Abstract
Enantioselective synthesis through photocatalysis is one of the highly preferred approaches towards preparation of optically active compounds. This review elaborates and critically analyzes the different strategies of photocatalytic enantioselective reactions through H-bonding, transition metal catalysis, phase-transfer catalysis (PTC), chiral Lewis acid catalysis, N-heterocyclic carbene catalysis, and amine catalysis, and also explores ion pairs. In addition, it explains the different catalysis modes with multifunctional approaches for enantioselective photocatalytic reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Enantioselective synthesis is the most interesting and widely studied area in modern synthetic organic chemistry, and it is also known as chiral synthesis and asymmetric synthesis. Asymmetric synthesis is preparation of an optically active product from an optically inactive substrate with or without the use of an enantiomer. Enantioselective synthesis became the main focus for organic synthetic chemistry after Sharpless, Knowles, and Noyori were honored with the Nobel Prize in chemistry for their innovative work in the field of asymmetric synthesis in 2001 [1,2,3]. Numerous methods have been reported for the synthesis of chiral compounds by enantioselective catalytic transformations. Enantioselective reaction is mostly attained using a chiral substrate, reagent, catalyst, and solvent. Synthesis of new chiral ligands has become important for researchers in synthetic organic chemistry [4, 5] due to similarity in their chemical and physical properties, except when they interact with chiral systems [6]. Van 't Hoff and Joseph Le Bel defined chirality by considering the chemical bonds in the tetrahedral asymmetric carbon atom, which has become known as the Le Bel–van 't Hoff rule [7]. In 1952, Kenyon and Ross (Scheme 1) reported a new reaction mechanism for Marckwald asymmetric synthesis. Brucine (an alkaloid natural product)-based catalysis was reported for enantioselective decarboxylation of 2-ethyl-2methylmalonic acid, and it was observed that a dialkaloidal salt of a disubstituted malonic acid produces an optically active product during decarboxylation. These kinds of partial asymmetric synthesis, when an asymmetric transition during crystallization is not possible, have been carried out [8].
In 1951, Johannes Bijvoet reported a physical method to define the configuration of organic compounds using X-ray crystallography. Dalglish subsequently introduced chiral chromatography, in which paper chromatography is used to separate the chiral amino acids [9]. In the early years, enantioselective synthesis was very slow due to limited sources for analysis, but nowadays it is commonly reported with a catalytic route due to the less stringent reaction conditions required and is also widely used in the chemical and pharmaceutical industries [10]. On the other hand, photocatalysis plays an important role in modern catalysis due to its low energy requirement, sustainability, broad applications, and environmentally friendly nature. Any photocatalytic reaction is able to decrease the barrier of activation energy in conventional fashion compared to thermal reaction (Fig. 1).
The use of photocatalysis in organic synthesis was first described in 1978 [11], but promising concepts have been more recently reported by Yoon [12], MacMillan [13], and Stephenson [14]. Both MacMillan [13] and Bach [15] reported innovative methods for enantioselective reaction using a photocatalytic pathway. Ciamician and his colleagues reported many kinds of photochemical reactions including photoreduction, photo-isomerization, photo-pinacolization, photo-cleavage, and photo-cycloaddition (Scheme 1) [16]. In 1908 he reported an example of intramolecular [2+2] photo-cycloaddition (Scheme 2), after which he reported many reactions which occurred in the presence of light [17]. Photocatalysts have also been reported for organic transformations such as C–C coupling reaction with splitting of alcohol into hydrogen [18] and oxidation of aromatic alcohols over hydrogen evolution [19, 20]. Comparative compilations have been reported to explain the wide role of photocatalysis in organic transformations as well [21,22,23,24].
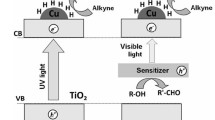
Photocatalysis is defined as when energetic photons have a role in the activation of catalysts [25]; it is a term that describes the use of light energy to generate chemical reactions. Metal surfaces such as TiO2, Fe2O3, CdS, and ZnO absorb radiation from sunlight, and electrons of the valence band migrate to the conduction band, which creates negative (e−) and positive (h+) hole pairs in the valence band (Scheme 3). The photo-generated hole pairs take part in oxidation and reduction reactions. Excitation of electrons completely depends on the wavelength of the light source [26].
2 Chiral photocatalyst
A reaction does not accelerate by itself with a photocatalyst, but in an excitation step, it binds with a substrate and produces intermediates. Due to the high activation energy and reactivity of intermediates in photocatalytic enantioselective reaction, it becomes difficult to achieve enantioenriched products. In chiral photocatalytic reaction, stereo-control is a challenging task [27, 28] compared to traditional asymmetric catalysis such as metal-based catalysis [29, 30], organocatalysis [31,32,33], and enzyme catalysis [34, 35]. Currently, two strategies are available to explain the photocatalytic pathway for enantioselective reaction. The first is dual catalysis involving two catalyst systems, and the second utilizes a bifunctional catalyst. In dual catalysis, a chiral photocatalyst introduces a chiral catalyst with ground-state activation for enantioselective bond formation and non-excited activation of substrates. Many catalysts are reported as dual catalysts for enantioselective photocatalytic reaction, such as enamine catalysis [13, 36], Brønsted acids [37, 38], transition metals [39], Lewis acids [40], N-heterocyclic carbenes [41], enzymes [42], and the activation of α,β-unsaturated carbonyl compound [43]. On the other hand, a single catalyst is used for excitation and stereoselective control in bifunctional catalysis. Figure 2 shows the historical timeline of photocatalytic enantioselective reaction from 2008 to 2017.
