Abstract
Air pollution is one of the major health threats for urban dwellers due to increased concentration of pollutants and vehicular traffic. Approximately 7.0 million premature deaths in the world are associated with air pollution. Although stringent emission standards have been introduced, no significant reduction in air quality index in metro cities has been reported. Alternative solutions such as photocatalytic materials can be utilized as an innovative strategy to reduce the concentration of pollutants. Photocatalytic materials can be used in photocatalytic concrete pavements, which decrease the concentration of NOx by photocatalytic activity. A systematic review of photocatalytic materials and its application in concrete pavements has been introduced. The laboratory and field investigations of photocatalytic concrete pavements have been discussed. It was found that NOx reduction up to 60 and 31%, respectively, was observed in laboratory and field conditions. Titanium dioxide (TiO2) is the mostly commonly used photocatalytic additive in the range of 3–10% by cement weight. The review found that the photocatalytic concrete pavements have not gained significant attention unlike other pavement materials. The demerits of TiO2 for field application and research lacunae in this domain have been discussed and based on which, future scope of research is proposed. It is envisaged that photocatalytic concrete pavements can play a significant role in reducing air pollutant concentration in urban areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air is one of the significant elements for the life to sustain. The quality of air in the atmosphere plays a crucial role in influencing the well-being. However, with the industrialization, the quality of air has significantly worsened over the course of time [1]. In addition to industrialization, one of the other significant factors, which have contributed to lowering the air quality, is vehicle emission from transportation activities, and in particular, on-road vehicles [2]. According to World Health Organization (WHO), 99% of the World’s population breathes air, whose quality levels are below the prescribed limits [3]. The air quality is measured using a parameter “Air quality index (AQI)”, which ranges from 0 to 500, where higher the AQI indicates unhealthier air [4]. The motor vehicle emissions are one of the prime contributors towards higher AQI [5]. The motor vehicles emit gases such as carbon dioxide (CO2), carbon monoxide (CO), particular matters such as PM2.5 and PM 10, and toxic gases such as nitrous oxide (NOx) and sulphur oxide (SOx) [6]. Among these gases, CO2 contributes to the global warming leading to depletion of ozone but has relatively less impact on respiratory system of urban dwellers. On the contrary, NOx and SOx are toxic in nature and are known to cause short- and long-term respiratory disorders along with PM2.5 and PM10. Several studies have shown good association with exposure to the vehicle pollutants and respiratory disorders such as chronic obstructive pulmonary disease, upper respiratory tract infection, asthma, and bronchitis. [7,8,9,10,11,12,13]. Further, the extent of air pollution varies spatially depending on the type of settlement, where urban dwellers are more affected from respiratory disorders compared to rural dwellers [14, 15]. The concentration of vehicle pollutants is higher in urban areas due to higher vehicular population and canopy-type built environment. The study by Schneidemesser et al. [16] found that the presence of trucks, buses, and mopeds increased the particulate matter concentrations by 40% and this increased with traffic congestions. This clearly indicates that with the increase in number of vehicles, traffic congestions during peak hours, presence of traffic signals at the intersections, etc., are bound to increase the vehicle pollutant concentration further deteriorating the air quality, especially in urban scenario.
In order to reduce the emissions from the vehicles, emission standards are in effect in different countries. In Europe, Euro 6/VI emission standards are adopted and different emissions standards are provided for gasoline and diesel vehicles [17]. The G20 countries, which account for 90% of global vehicle sales, also adopt a similar emission standards. These emissions standards have been revised and made more stringent to curb the contribution of air pollution from vehicles. The emission standards for Euro 5 and Euro 6 for diesel vehicles are shown in Table 1.
Although emission standards are in force, still the problem of air pollution persists and has been one of the major causes of pollution related deaths. Some cities have adopted traffic congestion mitigation measures, such as car-pooling methods, public transport, but still there is no significant drop in the concentration of pollutants [18].
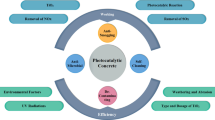
There is a need to look for solution to curb air pollution problem due to vehicle emissions from different dimension apart from the solutions provided by administrative and economical aspects. One such material technology, which can be considered as a supplement to the existing methods of air pollution mitigation, is photocatalytic concrete [19]. The main objective of this review is to critically assess the performance of photocatalytic concrete in pavement applications as one of the solutions to reduce air pollution from vehicle emissions. The outline of the review is shown in Fig. 1.
Significance of the review
There has been rapid growth in world’s population and economic development, which has led to an increase in transportation infrastructure. With the increase in transportation infrastructure, vehicular emission has increased at an alarming rate. Almost 29% of the greenhouse gases (GHGs) are caused due to vehicular emissions [20]. Nearly 10% of work-related diseases are chronic respiratory disease (CRD), majorly caused by air pollution. The increment in the population has increased the demand to travel, which has increased the transportation on the roads. If seen closely, the drivers of buses, autos, and rickshaws are a significant group of people who are continuously exposed to the pollution effect and hence are more prone to get infected with CRDs. Out of all the pollutants, nitrous oxide (NOx) has the most detrimental impact and has the second-highest societal impact cost after particulate matter (PM) [21]. As far as road infrastructure is considered, it contributes a significant part to the urban sprawl. The total area coverage of roads in the urban areas is nearly 40–60% [22]. With this advantage, several kinds of research have been carried over self-cleaning and air-purifying pavements to reduce air pollution. This review is related to the applicability of photocatalytic concrete in pavements to reduce NOx pollutant from the atmosphere.
