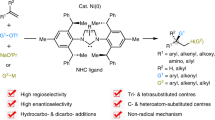
Abstract
This review article discusses historical and contemporary research studies of asymmetric allylic oxidation of olefins using homogeneous and heterogeneous copper complexes of various kinds of oxazoline-based ligands, until the end of 2021. It is revealed that this strategy is a powerful method to form a new stereogenic center bearing an oxygen substituent adjacent to an unchanged C=C bond. Enantioselectivities as well as chemical yields, and also the reactivity, are strongly dependent on the type of substrate, oxidant, the copper salt and its oxidation state, ligand structure, temperature, nature of the solvent, and additives such as phenylhydrazine and porous materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In 1958, Kharasch and Sosnovsky [1,2,3] introduced non-stereoselective allylic oxidation of olefins using tert-butyl peroxybenzoate and catalytic amounts of copper (I) bromide in refluxing benzene. This process led to the formation of allylic esters with moderate-to-high yields (50–80%) and relatively good regioselectivity of the internal secondary ester over the terminal primary ester at a ratio of 9:1 (Scheme 1).
The asymmetric version of allylic C–H bond oxidation of olefins (known as enantioselective Kharasch–Sosnovsky reaction) giving access to chiral allylic esters and, after hydrolysis or reduction, chiral allylic alcohols, is a valuable asymmetric transformation for synthetic organic chemists, where, unlike the hydroxylation and epoxidation, the second functional group forms while the double bond remains unchanged. The resulting olefinic products can also be further functionalized to afford a variety of desirable compounds. This enantioselective process is attractive, as it employs simple and inexpensive olefins. The reaction is carried out in the presence of a catalytic amount of various types of metal catalysts, particularly copper salts [4,5,6,7,8,9].
This enantioselective transformation is depends largely on the nature of the ligand, perester, type of metal and its oxidation state, counter anion, temperature, solvent, and additive. These factors affect the rate, enantioselectivity and also the yield of the reaction. There is a special focus on chiral copper catalysts because of their superior efficiency in terms of reactivity, yield, and selectivity. It worth mentioning that the active copper species involved in the allylic oxidation reactions is copper (I), so common copper salts that are used in this reaction are copper (I) salts. However, in some cases, copper (II) salts show satisfactory results. Furthermore, experimental results have shown that Cu(CH3CN)4PF6, CuOTf and Cu(OTf)2 are frequently used copper salts that are capable of complexing with the appropriate ligands for construction of efficient chiral catalysts [4,5,6,7,8,9] (Scheme 2).
Although this review focuses on oxazoline-based copper catalysts, it has to be mentioned that other various chiral copper complexes have been used as catalysts in the Kharasch–Sosnovsky reaction. Chiral carboxylic acids [10], proline [11,12,13] and bicyclic amino acids based on the proline skeleton [14, 15] have given modest enantioselectivities and yields. For instance, cyclohexene and cyclopentene in the presence of a copper-bicyclic proline complex using t-butyl perbenzoate at room temperature were oxidized to the corresponding (S)-benzoates in moderate yields with enantioselectivities of 60% and 65% ee, respectively (Scheme 3). It should be noted that this type of chiral ligands has been rarely studied in allylic oxidation, presumably because of the higher efficiency of oxazoline ligands.
Furthermore, in recent years, new non-oxazoline ligands like (iminophosphoranyl)ferrocenes [16], C2-symmetric 1,10-phenanthrolines [17], C1- and C2-symmetric bipyridineligands [18], aza-arene cis-dihydrodiol-derived 2,2′-bipyridine ligands [19], aldimine ligands [20], C1-pyridyl–thiazole ligands [21], N,N-bidentate and N,N,N-tridentate pyridine-based ligands [22], diamino alcohols [23], polyfluorous proline [24, 25] and proline [26, 27] have been employed, which, in some cases, are able to provide chiral products with enantioselectivities and yields in almost the same ranges as oxazoline-based ligands. However, in general, oxazoline moieties have proved to be the chosen ligands due to their specific characteristics, which make them one of the most privileged chiral ligand classes in a wide range of enantioselective transformations. Their accessibility, as well as the potential to modify the structure, can be considered as remarkable aspects of oxazoline ligands, which allow fine-tuning of the ligand structure for a specific application. Besides, the presence of a stereocenter close to the donor nitrogen atom creates a well-ordered chiral environment at the catalytic site [28, 29]. Accordingly, oxazoline-based ligands have been applied frequently in asymmetric allylic oxidation and provided chiral allylic esters in excellent yields and enantioselectivities. These versatile ligands can be prepared easily from reaction of carboxylic acids or derivatives thereof and also nitriles with chiral β-amino alcohols, usually obtained from the reduction of natural amino acids, although different synthesis methods have been developed [30,31,32,33,34,35,36,37,38,39,40,41,42] (Scheme 4).