3 Enantioselective Photocatalysis
3.1 Hydrogen Bonding
H-bonding catalysis provides an excellent platform for asymmetric synthesis. In H-bond-based enantioselective transformations, the orientation of a substrate with chiral moiety is stereochemically controlled by H-bonds [56]. Cao et al. reported dual asymmetric catalysis by nickel metal and organocatalyst with insertion of sulfur dioxide, which provides three-component asymmetric sulfonylation by C–H functionalization of cycloalkanes, alkanes, toluene derivatives, and ether for preparation of enantioenriched products under mild condition with 95% enantioselectivity. This technique has high advantage in organic synthesis since it uses low-cost materials and is cost-effective (Scheme 4). It is a multicomponent reaction which is followed by α,β-unsaturated carbonyl molecules that act as Michael acceptors [57].
H-bond-based enantioselective catalyst was also reported as a sensitizing receptor for photochemical transformations, in which a bifunctional photocatalyst is used in an intramolecular enone–olefin photo[2+2]-cycloaddition of quinolone. The reactant was completely converted into related product without any loss of material with enantioselectivity up to 22% (Scheme 5) [58].
Alonso et al. synthesized thioxanthone from methyl thiosalicylate, which was used as sensitizer for enantioselective photo[2+2]-cycloaddition reaction by quinolone. The compound was readily converted into related product after 1 h at –25 °C with 95% yields and 94% enantiomeric excess (ee) (Scheme 6) [59].
Chiral triplet sensitizer was also reported for the intermolecular reaction of pyridines and acetylene dicarboxylate derivatives followed by [2+2] cycloaddition reaction, which provided 88% yield of related compounds with 92% ee [60]. This transformation occurred via non-covalent interactions (H-bonding) between the substrate and chiral catalyst under photochemical conditions (Scheme 7).
Furthermore, photo [2+2] cycloaddition of quinolones involving H-bonding at λ = 419 nm was also reported in which chiral thioxanthone catalyst (10 mol%) was required with electron-deficient olefins. High enantiomeric excess of related compounds with region- and diastereoselectivity (94% yield and 95% ee) was observed (Scheme 8) [61].
Furthermore, H-bonding-based enantioselective application was reported by Hölzl-Hobmeier et al. for catalytic deracemization of chiral allenes. Recently, Bach revealed an extraordinary photochemical transformation by an enantioselective catalyst with visible light which showed photochemical deracemization of chiral compounds, providing good enantioselectivity (98–97% ee) of the related compound using a chiral sensitizer at room temperature (Scheme 9) [62].
Wimberger et al. reported the photochemical deracemization of chiral sulfoxides by xanthone sensitizer using H-bonding. The racemic mixture of chiral benzothiazinone-1-oxides was completely converted into single enantiomer without loss of material. Reactant was fully deracemized with excellent yield and 29% enantioselectivity (Scheme 10) [63].
H-bonding was also introduced in [2+2] intramolecular photo-cycloaddition of 4-alkenyl-substituted coumarins, which was catalyzed by atropisomeric thiourea-based organo-photocatalyst, and it required lower energies than the substrate to be excited and converted into the related enantioenriched product. Binaphthyl-based thiourea compounds are good absorbers of light, and due to this, they can promote photochemical reactions with high enantiomer excess of related compound. 2-Amino-2′-hydroxy-1-1′-bi-naphthalene has been used as organocatalyst in the asymmetric photo-cycloaddition of substituted coumarins to obtain the desired product with high enantioselectivity (Scheme 11) [64].
Skubi et al. reported a highly efficient photocatalyst that attracts prochiral quinolones via hydrogen bonding and π–π interactions, and also described a photocatalyst iridium sensitizer with bifunctional chiral H-bonding. The intramolecular photo-cycloaddition of 3-alkoxyquinolone was optimized to demonstrate the efficiency of this photocatalytic system at −70 C, providing 91% ee of the desired product (Scheme 12) [65].
Liu et al. carried out an asymmetric organocatalytic reaction using benzofuran-2(3H)-ones and naphthoquinones. l-Leucine-derived tertiary amine-thiourea and squared amide-based catalyst was reported for the arylation of benzofuranones with high yield and enantioselectivity by asymmetric H-bond-based photocatalysis and provided the desired product in 94% yield with 98% ee (Scheme 13) [66].
Shin et al. demonstrated the deracemization approach in which amine derivatives undergo spontaneous reaction in the presence of visible light via the tri-catalytic method based on phosphate using a redox process and producing an equilibrium product distribution across substrate enantiomers. Desired products were obtained with 99% yield and 95% ee (Scheme14) [67].
3.1.1 Transition Metal Catalysis
Transition metal catalysts have become an important part of fundamental research in organic synthesis. Knowles et al. were awarded the Nobel Prize in 2001 for stereoselective catalysis [68]. The combination of photocatalyst with transition metal complexes has gained attention due to its novelty in organic synthetic chemistry.
Nickel is the most important and widely used metal in asymmetric and cross-coupling reactions because it has single-electron redox potentials and easily undergoes β-hydride elimination. Molander et al. reported a photoredox catalyst with nickel metal which was used for organocarbon cross-coupling reaction between benzyl potassium trifluoroborate salt as radical precursor and methyl 3-bromobenzoate under mild reaction conditions in the presence of 4,4-di-tert-butyl-2,2′-bipyridine (dtbbpy), and observed 52% yield with 50% ee of desired product (Scheme 15) [69]. In this reaction, lutidine was used as base, while in other nickel-based photocatalytic reactions, Cs2CO3 was used as base and chiral catalyst prepared by the combination of nickel and photoredox catalyst for enantioselective decarboxylative arylation of α-amino acids in mild reaction conditions (Scheme 16) [70]. In this reaction, N-Boc-leucine was used to obtain benzylic amines with 84% yield and 92% ee.
The combination of nickel and photoredox catalyst was also reported for the enantioselective desymmetrization of cyclic meso-anhydrides with high enantioselectivity. Benzyl trifluoroborate salts as a radical source, carboxylic anhydride as a substrate, (S,S)-PhBox as chiral ligand, and Ni(COD)2 and the organophotocatalyst 4-CzIPN were used in reaction and provided the final product with 85% yield (Scheme 17) [71] with the involved mechanism of decarboxylation and Ni–C bond hemolysis of a Ni (II) adduct. This is a smooth production of desymmetrized anhydride on a large scale.