Vehicular pollutants and health impacts
Studies have shown that there has been an average consumption of 100 million tons of fuel every year [23]. The emissions due to the utilization of these fuel include pollutants such as carbon dioxide (CO2), carbon monoxide (CO), particulate matter (PM) and nitrous oxide (NOx). One of the major reasons for air pollution is the increasing travel demand of the people, which increases the transportation mobility on the roads. This hiked mobility of the people is converting into a high price in terms of its side effects. The major pollutants are CO2, CO, PM and NOx. The primary reasons for the escalation of vehicular pollution are as follows:
-
Increased vehicle density in urban areas [24]
-
Use of older vehicles [6]
-
The dominance of cars and two-wheelers [25]
-
Insufficient public transportation system [26]
-
Inadequate land use planning [27]
-
Improper road conditions [28]
A study conducted by Singh et al. [23] estimated the pollution load from different metro cities in India, which is one of the fastest developing countries and also has highest social cost of carbon [29]. The pollution load estimated for the year 2020 is shown in Table 2. The pollution load corresponds to Euro 4 emission standards, which is equivalent to BS-IV in India. It can be clearly seen the magnitude of the different pollutants being emitted from gasoline and diesel vehicles in one year. These pollutants will have severe implications on the respiratory health of the urban dwellers [30].
The transportation sector contributes nearly 55% of NOx production [20], out of which 35% of the NOx production is from heavy vehicles such as buses and trucks, and the rest is contributed by diesel engine cars. As compared to 2012, the pollution load has drastically increased, and reports suggest that the production of NOx per day in Delhi is nearly 17,000 kg/sq-km [23]. The damaging effect of these pollutants is more adverse as compared to CO2. A detailed pollutant-wise health effect is explained in Table 3. It can be seen that NOx has more negative effects both on the environment and health. Hence, there is a need to reduce the pollutants significantly at the site of production itself.
Photocatalysis
The photocatalysis is a process, where the reaction takes place in the presence of a catalyst activated by the light. The catalyst used in the process of photocatalysis is referred as photocatalyst, as these become active in the presence of light and increase the rate of reaction [31]. The first observation of the photocatalysis was perceived by Fujishima and Honda [32] in electrochemical photolysis of water using semiconductor in the presence of ultraviolet radiations. Most of the semiconductor materials possess photocatalytic property and are used in various engineering applications [33]. Among the various photocatalysts, titanium dioxide (TiO2) has been studied and applied extensively in the domain of civil engineering in cement concrete applications [34].
Photocatalytic additives
The photocatalytic property in concrete is achieved by the addition of photocatalytic material/additives such as titanium dioxide (TiO2), zinc oxide (ZnO), zinc sulphide (ZnS), carbon nitride (C3N4), and silicon carbide (SiC). Each photocatalytic material is characterized by band gap energy value, which is the energy required for the electron to move from valence band to conduction band. The typical band gap energy value for different photocatalytic additives is shown in Table 4. However, among several photocatalytic additives, TiO2 has gained significant attention owing to its non-toxicity, cost-effective, and stability against photochemical corrosion.
The band gap energy value indicates the minimum energy required for the electron to shift from valence band to conduction band, where this energy is derived from the light source. In the ambient conditions, the solar radiations act as light source providing the required energy for electron to shift from valence band to conduction band corresponding to their band gap energy value. According to the Planck-Einstein relation (Eq. 1), the band gap energy is inversely proportional to wavelength of the light.
where
E = Energy Band Gap, eV; \(h\) = Planck’s Constant; \(c\) = Speed of light, m/s; \(\lambda\) = Wavelength, nm.
Substituting the band gap energy value for TiO2 in Eq. 1 results in wavelength of 387 nm, which falls in the domain of ultraviolet radiations (UV-A) of electromagnetic spectrum as shown in Fig. 2. In other words, this indicates that photocatalytic activity in TiO2 can be only activated in the presence of UV-A radiations. The wavelength of light higher than UV-A such as visible light does not result in photocatalytic activity as the energy acquired will not be sufficient to shift electron from valence band to conduction band.
Photocatalytic concrete
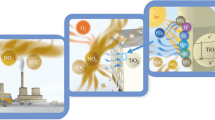
Photocatalytic concrete is a special concrete, which possess photocatalytic property that can provide self-cleaning characteristics to the concrete [35]. The photocatalytic concrete has the ability to reduce the concentration of harmful gases such as NOx and hence has been found applications in the pavements. Road infrastructure constitutes a major part in the urban sprawl. The total area coverage of roads in the urban areas is nearly 40–60% on an average. Several investigations have been carried over self-cleaning [22] and air-purifying pavements in order to reduce the air pollution. The major catalyst used here is TiO2 and its efficiency to reduce pollutant concentration especially NOx in the presence of ultraviolet (UV) radiation will be reviewed in detail in subsequent sections.
The schematic shown in Fig. 3 shows the functioning of photocatalytic concrete in NOx concentration reduction. The photocatalytic reaction in the concrete is a result of series of reduction–oxidation reactions (redox), which takes place on the surface and also at a certain distance from the surface. When the electron–hole pair is generated in the TiO2 and no recombination takes place, the first stage of redox reaction takes place with water or oxygen forming free radicals. Further, the electron–hole pair can also initiate redox reactions with pollutants, which is regarded as direct redox reaction. The free radicals, which are formed, tend to detach from the surface forming reactive oxygen species (ROS). ROS are generally in the form of.OH,.O2−, HO2., H2O2. ROS decompose the organic and inorganic pollutants thus reducing the adverse effect of the pollutants. Based on this, the photocatalytic reaction process can be classified as direct redox reaction, radical formation and redox reaction, radical-ROS formation and redox reaction [36]. The typical reduction and oxidation reactions, which takes place in the presence of UV light and TiO2 as photocatalyst, are shown below.