In the present review, we will summarize all the progress in asymmetric allylic oxidations of olefins catalyzed by chiral oxazoline-based homogeneous and heterogeneous Cu catalysts until the end of 2021, including all aspects of this valuable asymmetric transformation.
2 The Mechanism of Allylic C–H Bond Oxidation of Olefins
The mechanism of allylic C–H bond oxidation of olefins has been studied extensively by several research groups, e.g., Kochi [43,44,45,46], Walling [47, 48], Beckwith [49], Salvatella [50], Slough [51], and Bao [52]. In the beginning, a chiral catalyst would be formed by addition of copper salt to the chiral ligand, then a multi-step mechanism occurs upon addition of the required compounds through a catalytic cycle with chang in the oxidation state of the Cu atom. In a non-coordinating solvent, the copper (I)-alkene complex is the main component [50, 51, 53]. The catalytic cycle begins by replacing the alkene with perester, tert-butyl perbenzoate, which coordinates to the copper (I) through two of its oxygen atoms to generate a perester-copper (I) complex. In a subsequent step, the copper (I) is oxidized to copper (III) and a crypto-alkoxyl radical is formed through oxidative-addition of the oxygen–oxygen bond of the perester [50]. It should be noted that there is ample evidence in the literature suggesting that the reaction proceeds via a free radical intermediate, and most of the mechanisms rely upon the participation of a tert-butoxyl free radical [43,44,45,46,47,48,49]. Hereupon, alkene is again coordinated to the copper (III) complex and thereafter, in a limiting step, allylic hydrogen of the alkene is abstracted intramolecularly by a crypto-alkoxyl radical. As a result, the obtained complex can release tert-butyl alcohol to form a key allyl-copper (III) reaction intermediate. Subsequently, by a pericyclic rearrangement of the resulting allyl-copper (III) intermediate through a stereospecific reductive-elimination including the migration of a π-bond, a product–catalyst complex can be formed. Finally, the initial alkene–catalyst complex is regenerated by releasing the chiral allylic ester as the reaction product through exchanging it with alkene (Scheme 5).
3 Asymmetric Allylic Oxidation Using Chiral Oxazoline Based Catalysts
3.1 Homogeneous Catalysts
3.1.1 Comprehensive Studies
In 1995, the use of oxazoline ligands in asymmetric allylic oxidation of olefins was investigated independently by the research groups of Pfaltz [54] and Andrus [55]. These groups studied chiral Cu-bisoxazoline complexes in the allylic oxidation of cyclic and linear olefins in the presence of peresters, leading to the corresponding chiral allylic esters in good enantioselectivities and yields. Pfaltz and coworkers evaluated asymmetric allylic oxidation of some substrates such as cyclopentene, cyclohexene, and cycloheptene using malonyl bisoxazoline 1a–c and pyridine bisoxazoline (Pybox) ligand 2a in the presence of copper salts such as copper (I) triflate, copper (II) triflate, and copper (I) hexafluorophosphate in polar solvents like acetone and acetonitrile at the temperature range of −20 to +50 °C [54]. The results revealed that conducting the reactions at low temperatures (Table 1, entries 4 and 8), especially in the presence of ligand 1c, containing bulky t-butyl groups at the stereogenic centers, led to the improvement in enantioselectivity. It seems that, under these circumstances, the bulkier the groups, the higher the enantioselectivities. In terms of copper salts and solvents, the best enantioselectivities were achieved using copper (I) triflate in bi-solvent systems acetonitrile/chloroform (3:1) (Table 1, entry 4) and acetone/chloroform (3:1) (Table 1, entry 8). Almost the same results were achieved using copper (I) hexafluorophosphate or copper (I) triflate, while copper (II) triflate showed low activity and enantioselectivity. Generally, counter ions and oxidation states of copper not only have a great impact on the enantioselectivity but could also affect the reactivity of the catalyst and the yields of the chiral products.