Fan et al. expanded the previously reported system by using TBADT chiral nickel-PHOX complex to synthesize oxindoles and their derivatives. The reaction between aryl carbamoyl and aldehydes proceeded under mild conditions for 9 h to obtain the desired product (Scheme 18) [72].
Recently, Qandil and Guan used a similar type of combination of photoredox and nickel for an asymmetric cross-coupling reaction to obtain α-aryl ester with 90% enantioselectivity and 94% yield with the racemic α-chloro ester and aryl iodides. Aryl esters are mostly used in anti-inflammatory drugs [73] (Scheme 19) [74].
In 2017, Lin and Liu reported a hybridized photocatalyst with copper catalysts to convert carboxylic acid into enantioenriched alkyl nitriles. Enantioselective decarboxylative cyanation reaction was reported with the combination of N-hydroxyphthalimide (NHP) esters and trimethylsilyl cyanide (TMSCN) to give chiral alkyl nitriles with 88% yield under irradiation of a 12 W blue light-emitting diode (LED), 10% CuBr, and 12 mol% relative ligand in DFM at room temperature using 2 mol% Ir(ppy)3 as photocatalyst (Scheme 20) [75]. Bioactive compounds are also synthesized with this protocol.
Lu and Xiao reported a palladium catalyst for enantioselective asymmetric [4+2] cycloaddition of diazoketones by reactive ketenes intermediate with vinyl benzoxazinanones to give heterocyclic compounds under mild reaction conditions in the presence of visible light, with high product yield and selectivity (Scheme 21) [76].
Inspired by the work of Hong-Hao et al. introduced chiral ligands which enhanced the enantioselectivity and yields of the related product. Palladium was used as co-catalyst with photoredox for the enantioselective monosubstituted allylic alkylation reaction with 4-alkyl-1,4-dihydropyridine in the presence of visible light with high enantioselectivity. By this reaction, photo-generated free radicals can be converted into a symmetric allyl alkylation (AAA) that directly uses hard nucleophiles as substitutes (Scheme 22) [77].
Harunobu et al. combined organo-photoredox with chiral chromium to form a hybrid catalyst for asymmetric allylation reaction of carbonyl compounds with inactivated hydrocarbon alkene to produce homoallylic alcohols with a diastereomeric ratio of > 20:1 in the presence of visible light at room temperature. Mg(ClO4)2 was also added to enhance both reactivity and enantioselectivity (Scheme 23) [78].
Similarly, Montgomery and Martin reported the reaction of carbon/hydrogen arylation and alkylation of benzamides using a dual catalytic system under mild reaction conditions (Scheme 24) [79]. iPrBiOx was used as ligand in the reaction to obtain the product at high levels of enantioselectivity.
3.1.2 Enzyme Catalysis
In previous years, biocatalysis has attracted attention in synthetic chemistry due to nonhazardous to the environment. Litman et al. combined photocatalysis and enzymatic catalysis to develop a method for asymmetric reaction of alkenes under photocatalytic conditions to form amino ester in 90% yield with 98% ee (Scheme 25) [42].
Subsequently, Hyster and Cooper’s group reported photoredox catalysis with enzyme catalysis. It was observed that when acetoxyteralone was added to a solution of Nicotiana tabacum (NtDBR) as a model enzyme, nicotinamide adenine dinucleotide phosphate (NADPH) NADP+ and rose bengal under the given conditions, the desired product was obtained m-methoxy-substituted ketone in low yield (Scheme 26) [80].
In 2020, Guan's group combined electrochemistry and organocatalysis to synthesize indolin-3-ones from substituted arlindoles with high enantioselectivity (up to 90% ee) (Scheme 27) [81]. It is also observed that without l-proline and electrical current, the reaction was completed successfully.
Schmidt and Betori reported the catalysis of C–H benzylic hydroxylation with a combination of photo-redox and enzyme catalyst (ketoreductases, KREDs) with excellent yield and good enantioselectivly (Scheme 28) [82].
Emmanuel et al. demonstrated enantioselective radical dehalogenation of lactones using nicotinamide-based enzyme keto-reductase (has a large active site) under irradiation with visible light with excellent enantioselectivity. NADH and NADPH were used as a source of hydrogen atoms. Reaction with Ralstonia species (RasADH) and reaction with Lactobacillus kefiri (LKADH) were reported (Scheme 29) [83].
Similarly, Cooper's group illustrated the photoexcitation of flavoenzymes for cyclization of α-chloroamides using nicotinamide adenine dinucleotide phosphate (NADP+) and glucose dehydrogenase (GDH-105) for good reactivity with a cyan LED. Ene-reductase (flavin-dependent) shows good catalytic activity (Scheme 30) [84].
In 2018, Wenger and Ward reported an enantioselective synthesis of amines from reduction of cyclic imines by photocatalyst enzyme in excellent yield. Na3[Ir(ppy)3] as a water-soluble iridium photocatalyst and ascorbic acid were used in the synthesis (Scheme 31) [85].
In 2022, reduction of ketones using chemo- and enantioselective photoenzymatic processes via BaNTR1 as a flavin-dependent nitroreductase, an oxidoreductases, was reported by Poelarends et al. BaNTR1 was composed of an enzyme from Bacillus amyloliquefaciens. Reduction of ketone was successful with high yield and excellent enantioselectivity using [Ru(bpy)3]Cl2 and bmGDH with blue LEDs under aerobic conditions (Scheme 32) [86].
3.1.3 Phase-Transfer Catalysis (PTC)
PTC is widely used in asymmetric reactions, where PTC is combined with photocatalysis to obtain chiral molecules. In 2012, Gao and Meng’s group reported the first example of PTC combined with photocatalysis to synthesize the β-keto ester by PTC using molecular oxygen, achieving high yield with good enantioselectivity. Between the reaction, K2HPO4 is also added to maximize the yield of the obtained product (Scheme 33) [87].