Reduction reactions as shown in Eqs. 2–9 lead to the formation of N2 by decomposing NOx as experimentally verified by [37].
where
\(hv\) = Incident Photon
\(e^{ - }\) = Electron
\(h^{ + }\) = Hole
It should be noted here from Eqs. 2–9 that no free radicals or reactive oxygen species are present in the reactions. In other words, reduction reactions takes place by the formation of electron–hole pair generation and hence increasing the recombination time can improve the reduction reactions as stated in the later sections.
The oxidation reactions leads to the formation of nitric acid (HNO3), which gets deposited on the surface and can be cleaned easily [38]. Equations 10–18 show the oxidation reactions in the presence of ROS. Equation 10 indicates the generation of charge carrier, while Eqs. 11 and 12 show the formation of ROS. Equations 13–16 show the oxidation reactions. Most of the previous studies have highlighted the formation of HNO3 due to oxidation on pavement. However, reduction reactions in TiO2 are seldom due to high electron–hole pair recombination rate.
Effect of photocatalytic additive content
The efficiency of the photocatalytic concrete highly depends on the quantity of TiO2 and exposure conditions. The past studies have used photocatalytic concrete as thin layer of concrete on existing pavements as only the concrete surface takes part in photocatalytic action. Poon and Cheung [39] developed paver blocks of size 200 by 100 mm, where they were provided with 5-mm-thick photocatalytic concrete on the top. The dosage of TiO2 in the concrete was varied from 0 to 10% per cent in the interval of two per cent. The testing for NOx degradation was conducted in a close chamber where specimen were tested over 28, 56 and 90 days, respectively. The results showed that the NOx removal decreased as the age of the specimen increased but it was constant throughout the life after that. The decrease was around 8% and was majorly due to carbonization of cement. The sample with 10% of titanium dioxide showed the best removal efficiency as compared to the lower content. This study indicated that the photocatalytic activity will reduce with time and hence renewal will be required.
Dylla et al. [40] evaluated the efficiency and cost-effectiveness of TiO2 coating placed over a concrete pavement. The coating consisted of TiO2, cement, fines, and water. The dosage of TiO2 varied between 3 and 5% by weight of cement. It was seen that the specimen coating with 5% of TiO2 with no fines showed the maximum NOx removal efficiency up to 47% as compared to others dosages. This study indicated that applying a coating of TiO2-based cement on the existing surface of concrete pavements will function better than adding it into the concrete.
In another interesting study, Asadi et al. [41] utilized TiO2 in pervious concrete for the first time. The main aim of incorporating TiO2 in pervious concrete was attributed to the increased surface texture, which increases the reaction sites and also it allows sunlight to penetrate deeper into the concrete increasing the NOx removal efficiency. Pervious concrete was prepared in two layers, where photocatalytic concrete was provided in top 12.5, 25, 50 and 75 mm to study the effect of thickness on NOx removal. Similar to Dylla et al. [40], the TiO2 content was varied from 3 to 5%. The results indicated that highest NOx removal efficiency was achieved when void ratio was 27% and thickness of photocatalytic concrete was 75 mm. This study clearly indicated that pervious concrete provides added benefit for NOx reduction due to porous structure and deeper penetration of sunlight.
Janus et al. [42] concentrated on properties of TiO2 and their influence on performance characteristics of concrete. Two types of TiO2, calcined at 300 and 600 °C, were used in the range of 1–3%. The results indicated that compressive and flexural strength increased by 10 and 8%, when 3% TiO2 calcined at 300 °C was added. However, considering the NOx removal efficiency, 3% TiO2 calcined at 600 °C was found to be optimal.
Effect of physical parameters on photocatalytic concrete activity
The photocatalysis process is highly affected by both internal and external factors. Internal factors include the properties of the materials, whereas the external factor include physical surrounding characteristic where the reaction is taking place.
Temperature Temperature do not have a significant effect on the process but few of the studies carried out previously reveal that as the temperature increases, the efficiency of reaction decreases [43]. In contrast, some of the research studies concluded that temperature increases the efficiency of photocatalytic activity. Overall temperature can be considered as a neutral parameter.
Relative humidity Relative humidity is considered as one of the important influencing factors for photocatalytic activity. The increased water molecules due to higher RH decreases the reaction sites, thus reducing the efficiency of photocatalysis [Kakinoki et al. 2004]. Due to hydrophilic nature of photocatalyst, they tend to absorb water from atmosphere. This in turn forms layer on the surface of photocatalyst, thereby providing hindrance absorption of pollutant. [44] carried out the experiment at various conditions. The results revealed that the photocatalytic activity was highly significant at humidity level 50%, and beyond it, the photocatalytic activity decreased. [41] found that for TiO2 photocatalytic pervious concrete, the NOx removal efficiency decreased from 80 to 10%, when the RH increased from 20 to 80%. Similarly, [45] also found that the NOx removal efficiency decreases from 55 to 7.5%, when the RH increased from 20 to 80%. Therefore, as the humidity increases, the removal efficiency decreases and vice versa.
The flow rate of the pollutants The flow rate of the pollutants controls the time of exposure for photocatalytic activity. Higher flow rate decreases the availability of pollutants for reaction. [44] found that there was a significant reduction in NOx removal from 64.29 to 28.88%, when the flow rate of gas increased from 3.0 to 5.0 l/min. [41] varied the flow rate from 3 to 9 l/min for photocatalytic pervious concrete. The increased flow rate resulted in decreased NOx removal efficiency from 59 to 19%. [45] found that when the flow rate of NOx was increased from 1.5 to 3.0 l/min, the removal efficiency decreased from 65 to 38%.