Employing 1-methyl cyclohexene as a substituted cycloolefine resulted in allylic esters with good enantiomeric excess, but low regioselectivity. It should be noted that the chiral allylic esters that formed through oxidation of the methyl-substituted C atom were obtained in poor yield but high enantiomeric excess (Scheme 6).
In a similar study by Andrus and colleagues [55], when the reaction was carried out in the presence of 5 mol% of bisoxazoline 1c-CuOTf as catalyst at −20 °C in CH3CN instead of a bi-solvent system, chiral allylic esters were obtained with moderate yields but in almost the same enantioselectivities reported by Pfaltz et al. [54]. Besides, it is noteworthy that the reaction was completed in a shorter time. Further studies have revealed that the oxazoline ligand 1c could not be recycled and reused, presumably due to its decomposition during the oxidation process. It had been assumed that, the CH2 group in the oxazoline ring has the potential to be oxidized under reaction conditions, which can affect catalyst efficiency. To avoid this problem, Andrus designed bis-gem-dimethyl bisoxazolines 3a and 3b, which were stable ligands under reaction conditions, by replacing the both hydrogens with methyl groups (Table 2). In the presence of ligand 3a, oxidation of cyclopentene proceeded with 81% enantiomeric excess, whereas this substrate with ligand 3b was oxidized in much lower enantiomeric excess of 42% (Table 2, entries 2 and 3). In the case of cyclohexene, ligand 3b showed much better efficiency than 3a, but, in contrast to cyclopentene, the best enantioselectivity was obtained with 1c (Table 2, entries 4–8). The enantioselectivities were even lower for cyclooctene (Table 2, entries 9 and 10). This drop in enantiomeric excess could be explained by the fact that the bigger ring systems might be too bulky to fit in the catalyst complex cavity, which is determined by two substituents on the bisoxazoline.
However, acyclic substrates such as allylbenzene and 1-octene did not give satisfactory results. Allylic oxidation of allylbenzene using ligand 1c-copper (I) in acetonitrile at low temperature proceeded with 50% yield, and the corresponding chiral allylic ester was obtained in racemic form. Surprisingly, at higher temperatures (55 °C) in the non-polar solvent of benzene, the enantioselectivity increased to 36% and 30% for both allylbenzene and 1-octene, respectively (Scheme 7).
A selectivity model, proposed for the reactions of cyclohexene and acyclic substrates [55], is shown in Fig. 1. The Cu(III) intermediates adopt a distorted square planar coordination geometry. The rearrangement leading to the product delivers the benzoate to the internal position of the acyclic olefin or to the distal position of the cycloalkene. In the favored transition states, the benzoate and allyl groups are positioned in such a way that repulsive steric interactions with the adjacent tert-butyl groups of the bisoxazoline ligand are minimized. With cyclic alkenes, the reaction of the Cu(II) intermediate with the allyl radical can produce two diastereomeric allyl complexes. The enantioselectivity in this case may result from stereoselective formation of the Cu(III)-allyl complex followed by a stereospecific rearrangement. Alternatively, if the two diastereomeric Cu(III) complexes equilibrate rapidly, the enantioselectivity may be induced by kinetic discrimination of the two possible rearrangement pathways leading to the major and minor product enantiomers. In the reaction of acyclic terminal olefins, only one allyl complex is formed. In this case, the rearrangement must be the enantioselective step. The lower enantioselectivity of acyclic olefins may be explained by their greater conformational flexibility, making both olefin faces easily accessible.
As it was observed, allylic oxidation of cycloolefins generally proceeds slowly. To solve this challenging issue, Andrus introduced a new category of peresters containing withdrawing substituents on the phenyl ring. It was supposed that withdrawing substitutions can weaken the oxygen–oxygen bond in the perester, causing acceleration of tert-butoxy radical formation, and consequently increasing the rate of reaction. In this case, reactivity was not increased; however, the yields and ees were similar to those obtained using unsubstituted perester [54, 55] (Table 3 vs Tables 1, 2). It was also found that enantiomeric excesses will be simply enhanced when the mole percentage of the copper–ligand complex was raised. In the oxidation of cyclohexene with para-nitroperbenzoate, for example, the increasing amount of catalyst 1c from 5 mol% to 15 mol% not only improved stereoselectivity from 63% to 76% but also enhanced the yield considerably (Table 3, entry 7 vs 9). Furthermore, the effect of counter anions of copper (I) salts on yield and enantioselectivity revealed that Cu(CH3CN)4PF6 is preferred over other Cu salts. It would seem that, due to stronger interactions between more acidic copper salt and allyl radical, high stereoselectivity will be obtained. In optimum conditions at −20 °C and in acetonitrile solvent, the best results (66% ee, 76% yield) for cyclopentene were obtained when o-chloroperester and ligand 1c were employed (Table 3, entry 5), whereas the combination of p-chloroperester and ligand 1a was suitable for cyclohexene (75% ee, 83% yield) (Table 3, entry 2) [56].