Similarly, another phase transfer catalyst was reported for perfluoroalkylation of β-ketoesters by Melchiorre et al. (Scheme 34) [88].
3.1.4 Lewis Acid Catalysis
This type of catalysis helps in controlling the stereochemistry in organic reactions. Yoon’s group combined transition metal photoredox catalysis with Lewis acid catalysis for the reaction between α-silymethyl aniline and crotonyl oxazolidinone under visible light with mild conditions (Scheme 35) [89].
Blum et al. in 2016 described two catalysts, Sc-PyBox complex and tris(bipyridyl) ruthenium photosensitizer, for an asymmetric [2+2] photocycloaddition reaction of 2-hydroxy chalcones with 90% yields (Scheme 36) [90].
In 2016, Huang et al. [91] combined a series of chiral-at-metal rhodium-based complexes for asymmetric reaction of azides and ethyl diazo compounds to obtain an alkylation product with high yield under photoredox conditions (Scheme 37) [92].
Later in 2019, Sheng Wang's group used the combination of a rhodium metal photocatalyst with chiral Lewis acid to catalyze the coupling reaction between substituted acyl imidazoles and γ-ketoamides (fluorine-substituted) and obtained γ-keto acid derivatives in high yield and 97% ee (Scheme 38) [93].
Lu and Xiao's group reported enantioselective asymmetric fluoro-alkylation reactions of β-ketoesters by a dual-based catalyst with 67% yield. NiBr2 glyme was used to improve the yield of desired product. During the reaction, it was observed that when ethyl di-fluoroacetate was added, the yield and enantioselectivity were increased (Scheme 39) [94].
Chen et al. in 2018 reported the enantioselective reaction of vicinal amino alcohol via catalysis of rhodium metal photocatalyst in the presence of N,N-dioxide ligands under mild reaction conditions and simple procedure with high yield (Scheme 40) [40].
Yoon's group combined the two catalysts Ru(bpy)32+ for enantioselective synthesis of 1,2-cis and 1,2-trans isomers using chiral ligand L and Eu(OTf)3 complex in good yield with high ee under visible light. The reaction was conducted for 24 h (Scheme 41) [95].
Meggers and Huo also catalyzed alkyl radicals by enantioselective addition reaction using bis-cyclometalated rhodium under mild reaction conditions (Scheme 42) [96].
Similarly, Hui et al. gave an example of this type of asymmetric photocatalytic reaction method for fluorine containing keto acids using a dual catalytic strategy with excellent stereoselectivity. Organic compounds containing fluorine are widely used in the pharmaceutical industry (Scheme 43) [97].
Wu and his group also used a dual catalysis system by combining the neutral eosin Y with rhodium metal complex to synthesize substituted dimethylpyrazoles with high yield from aldehydes used as acyl radicals under photocatalytic conditions (Scheme 44) [98].
Recently, Uchikura et al. reported an asymmetric radical addition using chiral phosphoric acid-imine complex as photoredox catalyst. Reaction occurred between N-3,4,5-trimethoxyphenyl (TMP) and benzothiazolines (radical precursors) in the presence of photoredox catalysis and mesitylene solvent under photo-irradiation conditions. MS4A is also used to increase the enantioselectivity to up to 90% ee of the desired product (Scheme 45) [99].
3.1.5 N-Heterocyclic Carbene Catalysis (NHC)
In 2012, Rovis and DiRocoo reported the first NHC catalysis with photoredox catalysts to α-acylation asymmetric catalysis of tertiary amines. To obtain α-amino ketone, butanal was added to N-phenyl tetrahydroisoquinoline, and to improve the yield of the obtained product, m-dinitrobenzene (m-DNB) was added using rhodium catalyst and chiral NHC under mild reaction conditions with good yield (Scheme 46) [41].
NHC was also introduced in an asymmetric Stetter reaction. The Stetter reaction is an efficient method for asymmetric formation of C–C bonds. Spiro-cyclic furanone-containing natural product was synthesized under UV irradiation in the presence of triazolium salt and sodium acetate with 80% yield and 99% ee (Scheme 47) [100]. In this reaction, amino-indanol and pyrrolidine-derived trizolium NHC was used.
3.1.6 Amine Catalysis
In recent years, the success in using chiral amines in organocatalysis has grown rapidly. Enamine and iminium catalysis are two types of amine catalysis. Enamine catalysis has the advantage of great enantiocontrol, which is obtained using chiral amines as catalysts.
In 2008, Nicewicz and MacMillan demonstrated the enamine catalytic reaction of organocatalysis and photoredox catalysis to catalyze the enantioselective asymmetric alkylation reaction of aldehyde [13] using ruthenium metal complex as the photocatalyst and chiral imidazolidinone as the organocatalyst, where several aldehydes reacted with an alkyl bromide (Scheme 48).
Tung and his group used the triple catalytic method for enantioselective cross-dehydrogenative coupling (CDC) of tertiary amines to obtain ketones (Scheme 49) [101]. It was observed that when m-NO2C6H4COOH was added to the reaction, the yield of aniline was also increased.
Yunbo et al. catalyzed the asymmetric α-photoalkylation reaction of β-ketocarbonyl compounds using amine catalyst. By combining photoredox catalysis and primary amine catalysis, they reported the production of all carbon quaternary stereo-centers under thermal conditions [102] (Scheme 50).
Similarly, Miquel and his group reported another example of CDC reaction between aldehyde and xanthenes using organocatalyst and [Ru(bpy)3]PF3 in the presence of visible light under mild reaction conditions, resulting in high yield with high enantioselectivity [103] (Scheme 51).
In addition, Hou et al. catalyzed the asymmetric reaction of tetrahydroisoquinolines with cyclic ketones by combining the chiral amine and iridium photocatalyst, resulting in good yield and diastereoselectivity [104] (Scheme 52).