Field studies related to photocatalytic concrete
The laboratory studies on photocatalytic concrete depicted the influence of test parameters on the NOx removal efficiency. However, these tests were conducted on controlled mode under specific conditions. Several studies have implemented photocatalytic concrete in pavements and have quantified the NOx removal efficiency by various means under realistic conditions. [46] carried out full-scale demonstration of photocatalytic concrete pavement in Hengelo, Netherlands. The pavement consisted of paver blocks of 75 mm thickness, where 70 mm was conventional concrete, while the 5.0 mm was photocatalytic concrete with TiO2. The field data indicated that under ideal conditions, photocatalytic concrete could reduce the NOx concentration by 42% compared to the control pavement. Further, a significant variation was observed in the results due to the influence of several factors. In the year 2010, Hassan et al. [45] applied TiO2-based water solution in spray form on existing concrete pavement in Louisiana State. The NOx removal efficiency in field condition was determined using two methods. In the first method, concentration of NOx was determined before and after application of TiO2 spray. The NOx removal efficiency varied on daily basis, where the highest reduction was found from 37 part per billion (ppb) to 7 ppb. In the second method, water was collected from the surface of pavement with and without TiO2 spray. The chemical test of water indicated that pavement treated with TiO2 spray had higher content of nitrates indicating NOx removal. In another study, Dylla et al. [47] applied TiO2 water-based spray at a rate of 1.5 to 2.0 ml/ft2. The study evaluated the NOx removal efficiency under field conditions as a function of wind speed, solar intensity, and relative humidity. Akin the laboratory studies, the field indicated that when RH was high, the removal efficiency was lower and vice versa. The peak reduction in NOx concentration was found in the mid-day, when the solar intensity was highest. Further, at higher wind speeds (> 2.5 m/s), the NOx removal efficiency was significantly lower. [48] conducted field evaluation of photocatalytic concrete pavement serving as walkway in the Florianopolis, Brazil. The photocatalytic concrete was laid in three thicknesses of 3, 6 and 10 mm, where the blocks were tested for efficiency in laboratory before installation in the field. After one year of service, few blocks were dismantled and tested again in laboratory. The study found reduction in efficiency of photocatalytic activity with time, and this can be rejuvenated by washing. Further, thinner the photocatalytic concrete, better was the efficiency, which was further enhanced with Anatase variant of TiO2. The paver blocks were prepared with two variants of TiO2: Rutile and Anatase. In similar direction, [49, 50] discusses the implementation of photocatalytic concrete pavement in Antwerp, Belgium. [51] determined the efficiency of photocatalytic concrete on pilot test sections in Warsaw. The study indicated that large variability in the NOx removal efficiency exists under field conditions due to the effect of various factors across the geographic locations. Based on the field measurements, the study found that on an average, 31% reduction in NOx concentration was achieved due to photocatalytic concrete paver blocks.
Developments in the photocatalytic activity of TiO2 and way forward
The past studies have used TiO2 as the photocatalytic additive in the concrete. However, TiO2 depicts photocatalytic activity only due to UV radiations, which constitute 3–5% of electromagnetic spectrum. Under visible light conditions, the efficiency of TiO2 decreases as the energy will not be sufficient to shift electron from valence band to conductance band (Eq. 1). In order to improve the efficiency in visible light conditions, researchers have adopted the various methods, which can decrease the bandgap energy value of TiO2.
Visible light excited photocatalytic additives
In order to improve the photocatalytic activity in the visible light conditions, photocatalytic materials, which can be excited in the visible light range, can be used. Visible light photocatalyst can be either developed using TiO2 as primary material using the method discussed below. On the other hand, non-TiO2-based visible light photocatalyst has been explored and developed. Some of the non-TiO2-based photocatalyst include tungsten trioxide (WO3), silver phosphate (Ag3PO4), bismuth vanadate (BiVO4), and graphitic carbon nitride (g-C3N4) [52]. The bang gap energy of non-TiO2 photocatalyst is shown in Table 5. The non-TiO2-based photocatalysts are currently being investigated for water treatment and hydrogen splitting, while its application for photocatalytic concrete has not been made yet.
Coupling and doping
Coupling is a process of attaching another semi-conducting material to TiO2 such that the electron remains in the conductance band for longer [53]. Doping is a process, where foreign materials are introduced into the lattice structure of TiO2, which internally develops different levels of band gap energy [54]. The schematic of TiO2 coupled with other metals such as zinc oxide and silicon carbide is shown in Fig. 4. It can be seen that the TiO2 (green circle) is coupled with low energy band gap material. Due to this coupling, the electrons easily shift to conductance band from valence band in the low energy band gap material. Further, instead of shifting to valence band, it remains in the conductance band due to coupling, thus increasing the electron–hole pair recombination duration.
The schematic of doped TiO2 is shown in Fig. 5. It can be seen that doping includes modifying the lattice structure of TiO2, which creates additional energy levels increasing the electron–hole pair recombination duration. The doping can be performed using noble metals such as gold (Au), silver (Ag), platinum (Pt), and palladium (Pd) and also non-metals and metals such as nitrogen (N), carbon (C), silicon (Si), iron (Fe), molybdenum (Mo), respectively. The coupling and doping increase the efficiency of photocatalytic activity as electrons will be excited even in the visible light conditions. A schematic of absorbance spectrum of TiO2 and coupled-doped TiO2 is shown in Fig. 6. It can be seen that by coupling-doping TiO2, the absorbance in the visible light region can be increased leading to photocatalytic activity.