In the same year, in addition to the Pfaltz [54] and Andrus [55] research groups, Katsuki was studying asymmetric allylic oxidation of olefins by applying Cu-oxazoline complexes. This group designed and synthesized a series of tetradentate tris-(oxazoline) ligands 4a–h containing alkyl and aryl substituents with steric and electronic effects on the oxazoline moiety [57, 58]. Investigation of this kind of ligands under various reaction conditions resulted in desired chiral allylic esters in moderate-to-excellent enantioselectivities. Using a wide range of solvents from non-polar to polar indicated that, contrary to the previous studies, which have suggested acetonitrile as a promising solvent in terms of selectivity, performing the reaction in acetone could contribute to better results. However, the findings did not reveal any clear link between the polarity of the solvent and asymmetric induction. Enantioselectivity of cyclopentene, for example, in toluene and DMF was found in the same range (66% ee and 68% ee), and even higher than acetonitrile (18% ee). Interestingly, inconsistent with Pfaltz and Andrus’ findings, Cu (II) gave higher asymmetric induction than Cu (I). It was observed that tris-(oxazoline) bearing aryl groups at the chiral center exhibits remarkable potential for higher ees and yields than other ligands, presumably due to attractive interactions between the aryl substituents and the allyl radical intermediates. Steric and electronic effects also did not lead to better results, although the p-methoxy group showed a slight increase in ee but the allylic ester was obtained in lower yield (Table 4, entries 6–12). In a comparative study among three different oxidizing reagents, benzoyl peroxide, t-butyl hydroperoxide, and t-butyl perbenzoate, it was demonstrated that t-butyl perbenzoate is a more suitable candidate for achieving chiral allylic products with reasonable enantiomeric excess and yield, which is similar to previous reports. For instance, although t-butyl hydroperoxide resulted in (S)-cyclopent-2-en-1-yl benzoate, with an acceptable level of enantioselectivity (68%), the yield was poor (21%) and undesired compounds were produced too. In the case of benzoyl peroxide, enantioselectivity was inferior (13%) as well. The effect of temperature on the yield and enantioselectivity of the reaction was also in accordance with earlier studies [54, 55]. In other words, enantioselectivity increased as the reaction temperature decreased. The highest enantioselectivity of 88% at –20 °C was obtained when cyclopentene was used as the substrate, although the yield was very low (11%). In larger substrates like cyclooctene, the corresponding ester with moderate enantioselectivity of 54% in 18% yield was obtained at room temperature. Further studies have shown that longer reaction times have a slightly negative impact on enantiomeric excess [58]. Katsuki et al. reasoned that the Lewis acidity of Cu (II)-4a complex, probably makes a contribution to partial racemization in the allylic esters. It was expected that the addition of water could reduce the Lewis acidity of Cu (II) via coordination to copper ion. However, the reaction in no way made progress in the presence of one equivalent of water to copper ion. Therefore, they deduced that if the process completes in a shorter time, racemization can diminish. To achive this aim, water was removed from the reaction mixture by adding 4 Å molecular sieves (MS). It was observed that MS have a remarkable effect on improving the enantioselectivity, yield and also reaction rate. For example, in the presence of MS, cyclopentene was oxidized in 16 h (compared with 40 h in the absence of MS) and (S)-cyclopen-2-yl benzoate was afforded with enantiomeric excess of 76% and 83% yield (Table 4, entry 3). Enantioselectivity at the temprature of −20 °C was increased to excellent 93% ee, although the yield dropped to 30% and the reaction was prolonged to 200 h (Table 4, entry 5). The same pattern was observed in cycloolefins larger than cyclopentene. In the presence of MS at room temperature, the corresponding chiral allylic esters were achieved in good-to-moderate ees, although the yields were very poor (Table 4, entries 13–15). Utilizing aryl substituents of peroxyesters had no impressive impact on reactivity and enantioselectivity. Apart from the o-methyl group, which slightly improved enantioselectivity, o-chloro resulted in lower ee and the p-nitro group decelerated the reaction rate. Oxidation of 1-methyl cyclopentene at 0 °C provided chiral regioisomeric esters with low to high enantiomeric excesses, closely similar to Pfaltz’s studies [54]. Furthermore, based on Andrus’ findings [55], acyclic olefin 1-octene gave allylic ester in both poor yield and ee at room temperature.