Another methodology was also introduced by Elena et al. to achieve similar transformation under photolytic conditions [105] (Scheme 53). Enamine intermediate forms an electron donor–acceptor (EDA) complex by using an alkylating agent.
3.1.7 Ion Pair
Daisuke et al. catalyzed the asymmetric coupling reaction of N-arylaminomethanes with N-sulfonyl aldimines using [Ir(ppy)2(L)]BArF in toluene under visible light in an argon atmosphere. Desired product was obtained with 90% yield and 95% enantioselectivity (Scheme 54) [37]. This protocol may be control the bond forming process of reactive radical intermediates.
Yang et al. reported the dual catalysis strategy for anti-Markovnikov hydroetherification of alkenols (Scheme 55) [106]. They also reported a comparative study with parental Fukuzumi catalyst, but satisfactory results were not obtained. Hence, the ion pair approach was found to be more reasonable to achieve enantioselective control of cation transformations of simple alkenes under photochemical conditions.
Gentry et al. synthesized alkoxyamine-substituted pyrroloindolines with high enantioselectivity. TEMPO was used to speed up the reaction procedure (Scheme 56) [107]. Iridium metal based photoredox and TRIP phosphate ions were used for synthesis of pyrroloinolines.
Morse et al. described the development of a photoredox catalyst system containing an oxidizing pyrylium salt with a chiral N-triflyl phosphor amide anion and enantioselective radical cation is created. Diels–Alder reaction performed intramolecular with alkenes, cyclopentadiene and desired product was obtained with yield 72% yield and good enantioslectivity (Scheme 57) [108].
4 Conclusion
Over the last 15 years, enantioselective photocatalysis has seen rapid growth in organic synthetic chemistry and its applications. We have reviewed the recent research papers reporting various catalytic methods and the use of different sources of visible light in enantioselective symmetric reactions. Different catalysts including hydrogen bonding, ion pairs, chiral transition metals, chiral amines, NHC, and enzymes were reviewed. These types of catalysts provide an excellent environment for visible light processes.
References
Noyori R (2002) Asymmetric catalysis: science and opportunities (Nobel lecture). Angew Chem Int Ed 41:2008–2022
Blaser HU, Pugin B, Spindler F (2012) Asymmetric hydrogenation. Top Organomet Chem 42:65–102
Sharpless KB (2002) Searching for new reactivity (Nobel Lecture). Angew Chem Int Ed 41:2024
Krendlinger E, Heinrichs FL (2001) Performance enhancers. Polym Paint Colour J 190:22–24
Yoon TP, Jacobsen EN (2003) Privileged chiral catalysts. Science 299:1691–1693
Smith SW (2009) Chiral toxicology: it’s the same thing only different. Toxicol Sci 110:4–30
Grossman RB (1989) Van’t Hoff, Le Bel, and the development of stereochemistry: a reassessment. J Chem Educ 66:30–33
Kenyon J, Ross WA (1952) A new reaction mechanism for the Marckwald asymmetric synthesis. J Chem Soc (Resumed) 20:2292–2299
Resolution TO (1952) Dalgliesh: the optical resoldion of 756. Opt Resol 20:3940–3942
Trost BM (2004) Asymmetric catalysis: an enabling science. Proc Natl Acad Sci USA 101:5348–5355
Hedstrand DM, Kruizinga WH, Kellogg RM (1978) Light induced and dye accelerated reductions of phenacyl onium salts by 1,4-dihydropyridines. Tetrahedron Lett 19:1255–1258
Ischay MA, Anzovino ME, Du J, Yoon TP (2008) Efficient visible light photocatalysis of [2+2] enone cycloadditions. J Am Chem Soc 130:12886–12887
Nicewicz DA, Macmillan DWC (2014) Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 77:5898
Narayanam JMR, Tucker JW, Stephenson CRJ (2009) Electron-transfer photoredox catalysis: development of a tin-free reductive dehalogenation reaction. J Am Chem Soc 131:8756–8757
Grimme S, Bach T, Bauer A, Westka F (2005) Catalytic enantioselective reactions driven by photoinduced electron transfer. Nature 436:1139–1140
Albini A, Fagnoni M (2008) 1908: Giacomo Ciamician and the concept of green chemistry. Chem Eur 20:63–66
Albini A, Dichiarante V (2009) The “belle epoque” of photochemistry. Photochem Photobiol Sci 20:248–254
Yu JL, Yu MQ, Jun YX (2022) Efficient splitting of alcohols into hydrogen and C-C coupled products over ultrathin Ni-doped ZnIn2S4 nanosheet photocatalyst. Chin J Catal 43(4):1084–1091
Yu-Lan Wu, Qi M-Y, Tan C-L, Tang Z-R, Yi-Jun Xu (2022) Photocatalytic selective oxidation of aromatic alcohols coupled with hydrogen evolution over CdS/WO3 composites. Chin J Catal 43(7):1851–1859
Li YH, Qi MY, Tang ZR, Xu YJ (2022) Coupling organic synthesis and hydrogen evolution over composite WO3/ZnIn2S4 Z-scheme photocatalyst. J Phys Chem C 126(4):1872–1880
Li JY, Li YH, Qi MY, Lin Q, Tang ZR (2022) Selective organic transformations over cadmium sulfide-based photocatalyst. ACS Catal 10(11):6262–6280
Yang MQ, Xu YJ (2013) Selective photoredox using graphene based composite photocatalyst. Phys Chem Chem Phys 15:19102–19118
Qi MY, Conte M, Anpo M, Tang ZR, Xu YJ (2021) Cooperative coupling of oxidative organic synthesis and hydrogen production over semiconductor-based photocatalyst. Chem Rev 121(21):13051–13085