Although there are no studies, where coupled-doped-TiO2 application in concrete pavement, there are a few studies conducted in concrete for building applications. [55] doped TiO2 with silver (Ag) and utilized in concrete to understand the self-cleaning nature of the concrete. TiO2 was modified with silver using the silver nitrate solution via the photo-deposition method. The amount of Ag used was nearly 0.11–0.98% of the total mass present in AgNO3. Slurry of 1% Ag- TiO2compound was prepared and sprayed on the concrete, and a dye-degradation test was carried out to check the photocatalytic activity of silver-doped titanium oxide. Analysis showed that slurry thus sprayed on the concrete showed an antifungal and self-cleaning effect of the concrete. Though the compound was successful in dye-degradation, it cannot be said that it can also degrade NOx from the atmosphere. Some additional investigation is required to the NOx decomposition of doped TiO2 which was not reflected in both pieces of research discussed above. Additionally, visible light decomposition of this doped titanium dioxide was also not discussed in the above research.
Seo and Kim [56] developed concrete walls, which had plastic bars to allow the light to pass through. The aim was to investigate the photocatalysis from the visible light excitation. In order to achieve this, the authors first doped the TiO2 with Cu, Cr and S by calcination at 1000 °C for 24 h. The calcined product was milled to produce fine powder of doped TiO2. The spectrometer analysis showed that the Cu-doped TiO2 was very efficient in exciting photocatalytic activity in the visible light environment. A reduction of 15.8% was achieved by Cu-doped TiO2, while Cr- and S-doped TiO2 showed very less degradation rate in the visible light environment. Khannyra et al. [57] investigated the photocatalytic activity in the visible light regime by doping TiO2 with copper. The copper was doped at 2, 5, 10 and 15%, and experiments were conducted under visible light range. In the visible light range of wavelength, TiO2 failed to absorb visible light unlike Cu-doped TiO2 as determined using UV spectrometer. This indicates that by doping copper, one can achieve the photocataysis in the visible light range. The experimental results indicated that the optimum % of copper was 5% even though %NO removed increased with increase in Cu content. The %NOx removed was highest in the case of 5% Cu. Coatings were developed with these materials and were applied for field testing. The results indicated that the region with 5% Cu had very less colour change compared to other coated regions indicating its self-cleaning abilities.
Leaching effect
The past studies have mostly focussed on assessing the pollutant reduction ability of photocatalytic concrete. However, the leaching of photocatalytic materials and nitrates from the photocatalytic pavements are not significantly investigated. Nitrates being the reaction product from photocatalytic activity will enter the groundwater and may increase its concentration in the long run. Studies have shown that higher concentration of nitrates in the drinking water may lead to adverse health issues such as infant methemoglobinemia, colorectal cancer, and thyroid disease [58]. Further, photocatalytic additives may also leach out from the pavement reducing the efficiency of photocatalysis. Relinque et al. [59] investigated the leaching potential in photocatalytic pavement by collecting rainwater runoff for 800 days. The concentration of titanium and nitrates along with pH of water and conductivity was assessed. The results indicated the photocatalytic concrete tiles depicted very low titanium leaching, while photocatalytic cement substrates percolated on asphalt layer showed higher leaching. Further, the nitrate concentration in leachate was in tandem with the photocatalytic reaction. The concentration of both the materials was found to be lower than limits prescribed for drinking water in Madrid city, where the maximum concentration of titanium was found to be only 60 μg/L. Very limited studies are available focussing on the long-term performance of photocatalytic pavements due to loss of photocatalytic materials and eutrophication potential of nitrates released from these pavements. Therefore, in addition to development of novel photocatalytic materials, this domain also provides future scope of research. Based on such studies, decisions can be taken on maintenance of photocatalytic pavements and life cycle analysis aspects.
Summary and conclusion
The main objective of this review article was to critically review the past studies on photocatalytic concrete and propose the way forward for future research as a sustainable application to reduce impact of air pollution. Unlike highways, the air pollutant concentration is found to be higher in urban area and is bound to increase with time due to increase in vehicular numbers. Although strict emission standards are introduced in many countries, still the air pollution problem persists and is known to affect to the health of urban dwellers. One of the innovative solutions to reduce the adverse effect of air pollution due to vehicular emission in urban areas is photocatalytic concrete pavements. The past studies have shown that up to 35–40% of NOx concentration was reduced in the presence of photocatalytic concrete pavements. Titanium dioxide (TiO2) was found to be the most commonly used photocatalytic material in concrete pavements. Several field studies have applied photocatalytic materials either in the form of spray or a thin layer of photocatalytic mortar as photocatalytic reaction is mainly confined to the surface. Interestingly, increasing the surface porosity, such as, in pervious concrete, can improve the photocatalytic reaction. Although TiO2 is used as a photocatalytic material, its performance is only limited in the presence of UV radiations, which constitutes only 3–5% of electromagnetic spectrum. In this regard, a large scope for research exists in this domain as stated below:
-
Development of novel photocatalytic materials, which can be excited in the visible light wavelength range.
-
Long-term photocatalytic performance of photocatalytic concrete pavement: the past studies have evaluated the performance over a short term. However, pavements are designed to serve 10–15 y, during which photocatalytic materials may leach out reducing the efficiency. Understanding and modelling the long-term performance will assist in decision-making related to maintenance and rejuvenation of photocatalytic reaction. Past studies have indicated washing with muriatic acid and spray application of the suspended TiO2 particles in water as some of the rejuvenation methods. However, the long-term performance of such rejuvenation methods is yet to be quantified [46, 60]
-
Environmental impact assessment: nitrates are deposited on the photocatalytic concrete pavements as product of photocatalytic reaction. The regular maintenance of pavements like washing and rainfall will carry the nitrates into the adjoining soil increasing the nitrates concentration of groundwater. Investigating the long-term impact of nitrates from such pavements will assist of formulating regulatory measures.
