post-treatment on the FSS, and an increase from baseline to post-treatment on the vigor–activity subscale of the POMS. One of the group × time interactions was significant. This reflected that the increase of the scores of the vigor–activity subscale of the POMS was steeper from baseline to post-treatment in the placebo compared to the bright light condition.
Side-effects
On the composite score of the LVSEQ, the mean score did not differ between the groups (bright light: m = 0.24, SD 0.26 vs. placebo: m = 0.13, SD 0.22; t = 1.61, df = 46, p = 0.12).
Discussion
Regarding sleep variables, the results indicate that the bright light therapy condition did not influence any sleep variables differently than the placebo condition. It is well established both in terms of mechanisms [3] and in clinical studies [34, 35] that bright light administered after nadir for the core body temperature has phase-advancing properties. The present study suggests that administering transcranial bright light at corresponding times does not have similar effects, as no differential effects were detected on timing of the sleep–wake period between the bright light and the placebo condition. Two recent studies have shown that transcranial bright light does not suppress the production of melatonin [20, 36] in contrast to light administered via the eyes [37]. Thus, so far data suggest that transcranial bright light has no phase entrainment properties. Alternatively, transcranial bright light may have phase entrainment properties, but through other mechanisms than light administration via the eyes [17]. Thus, it cannot be ruled out that timing of transcranial bright light has a different PRC than for light administered via the eyes. However, this has to be shown in future research before transcranial bright light can be recommended as treatment against circadian rhythm sleep–wake disorders. Another explanation for the negative findings is that phase advancement did occur, but that this was not detected by sleep diaries and actigraphy assessment due to personal preferences and external demands on sleep and wake times.
In the present study we did, however, find one significant different effect over time on mood between the bright light and the placebo condition. In contrast to the placebo condition, mood did not improve over time on the vigor–activity subscale in the bright light condition, as shown by the significant group × time interactions. This finding is in contrast to previous randomized controlled trials that showed that transcranial bright light could counteract winter depression [19] and alleviate sleepiness and improve mood in jet lag [38]. One explanation for the divergent finding may relate to the samples used in the winter depression study, the jet lag study and in the present study. Winter depression is characterized by increased sleep need and lethargy [39] and symptoms of sleepiness and impaired mood prevail in jet lag [40]. Hence, if transcranial bright light has activating effects, patients suffering from winter depression and jet lag may experience symptom relief as their level of activation may become normalized. In the present study the Hospital Anxiety and Depression Scale was administered at inclusion and of the 50 participants, only three indicated mild depression and one indicated moderate depression (results not shown). In addition, the study took place during ongoing university classes, which significantly reduced the study participants’ opportunities for time-zone crossing traveling. Hence, it is not likely that neither winter depression nor jet lag was present in the sample as a whole. If transcranial bright light has activating properties, the level of activation of subjects without depression and jet lag may not be of significance.
Some notions in terms of limitations and strengths of the present study are warranted. None of the participants of the present study were diagnosed with circadian rhythm sleep–wake disorders, hence, lack of effects on the sleep–wake parameters may be attributed to floor effects. Still, their final wake-up time on free days/weekends at baseline was close to 12:00 noon, suggesting that their sleep–wake rhythm was delayed. Thus, they should have a significant potential for phase advancement of their sleep–wake rhythm. Measures of the core body temperature or dim light melatonin onset were not included, thus objective and direct measures of the circadian rhythm were lacking. However, the sleep–wake parameters were assessed both by sleep diaries and actigraphy. As such measures do correlate with objective measures of the circadian rhythm [21, 22] it is unlikely that advancement of the circadian rhythm did take place without this being reflected in the sleep–wake data, especially since motivation to phase advance the sleep–wake rhythms was an inclusion criterion. There were some missing data in the data set, resulting in analyses of data from <50 participants for all outcome variables. However, in all instances the total number of participants left for analyzing was above the number of participants needed according to the a priori power analysis reported in the methods section. In terms of assets of the present study, the use of a double-blinded randomized control trial should be emphasized. The difference between the light intensity across the two conditions was 80-fold, thus lack of differential effects is unlikely to be attributable to small differences between the two conditions. Another strength of the present study was the use of a compliance log, including only those who used the Valkee unit as prescribed at least nine of 14 days. The use of standardized and validated instruments was also strengths of the present study.
It is concluded that transcranial bright light did not alter the sleep–wake parameters differently than placebo. Transcranial bright light was associated with a less favorable development from baseline to post-treatment on one mood parameter.
References
Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81.
Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiol Int. 2003;20:741–74.
Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol (Lond). 2003;549:945–52.
Rajaratnam SM, Arendt J. Health in a 24-h society. Lancet. 2001;358:999–1005.
Bjorvatn B, Pallesen S. A practical approach to circadian rhythm sleep disorders. Sleep Med Rev. 2009;13:47–60.
Folkard S, Monk TH. Shiftwork and performance. Hum Factors. 1979;21:489–92.
Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–70.
Terman M, Terman JS. Light therapy. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. St. Louis: Elsevier Saunders; 2011. p. 1682–95.
Ruger M, St Hilaire MA, Brainard GC, Khalsa SBS, Kronauer RE, Czeisler CA, et al. Human phase response curve to a single 6.5 h pulse of short-wavelength light. J Physiol (Lond). 2013;591:353–63.
Cole RJ, Smith JS, Alcala YC, Elliott JA, Kripke DF. Bright-light mask treatment of delayed sleep phase syndrome. J Biol Rhythms. 2002;17:89–101.