Zhang N, Zhang Y, Yang MQ, Xu YJ (2013) Progress on graphene-based composite photocatalyst for selective organic synthesis. Curr Org Chem 17(21):2503–2515
Hashimoto K, Irie H, Fujishima A (2005) TiO2 photocatalysis: a historical overview and future prospects. Jpn J Appl Phys 44:8269–8285
Ahmed S, Rasul MG, Martens WN (2010) Heterogeneous photocatalytic degradation of phenols in wastewater: a review on current status and developments. Desalination 261:3–18
Brimioulle R, Lenhart D, Maturi MM, Bach T (2015) Enantioselective catalysis of photochemical reactions. Angew Chem Int Ed 54:3872–3890
Wende RC, Schreiner PR (2012) Evolution of asymmetric organocatalysis: multi- and retrocatalysis. Green Chem 14:1821–1849
Fairlamb IJS (2005) Transition metals in organic synthesis. ChemInform 36:113–148
Mazet C (2012) Privileged chiral ligands and catalysts. Angew Chem Int Ed 51:305–305
Evans PA (2005) Ruthenium in organic synthesis transition metals for organic synthesis metal-catalyzed cross-coupling reactions modern aldol reactions. Willey, New York, p 56
Dondoni A, Massi A (2008) Asymmetric organocatalysis: from infancy to adolescence. Angew Chem Int Ed 47:4638–4660
List B (2007) Introduction: organocatalysis. Chem Rev 107:5413–5415
Liang J, Mundorff E, Voladri R, Jenne S, Gilson L, Conway A, Krebber A, Wong J, Huisman G, Truesdell S, Lalonde J (2010) Highly enantioselective reduction of a small heterocyclic ketone: biocatalytic reduction of tetrahydrothiophene-3-one to the corresponding (R)-alcohol. Org Process Res Dev 14:188–192
Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K (2012) Engineering the third wave of biocatalysis. Nature 485:185–194
Silvi M, Melchiorre P (2018) Enhancing the potential of enantioselective organocatalysis with light. Nature 554:41–49
Uraguchi D, Kinoshita N, Kizu T, Ooi T (2015) Synergistic catalysis of ionic Brønsted acid and photosensitizer for a redox neutral asymmetric α-coupling of N-arylaminomethanes with aldimines. J Am Chem Soc 137:13768–13771
Li J, Kong M, Qiao B, Lee R, Zhao X, Jiang Z (2018) Formal enantioconvergent substitution of alkyl halides via catalytic asymmetric photoredox radical coupling. Nat Commun 9:1–9
Gutierrez O, Tellis JC, Primer DN, Molander GA, Kozlowski MC (2015) Nickel-catalyzed cross-coupling of photoredox-generated radicals: uncovering a general manifold for stereoconvergence in nickel-catalyzed cross-couplings. J Am Chem Soc 137:4896–4899
Ye CX, Melcamu YY, Li HH, Cheng JT, Zhang TT, Ruan YP, Zheng X, Lu X, Huang PQ (2018) Dual catalysis for enantioselective convergent synthesis of enantiopure vicinal amino alcohols. Nat Commun 9:1–9
Dirocco DA, Rovis T (2012) Catalytic asymmetric ±-acylation of tertiary amines mediated by a dual catalysis mode: N-heterocyclic carbene and photoredox catalysis. J Am Chem Soc 134:8094–8097
Litman ZC, Wang Y, Zhao H, Hartwig JF (2018) Cooperative asymmetric reactions combining photocatalysis and enzymatic catalysis. Nature 560:355–359
Brenninger C, Jolliffe JD, Bach T (2018) Chromophore activation of α, β-unsaturated carbonyl compounds and its application to enantioselective photochemical reactions. Angew Chem Int Ed 57:14338–14349
David A, Nicewicz MWC (2008) Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 20:322
David A, Scott ME, MacMillan WC (2009) Enantioselective r-trifluoromethylation of aldehydes via photoredox organocatalysis. J Am Chem Sci 131:10875–10877
Yoon TP, Ischay MA, Du J (2010) Visible light photocatalysis as a greener approach to photochemical synthesis. Nat Chem 2:25
Huong X, Kang MJ, Kim SJ, Park ED, Jang HY (2011) Green organophotocatalysis. TiO2-induced enantioselective a-oxyamination of aldehydes. Catal Sci Technol 1:923–926
Neumann M, Zeitler K (2012) Application of microflow conditions to visible light photoredox catalysis. Org Lett 14(11):2658–2661
Hong BH, Lin CW, Liao WK, Lee GH (2013) Sequential asymmetric catalysis in Michael–Michael–Michael–Aldol reactions: merging organocatalysis with photoredox catalysis in a one-pot enantioselective synthesis of highly functionalized decalines bearing a quaternary carbon stereocenter. Org Lett 20:25
Du J, Skubi KL, Schultz DM, Yoon TP (2014) A dual-catalysis approach to enantioselective [2 + 2] photocycloadditions using visible light. Reports 20:344
Gualandi A, Marchini M, Mengozzi L, Natali M, Lucarini M, Ceroni P, Cozzi PG (2015) Organocatalytic enantioselective alkylation of aldehydes with [Fe(bpy)3]Br 2 catalyst and visible light. ACS Catal 15:25
Amador AG, Sherbrook EM, Yoon TP (2016) Enantioselective photocatalytic [3+2] cycloadditions of aryl cyclopropyl ketones. J Am Chem Soc 5:25
Wang D, Zhu N, Chen P, Lin Z, Liu G (2017) Enantioselective decarboxylative cyanation employing cooperative photoredox catalysis and copper catalysis. J Am Chem Soc 5:25
Ye CX, Melcamu YY, Li HH, Cheng JT, Zhang TT, Ruan YP, Zheng X, Xin Lu, Huang PQ (2018) Dual catalysis for enantioselective convergent synthesis of enantiopure vicinal amino alcohols. Communications 20:25