-
Life cycle cost analysis (LCCA): photocatalytic materials result in additional material cost increasing the overall cost of project. Further, the regular maintenance associated with photocatalytic concrete pavements such as rejuvenation by spraying photocatalytic materials will also incur additional cost. Therefore, there is a need to perform cost analysis for a given design period including construction and maintenance of photocatalytic concrete pavements. The benefits associated with reduction in NOx concentration should be converted to monetary values to obtain realistic life cycle cost analysis.
References
Manisalidis I, Stavropoulou E, Stavropoulou A, Bezirtzoglou E (2020) Environmental and health impacts of air pollution: a review. Front Public Health. https://doi.org/10.3389/fpubh.2020.00014
Anenberg S, Miller J, Henze D, Minjares R (2019) A global snapshot of the air pollution-related health impacts of transportation sector emissions in 2010 and 2015, international council on clean transportation (ICCT). DC, USA, Washington
WHO (2022) https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health, Accessed on 12 Mar 2022
Suman (2021) Air quality indices: a review of methods to interpret air quality status. Mater Today Proc 34:863–868. https://doi.org/10.1016/j.matpr.2020.07.141
Walsh MP, Moore CA (1989) Motor vehicle contribution to global and transported air pollution. Stud Environ Sci 35:387–404. https://doi.org/10.1016/S0166-1116(08)70606-X
Song X, Hao Y (2021) Research on the vehicle emission characteristics and its prevention and control strategy in the central plains urban agglomeration China. Sustainability. https://doi.org/10.3390/su13031119
Buckeridge DL, Glazier R, Harvey BJ, Escobar M, Amrhein C, Frank J (2002) Effect of motor vehicle emissions on respiratory health in an urban area. Environ Health Perspect 110(3):293–300. https://doi.org/10.1289/ehp.02110293
Burr ML, Karani G, Davies B (2004) Effects on respiratory health of a reduction in air pollution from vehicle exhaust emissions. Occup Environ Med 61:212–218. https://doi.org/10.1136/oem.2002.003244
César AC, Carvalho JA, Nascimento LF (2015) Association between NOx exposure and deaths caused by respiratory diseases in a medium-sized Brazilian city. Braz J Med Biol Res Revista brasileira de pesquisas medicas e biologicas 48(12):1130–1135. https://doi.org/10.1590/1414-431X20154396
Heberle SM, da Costa GM, Barros N, Rosa M (2018) The effects of atmospheric pollution in respiratory health. In: Hussain C (ed) Handbook of environmental materials management. Springer, Cham
Mbelambela EP, Hirota R, Eitoku M, Muchanga SMJ, Kiyosawa H, Yasumitsu-Lovell K, Lawanga OL, Suganuma N (2017) Occupation exposed to road-traffic emissions and respiratory health among congolese transit workers, particularly bus conductors, in Kinshasa: a cross-sectional study. Environ Health Prev Med 22(1):11. https://doi.org/10.1186/s12199-017-0608-9
Mohandas S, Francis PT, Rakesh PS, Libin Antony PF (2019) Assessment of respiratory morbidity among bus drivers and conductors of the state road transport corporation, Kochi. Kerala J Family Med Prim Care 8(12):3887–3892. https://doi.org/10.4103/jfmpc.jfmpc_548_19.PMID:31879631;PMCID:PMC6924213
Luo Z, Wang Y, Lv Z, He T, Zhao J, Wang Y, Gao F, Zhang Z, Liu H (2022) Impacts of vehicle emission on air quality and human health in China. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.152655
Khandar C, Kosankar S (2014) A review of vehicular pollution in urban India and its effects on human health. J Adv Lab Res Biol. 5, E-ISSN: 0976–7614
Sciaraffa R, Borghini A, Montuschi P, Gerosa GA, Ricciardi W, Moscato U (2017) Impact of air pollution on respiratory diseases in urban areas: a systematic review: daniele Ignazio La Milia. Eur J Public Health. https://doi.org/10.1093/eurpub/ckx189.117
Schneidemesser E, Steinmar K, Weatherland EC, Bonn B, Gerwig H, Quedenau J (2019) Air pollution at human scales in an urban environment: impact of local environment and vehicles on particle number concentrations. Sci Total Environ 688:691–700. https://doi.org/10.1016/j.scitotenv.2019.06.309
William M, Minjares R (2016) A technical summary of Euro 6/VI vehicle emission standards, Int Council Clean Trans. 1–12
Wang J, Wu Q, Liu J, Yang H, Yin M, Chen S, Guo P, Ren J, Luo X, Linghu W, Huang Q (2019) Vehicle emission and atmospheric pollution in China: problems progress and prospects. PeerJ 7:e6932. https://doi.org/10.7717/peerj.6932
Boonen E, Beeldens A (2014) Recent photocatalytic applications for air purification in Belgium. Coatings 4:553–573. https://doi.org/10.3390/coatings4030553
Fan V, Yee SP, Klemeš JJ, Lee CT (2018) A review on air emissions assessment: transportation. J Clean Prod 194:673–684
Čokorilo O, Ivković I, Kaplanović S (2019) Prediction of exhaust emission costs in air and road transportation. Sustainability 11(17):4688. https://doi.org/10.3390/su11174688