Lack L, Bramwell T, Wright H, Kemp K. Morning blue light can advance the melatonin rhythm in mild delayed sleep phase syndrome. Sleep Biol Rhythms. 2007;5:78–80.
Sharkey KM, Carskadon MA, Figueiro MG, Zhu Y, Rea MS. Effects of an advanced sleep schedule and morning short wavelength light exposure on circadian phase in young adults with late sleep schedules. Sleep Med. 2011;12:685–92.
Terman M, Terman JS. Circadian rhythm phase advance with dawn simulation and negative air ionization for seasonal affective disorder. Am J Psychiatry. 2006;163:2126–33.
Boulos Z, Macchi MM, Sturchler MP, Stewart KT, Brainard GC, Suhner A, et al. Light visor treatment for jet lag after westward travel across six time zones. Aviat Space Environ Med. 2002;73:953–63.
Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10:647–63.
Ruger M, Gordijn MCM, Beersma DGM, de Vries B, Daan S. Acute and phase-shifting effects of ocular and extraocular light in human circadian physiology. J Biol Rhythms. 2003;18:409–19.
Starck T, Nissilä J, Aunio A, Abou-Elseoud A, Remes J, Nikkinen J, et al. Stimulating brain tissue with bright light alters functional connectivity in brain at the resting state. World J Neurosci. 2012;2:81–90.
Timonen M, Nissila J, Liettu A, Jokelainen J, Jurvelin H, Aunio A, et al. Can transcranial brain-targeted bright light treatment via ear canals be effective in relieving symptoms in seasonal affective disorder? A pilot study. Med Hypotheses. 2012;78:511–5.
Jurvelin H, Takala T, Nissila J, Timonen M, Ruger M, Jokelainen J, et al. Transcranial bright light treatment via the ear canals in seasonal affective disorder: a randomized, double-blind dose-response study. BMC Psychiatry. 2014;14:article no. 288.
Bromundt V, Frey S, Odermatt J, Cajochen C. Extraocular light via the ear canal does not acutely affect human circadian physiology, alertness and psychomotor vigilance performance. Chronobiol Int. 2014;31:343–8.
Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14:229–37.
Wright H, Lack L, Bootzin RR. Relationships between dim light melatonin onset and the timing of sleep in sleep onset insomniacs. Sleep Biol Rhythms. 2006;4:78–80.
Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’Hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–88.
Saxvig IW, Pallesen S, Wilhelmsen-Langeland A, Molde H, Bjorvatn B. Prevalence and correlates of delayed sleep phase in high school students. Sleep Med. 2012;13:193–9.
Short MA, Gradisar M, Lack LC, Wright HR, Dohnt H. The sleep patterns and well-being of Australian adolescents. J Adolesc. 2013;36:103–10.
Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90.
Hirschfeld RMA, Williams JBW, Spitzer RL, Calabrese JR, Flynn L, Keck PE, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the mood disorder questionnaire. Am J Psychiatry. 2000;157:1873–5.
Krupp LB, Larocca NG, Muirnash J, Steinberg AD. The Fatigue Severity Scale—application to patients with multiple-sclerosis and systemic lupus-erythematosus. Arch Neurol. 1989;46:1121–3.
Norcross JC, Guadagnoli E, Prochaska JO. Factor structure of the Profile of Mood States (POMS)—2 partial replications. J Clin Psychol. 1984;40:1270–7.
Orne MT. On the social psychology of the psychological experiment: with particular reference to demand characteristics and their implications. Am Psychol. 1962;17:776–83.
Levitt AJ, Joffe RT, Moul DE, Lam RW, Teicher MH, Lebegue B, et al. Side-effects of light therapy in seasonal affective-disorder. Am J Psychiatry. 1993;150:650–2.
Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–67.
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Saxvig IW, Wilhelmsen-Langeland A, Pallesen S, Vedaa O, Nordhus IH, Bjorvatn B. A randomized controlled trial with bright light and melatonin for delayed sleep phase disorder: effects on subjective and objective sleep. Chronobiol Int. 2014;31:72–86.
Gradisar M, Dohnt H, Gardner G, Paine S, Starkey K, Menne A, et al. A randomized controlled trial of cognitive-behavior therapy plus bright light therapy for adolescent delayed sleep phase disorder. Sleep. 2011;34:1671–80.
Jurvelin H, Takala T, Heberg L, Nissila J, Ruger M, Leppaluoto J, et al. Transcranial bright light exposure via ear canals does not suppress nocturnal melatonin in healthy adults—a single-blind, sham-controlled, crossover trial. Chronobiol Int. 2014;31:855–60.
Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–9.
Jurvelin H, Jokelainen J, Takala T. Transcranial bright light and symptoms of jet lag: a randomized, placebo-controlled trial. Aerosp Med Hum Perform. 2015;86(4):1–8.
Magnusson A, Partonen T. The diagnosis, symptomatology, and epidemiology of seasonal affective disorder. CNS Spectr. 2005;10:625–34.
Haimov I, Arendt J. The prevention and treatment of jet lag. Sleep Med Rev. 1999;3:229–40.
Acknowledgments
The authors received 12,000 Euros from Valkee® covering expenses related to the conduct of the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pallesen, S., Nødtvedt, Ø., Saxvig, I.W. et al. A new light source (Valkee©) does not alter sleep–wake parameters and does not improve mood in phase delayed subjects. Sleep Biol. Rhythms 14, 97–105 (2016). https://doi.org/10.1007/s41105-015-0027-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-015-0027-5