Han B, Li Y, Ying Yu, Gong L (2019) Photocatalytic enantioselective α-aminoalkylationof acyclic imine derivatives by a chiral copper catalyst. Nat Commun 20:25
Crimmins MT, Pace JM, Nantermet PG, Kim-Meade AS, Thomas JB, Watterson SH, Wagman AS (2000) The total synthesis of (±) -Ginkgolide B. J Am Chem Soc 35:8453–8463
Cao S, Hong W, Ye Z, Gong L (2021) Photocatalytic three-component asymmetric sulfonylation via direct C(sp3)-H functionalization. Nat Commun 20:12
Cauble DF, Lynch V, Krische MJ (2003) Studies on the enantioselective catalysis of photochemically promoted transformations: “ Sensitizing Receptors ” as chiral catalysts exist for which catalytic enantioselective variants do not. J Org Chem 68:15–21
Alonso R, Bach T (2014) A chiral thioxanthone as an organocatalyst for enantioselective [2+2] photocycloaddition reactions induced by visible light. Angew Chem Int Ed 53:4368–4371
Maturi MM, Bach T (2014) Enantioselective catalysis of the intermolecular [2+2] photocycloaddition between 2-pyridones and acetylenedicarboxylates. Angew Chem Int Ed 53:7661–7664
Tröster A, Alonso R, Bauer A, Bach T (2016) Enantioselective intermolecular [2 + 2] photocycloaddition reactions of 2(1H)-quinolones induced by visible light irradiation. J Am Chem Soc 138:7808–7811
Hölzl-Hobmeier A, Bauer A, Silva AV, Stefan HM, Christoph B, Thorsten B (2018) Catalytic deracemization of chiral allenes by sensitized excitation with visible light. Nature 564:240–243
Wimberger L, Kratz T, Bach T (2019) Photochemical deracemization of chiral sulfoxides catalyzed by a hydrogen-bonding xanthone sensitizer. Synthesis (Germany) 51:4417–4424
Vallavoju N, Selvakumar S, Jockusch S (2014) Enantioselective organo-photocatalysis mediated by atropisomeric thiourea derivatives. Angew Chem Int Ed 53:5604–5608
Skubi KL, Kidd JB, Jung H, Ilia GA, Hyun BM, Tehshik PY (2017) Enantioselective excited-state photoreactions controlled by a chiral hydrogen-bonding iridium sensitizer. J Am Chem Soc 139:17186–17192
Liu Y, Li J, Ye X, Jiang Z (2016) Organocatalytic asymmetric formal arylation of benzofuran-2(3: H)-ones with cooperative visible light photocatalysis. Chem Commun 52:13955–13958
Shin NY, Ryss JM, Zhang X, Miller SJ, Knowles RR (2019) Light driven deracemization enabled by excited state electron transfer. Science 366:364–369
Ault A, College C, Vernon M (2002) The Nobel prize in chemistry for 2001 from pure enantiomers of natural occurrence, p 79
Tellis JC, Primer DN, Molander GA (2014) Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science 345:433–436
Zuo Z, Cong H, Li W, Gregory FC, David MWC (2015) Enantioselective decarboxylative arylation of α-amino acids via the merger of photoredox and nickel catalysis. J Am Chem Soc 20:8–11
Stache EE, Rovis T, Doyle AG (2017) Asymmetric synthesis hot paper dual nickel- and photoredox-catalyzed enantioselective desymmetrization of cyclic meso-anhydrides. Communications 56:3679–3683
Fan P, Lan Y, Zhang C, Wang C (2020) Communication nickel/photo-cocatalyzed asymmetric acyl-carbamoylation of alkenes nickel/photo-cocatalyzed asymmetric acyl-carbamoylation of alkenes. J Am Chem Soc 142:2180–2186
Qandil AM (2012) Prodrugs of nonsteroidal anti-inflammatory drugs (NSAIDs), more than meets the eye: a critical review. Int J Mol Sci 13:1744–1774
Guan H, Zhang Q, Walsh PJ, Mao J (2020) Nickel/photoredox-catalyzed asymmetric reductive cross-coupling of racemic a-chloro esters with aryl iodides. Angew Chem Int Ed 57:5172–5177
Wang D, Zhu N, Chen P, Guosheng L (2017) Enantioselective decarboxylative cyanation employing cooperative photoredox catalysis and copper catalysis. J Am Chem Soc 139:15632–15635
Wei Y, Liu J, Chen HW, Lu LQ, Xiao WJ (2017) Sequential visible-light photoactivation and palladium catalysis enabling enantioselective [4 + 2 ] cycloadditions. J Am Chem Soc 139:14707–14713
Zhang H, Zhao J, Yu S (2018) Enantioselective Allylic Alkylation with 4-Alkyl-1,4-dihydro-pyridines Enabled by Photoredox/Palladium Cocatalysis. J Am Chem Soc 140:16914–16919
Harunobu M, Shun T, Hiromu F, Kei O, Motomu K (2019) Catalytic asymmetric allylation of aldehydes with alkenes through allylic C(sp3)-H functionalization mediated by organophotoredox and chiral chromium hybrid catalysis. Chem Sci 10:3459–3465
Rand AW, Yin H, Xu L, Jessica G, Montero M, Ciro RR, John M, Ruben M (2020) A dual catalytic platform for enabling sp3 a C–H arylation & alkylation of benzamides. ACS Catal 10:4671–4676
Biegasiewicz KF, Cooper SJ, Emmanuel MA, Todd HK (2018) Catalytic promiscuity enabled by photoredox catalysis in nicotinamide-dependent oxidoreductases. Nat Chem 10:770–775
Lu FY, Chen YJ, Chen Y, Hong YH (2020) Highly enantioselective electrosynthesis of C2-quaternary indolin-3-ones. Chem Commun 56:623–626
Betori RC, May CM, Scheidt KA (2019) Combined photoredox/enzymatic C−H benzylic hydroxylations. Angew Chem Int Ed 58:16490–16494
Emmanuel MA, Greenberg NR, Oblinsky DG, Hyster TK (2016) Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 540:414–417