De Marco T, Fava G, Guerrini GL, Manganelli G, Moriconi G, Riderelli L (2013) Use of photocatalytic products for sustainable construction development. In: Third international conference on sustainable construction materials and technologies
Singh N, Mishra T, Banerjee R (2021) Emission inventory for road transport in India in 2020 framework and post factor policy impact assessment. Environ Sci Pollut Res 29:20844–20863
Harrison RM, Vu TV, Jafar H, Shi Z (2021) More mileage in reducing urban air pollution from road traffic. Environ Int. https://doi.org/10.1016/j.envint.2020.106329
Holnicki P, Nahorski Z, Kaluszko A (2021) Impact of vehicle fleet modernization on the traffic-originated air pollution in an urban area—a case study. Atmosphere. https://doi.org/10.3390/atmos12121581
Gonzalez L, Perdiguero J, Sanz A (2021) Impact of public transport strikes on traffic and pollution in the city of Barcelona. Trans Res Part D Trans Environ. https://doi.org/10.1016/j.trd.2021.102952
Nieuwenhuijsen MJ (2020) Urban and transport planning pathways to carbon neutral, liveable and healthy cities. A Rev Curr Evidence Environ Int. https://doi.org/10.1016/j.envint.2020.105661
Qiao F, Nabi M, Li Q, Yu L (2020) Estimating light-duty vehicle emission factors using random forest regression model with pavement roughness. Trans Res Rec 2674(8):37–52. https://doi.org/10.1177/0361198120922997
Ricke K, Drouet L, Caldeira K (2018) Country-level social cost of carbon. Nat Clim Chang 8:895–900. https://doi.org/10.1038/s41558-018-0282-y
Foster A, Kumar N (2011) Health Effects of air quality regulations in Delhi India. Atmos Environ 45(9):1675–1683. https://doi.org/10.1016/j.atmosenv.2011.01.005
Ohtani B (2014) Photocatalyst. In: Kreysa G, Ota K, Savinell RF (eds) Encyclopedia of applied electrochemistry. Springer, New York. https://doi.org/10.1007/978-1-4419-6996-5_497
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Serpone N, Emeline AV (2012) Semiconductor Photocatalysis—Past Present, and Future Outlook. J Phys Chem Lett 3:673–677. https://doi.org/10.1021/jz300071j
Alina P, Janina A, Bogdan L (2016) Application of titanium dioxide in cement and concrete technology. Key Eng Mater 687:243–249
Mircea D (2019) Self-cleaning concrete for landscaping applications. In: MATEC web of conferences, Vol 289 https://doi.org/10.1051/matecconf/201928905004
Ren H, Koshy P, Chen W, Qi S, Sorrell CC (2017) Photocatalytic materials and technologies for air purification. J Hazard Mater 325:340–366. https://doi.org/10.1016/j.jhazmat.2016.08.072
Boweing N, Walker GS, Harrison PG (2006) Photocatalytic decomposition and reduction reactions of nitric oxide over Degussa P25. Appl Catal B Environ 62:208–2016
Lasek J, Yu Y, Wu JCS (2013) Removal of NOx by photocatalytic processes. J Photochem Photobiol C Photochem Rev 14:29–52. https://doi.org/10.1016/j.jphotochemrev.2012.08.002
Poon CS, Cheung E (2007) NO removal efficiency of photocatalytic paving blocks prepared with recycled materials. Constr Build Mater 21:1746–1753
Dylla H, Hassan MM, Mohammad LN, Rupnow T, Wright E (2010) Evaluation of environmental effectiveness of titanium dioxide photocatalyst coating for concrete pavement. Trans Res Record 2164:46–51
Asadi S, Hassan MM, Kevern JT, Rupnow TD (2012) Development of photocatalytic pervious concrete pavement for air and storm water improvements. Trans Res Record 2290(1):161–167
Janus M, Mądraszewski S, Zając K, Kusiak-Nejman (2013) A new preparation method of cement with photocatalytic activity. Materials 40–50
Russell HS, Frederickson LB, Hertel O, Ellermann T, Jensen SS (2021) A review of photocatalytic materials for urban NOx remediation. Catalysts 11:675
Ballari MM, Hunger M, Hüsken G, Brouwers HJH (2010) NOx photocatalytic degradation employing concrete pavement containing titanium dioxide. Appl Catal B 95:245–254
Hassan M, Mohammad LN, Asadi S, Dylla H, Cooper S (2013) Sustainable photocatalytic asphalt pavements for mitigation of nitrogen oxide and sulfur dioxide vehicle emissions. J Mater Civ Eng ASCE. https://doi.org/10.1061/(ASCE)MT.1943-5533.0000613
Ballari MM, Brouwers HJH (2013) Full scale demonstration of air-purifying pavement. J Hazard Mater 254:406–414. https://doi.org/10.1016/j.jhazmat.2013.02.012
Dylla H, Hassan MM, Osborn D (2012) Field evaluation of ability of photocatalytic concrete pavements to remove nitrogen oxides. Trans Res Record J Trans Res Board 2290:154–160
De Melo JVS, Triches G, Gleize PJP, Villena J (2012) Development and evaluation of the efficiency of photocatalytic pavement blocks in the laboratory and after one year in the field. Constr Build Mater 37:310–319. https://doi.org/10.1016/j.conbuildmat.2012.07.073
Boonen E, Beeldens A (2013) Photocatalytic roads: from lab tests to real scale applications. Eur Trans Res Rev 5:79–89. https://doi.org/10.1007/s12544-012-0085-6
Boonen E, Beeldens A (2013) Photocatalytic roads: from lab tests to real scale applications. Eur Trans Res Rev 5(2):79–89
Witkowski H, Jackiewicz-Rek W, Chilmon K, Jarosławski J, Tryfon-Bojarska A, Gąsiński A (2019) Air purification performance of photocatalytic concrete paving blocks after seven years of service. Appl Sci 9:1735
Dong P, Xi X, Hou G (2015) Typical non–TiO2-based visible-light photocatalysts. Semicond Photocatal Mater Mech Appl. https://doi.org/10.5772/62889
de Oliveira C, Viana MM, Amaral MCS (2020) Coupling photocatalytic degradation using a green TiO2 catalyst to membrane bioreactor for petroleum refinery wastewater reclamation. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2019.101093
Khlyustova A, Sirotkin N, Kusova T, Kraev A, Titov V, Agafonov A (2020) Doped TiO2: the effect of doping elements on photocatalytic activity. Mater Adv 2020(1):1193–1201. https://doi.org/10.1039/D0MA00171F
Tryba B, Piszcz M, Morawski AW (2010) Photocatalytic and self-cleaning properties of Ag-doped TiO2. Open Mater Sci J 4:5–8
Seo S, Kim B (2020) Effect of Cu, Cr, S doped TiO2 for transparent plastic bar reinforced concrete. Appl Sci. https://doi.org/10.3390/app10207334
Khannyra S, Mosquera MJ, Addou M, Gil MLA (2021) Cu-TiO2/SiO2 photocatalysts for concrete-based building materials: Self-cleaning and air de-pollution performance. Constr Build Mater. https://doi.org/10.1016/j.conbuildmat.2021.125419
Ward MH, Jones RR, Brender JD, de Kok TM, Weyer PJ, Nolan BT, Villanueva CM, van Breda SG (2018) Drinking water nitrate and human health: an updated review. Int J Environ Res Public Health 15(7):1557. https://doi.org/10.3390/ijerph15071557.PMID:30041450;PMCID:PMC6068531
Relinque EJ, Grande M, Duran T, Castillo A, Castellote M (2020) Environmental impact of nano-functionalized construction materials: leaching of titanium and nitrates from photocatalytic pavements under outdoor conditions. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.140817
Bogutyn S, Arboleda C, Bordelon A, Tikalsky P (2015) Rejuvenation techniques for mortar containing photocatalytic TiO2 material. Constr Build Mater 96:96–101. https://doi.org/10.1016/j.conbuildmat.2015.07.192
Jain S, Khare A (2008) Urban air quality in mega cities: a case study of Delhi City using vulnerability analysis. Environ Monitor Assess 136:257–265. https://doi.org/10.1007/s10661-007-9681-7
Jadoon S, Nawazish S, Mahmood Q, Rafique A, Sohail S, Zaidi A (2022) Exploring health impacts of occupational exposure to carbon monoxide in the labour community of hattar industrial estate. Atmosphere 13(3):406. https://doi.org/10.3390/atmos13030406
Rall DP (1974) Review of the health effects of sulfur oxides. Environ Health Perspect 8:97–121. https://doi.org/10.1289/ehp.74897
Tong R, Liu J, Wang W, Fang Y (2020) Health effects of PM2.5 emissions from on-road vehicles during weekdays and weekends in Beijing, China. Atmos Environ. https://doi.org/10.1016/j.atmosenv.2019.117258
Coronado DR, Gattorno GR, Pesqueira ME, Cab C, de Coss R, Oskam G (2008) Phase-pure TiO2 nanoparticles: anatase, brookite and rutile. Nanotechnology. https://doi.org/10.1088/0957-4484/19/14/145605
Kamarulzaman N, Kasim MF, Rusdi R (2015) Band gap narrowing and widening of ZnO nanostructures and doped materials. Nanoscale Res Lett. https://doi.org/10.1186/s11671-015-1034-9
D’Amico P, Calzolari A, Ruini A (2017) New energy with ZnS: novel applications for a standard transparent compound. Sci Rep. https://doi.org/10.1038/s41598-017-17156-w
Xu Y, Gao S (2012) Band gap of C3N4 in the GW approximation. Int J Hydrogen Energy 37(15):11072–11080. https://doi.org/10.1016/j.ijhydene.2012.04.138
Sahu T, Ghosh B, Pradhan SK, Ganguly T (2012) Diverse role of silicon carbide in the domain of nanomaterials. Int J Electrochem. https://doi.org/10.1155/2012/271285
Borrero PP, Sato F, Medina AN, Baesso ML, Bento AC (2010) Optical band-gap determination of nanostructured WO3 film. Appl Phys Lett. https://doi.org/10.1063/1.3313945
Liu JJ, Fu XL, Chen SF, Zhu YF (2011) Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method. Appl Phys Lett. https://doi.org/10.1063/1.3660319
Nasir SNS, Mohammed NA, Tukimon MA, Noh M, Arzaee NA, Teridi M (2021) Direct extrapolation techniques on the energy band diagram of BiVO4 thin films. Phys B Condens Matter. https://doi.org/10.1016/j.physb.2020.412719
Ansari SP, Fawad A, Khan A, Cancar HD (2021) Carbon polymer hybrid supported nanomaterials for hydrogen production and storage application, Chapter-8. In: Nanomaterials for hydrogen storage applications, pp 133–152, https://doi.org/10.1016/B978-0-12-819476-8.00012-8
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest among the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chouhan, J., Chandrappa, A.K. A systematic review on photocatalytic concrete for pavement applications: an innovative solution to reduce air pollution. Innov. Infrastruct. Solut. 8, 90 (2023). https://doi.org/10.1007/s41062-023-01060-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41062-023-01060-6