Biegasiewicz KF, Cooper SJ, Gao X (2019) Photoexcitation of flavoenzymes enables a stereoselective radical cyclization. Science 364:1166–1169
Guo X, Okamoto Y, Schreier MR, Mirjam RS, Oliver SW (2018) Enantioselective synthesis of amines by combining photoredox and enzymatic catalysis in a cyclic reaction network. Chem Sci 9:5052–5056
Luján AP, Bhat MF, Saravanan T, Poelarends GJ (2022) Chemo- and enantioselective photoenzymatic ketone reductions using a promiscuous flavin-dependent nitroreductase. ChemCatChem 202200043:1–6
Lian M, Li Z, Cai Y, Meng Q, Gao PZ (2012) Enantioselective photooxygenation of b-keto esters by chiral phase-transfer catalysis using molecular oxygen. Asian J 20:1–6
Woźniak L, Murphy JJ, Melchiorre P (2015) Communication Photo-organocatalytic enantioselective perfluoroalkylation of β-ketoesters. J Am Chem Soc 137:5678–5681
Espelt LR, Mcpherson IS, Wiensch EM, Yoon TP (2014) Enantioselective conjugate additions of α-amino radicals via cooperative photoredox and lewis acid catalysis. J Am Chem Soc 137:2452–2455
Blum TR, Miller ZD, Bates DM, Yoon TP (2016) Energy transfer. Science 354:1391–1396
Amador AG, Yoon TP (2016) A chiral metal photocatalyst architecture for highly enantioselective photoreactions. Angew Chem Int Ed 55(7):2304–2306
Huang X, Webster RD, Harms K, Meggers E (2016) Asymmetric catalysis with organic azides and diazo compounds initiated by photoinduced electron transfer asymmetric catalysis with organic azides and diazo compounds initiated by photoinduced electron transfer. J Am Chem Soc 138(38):12636–12642
Li C, Cao Y-X, Jin R, Bian K-J, Qin Z-Y, Lan Q, Wang X-S (2019) Highly stereoselective nickel-catalyzed difluoroalkylation of aryl ketones to tetrasubstituted monofluoroalkenes and quaternary alkyl difluorides. Chem Sci 10:9285–9251
Liu J, Ding W, Zhou Q (2017) Enantioselective di-/per fl uoroalkylation of β-ketoesters enabled by cooperative photoredox/nickel catalysis. ACS Organ Lett 20:7–10
Giles AC, Rankin CH, Kerr RA, Yoon TP (2014) A dual-catalysis approach to enantioselective. Science 344:392–396
Huo H, Harms K, Meggers E (2016) Catalytic, enantioselective addition of alkyl radicals to alkenes via visible-light-activated photoredox catalysis with a chiral rhodium complex catalytic, enantioselective addition of alkyl radicals to alkenes via visible-light-activated photoredox. J Am Chem Sci 138(22):6936–6939
Liang H, Xu G, Feng Z, Peng FX (2018) Dual catalytic switchable divergent synthesis: an asymmetric visible-light photocatalytic approach to fluorine-containing keto acid frameworks. J Org Chem 84(1):60–72
Kuang Y, Wang K, Shi X, Eric M, Jie W (2019) Dual catalysis asymmetric synthesis of 1,4-dicarbonyl compounds from aldehydes by hydrogen atom transfer photocatalysis and chiral lewis acid catalysis. Angew Chem Int Ed 20:1–6
Uchikura T, Kamiyama N, Mouri T, Akiyama T (2022) Visible-light driven enantioselective radical addition to imines enabled by excitation of chiral phosphoric acid-imine complex. Chem Rexiv 20:1–5
Article E, Lathrop SP, Rovis T (2013) A photoisomerization-coupled asymmetric Stetter reaction: application to the total synthesis of three. Chem Sci 20:838
Yang Q, Zhang L, Ye C, Li WZ (2017) Visible-light-promoted asymmetric cross-dehydrogenative coupling of tertiary amines to ketones by synergistic multiple catalysis. Angew Chem Int Ed 56:3694–3698
Zhu Y, Zhang L, Luo S (2014) Asymmetric α-photoalkylation of β-ketocarbonyls by primary amine catalysis: facile access to acyclic all-carbon quaternary stereocenters. J Am Chem Soc 136(42):14642–14645
Larionov E, Mastandrea MM, Pericàs MA, Mquel A (2017) Asymmetric visible-light photoredox cross-dehydrogenative coupling of aldehydes with xanthenes asymmetric visible-light photoredox cross-dehydrogenative coupling of aldehydes with xanthenes. ACS Catal 20:7008–7013
Hou H, Zhu S, Atodiresei I, Rueping M (2018) Asymmetric organocatalysis and photoredox catalysis for the α-functionalization of tetrahydroisoquinolines. Eur J Organ Chem 20:1277–1280
Arceo E, Jurberg I, Álvarez-Fernández A et al (2013) Photochemical activity of a key donor–acceptor complex can drive stereoselective catalytic α-alkylation of aldehydes. Nat Chem 5(9):750–756
Yang Z, Li H, Li S, Zhang MT, Luo S (2017) A chiral ion-pair photoredox organocatalyst: enantioselective anti-Markovnikov hydroetherification of alkenols. Org Chem Front 4:1037–1041
Gentry EC, Rono LJ, Hale ME, Knowles RR (2018) Enantioselective synthesis of pyrroloindolines via noncovalent stabilization of indole radical cations and applications to the synthesis of alkaloid natural products. J Am Chem Soc 140(9):3394–3402
Morse PD, Nguyen TM, Cruz CL, Nicewicz DA (2018) Enantioselective counter-anions in photoredox catalysis: the asymmetric cation radical Diels–Alder reaction. Tetrahedron 74:3266–3272
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verma, R., Jindal, P., Prasad, J. et al. Recent Trends in Photocatalytic Enantioselective Reactions. Top Curr Chem (Z) 380, 48 (2022). https://doi.org/10.1007/s41061-022-00402-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-022-00402-9