The findings show that a combination of Cu (II)-4a complex and MS is only capable of inducing high enantiocontrol in allylic oxidation of cyclopentene. Hence, in a similar study Katsuki’s group [59] synthesized a different type of tridentate C3-symmetric tris-oxazoline ligands 5a-d by replacement of the central N atom in ligand 4 with methyne (CH). As in their former studies [57, 58], ligands with aryl substitutients at the oxazoline ring were able to induce better stereocontrol than alkyl ones. Morever, ligand 5b bearing an electron donating group (p-OMe) showed higher enentioselectivity (Table 5, entries 8, 13, 15 vs 16). It was found that, although ligands 4a and 5a,b have the same stereochemistry, the configuration of the resulting products was opposite, which presumably is due to different structures of the Cu(II) complexes with ligands of type 4a and 5a,b (Table 5, entries 1, 2 and 12). Interestingly, in contrast to tetradentate tris-oxazoline ligand 4a, in the presence of ligands 5a, MS exhibit an adverse effect on enantioselectivity (Table 5, entry 1 vs 3). Besides, using 1,2-dichloroethane as a non-polar solvent resulted in ralatively higher ee values in comparison with the previous findings in which acetonitrile or acetone exhibited promising results (Table 5, entries 3–7). However, non-polar aromatic solvents such as C6H6 and C6H5Cl have been found not to be effective for enantioselectivity (Table 5, entries 10, 11). In optimized conditions (Cu (II)-ligand 5b at low temperature), Katsuki could upgrade the enantioselectivity of the larger cycloolefins such cycloheptene and cyclooctene to higher enantioselectivity than what was obtained in their previous works (Table 5, entries 18–20). However, the chemical yield at low temperatures (e.g., 0 °C) for cyclooctene was only 25%.
Under these conditions, desymmetrization of racemic olefin 6 through a meso intermediate led to regioisomeric chiral allylic esters with good-to-high ee values (58–85%). However, the yields were poor. It seems that low regioselectivity in the hydrogen abstraction step is responsible for the formation of undesired products (Scheme 8).
In 1996, Singh et al. [60] were working on a series of tridentate gem-diphenyl Pybox 7a–d in which both hydrogens at C5 had been replaced with phenyl groups. Studying the effect of phenylhydrazine as a reductant agent for in situ generation of Cu (I) from Cu (II) may be considered as the leading characteristic of Singh’s works. Besides, in accordance with the first study of Katsuki with ligand 4a [58], it was observed that 4 Å MS had a considerable impact on asymmetric induction, although the reaction was completed in longer time period. In further research [61], they focused their attempts on decreasing the reaction time and improving ee and also chemical yields. Although, various species of copper salts such as CuCN, Cu (II) triflate and Cu (I) triflate were capable of catalyzing the reaction, reasonable ee was obtained only with 7c-Cu (I) triflate complex (Table 6, entries 1–5). Interestingly, it was observed that the procedure using Cu (I) formation has a significant effect on the rate of the reaction. In other words, the reaction in the presence of in situ generated Cu (I) from a combination of Cu(OTf)2 and phenylhydrazine is much faster than that of Cu (I) used directly from (CuOTf)2.PhH (Table 6, entries 3 vs 5). Moreover, the use of phenylhydrazine and Cu (I) together dramatically reduced the reaction time from several days to a few hours. In case of cyclohexene, for instance, employing 7c-(CuOTf)2.PhH in conjunction with phenylhydrazine considerably accelerated the reaction rate, and the time of reaction diminished from 144 to 5 h without much adverse effect on ee (Table 6, entry 3 vs 7). Although adding 4 Å MS in the absence of phenylhydrazine increased enantiomeric excess, the reactivity was drastically decreased (Table 6, entry 11). It is noteworthy that simultaneous use of MS (4 Å) and phenylhydrazine was beneficial in particular for ee. It appears that, due to the use of phenylhydrazine the reactivity and ee were increased; however, some slight decrease in yield was observed (Table 6, entries 10, 12, 13 vs 14, 16, 17). This situation resulted in lower yield as well as ee for cyclohexene (Table 6, entry 11 vs 15). Furthermore, replacement of isopropyl group at ligand 7c by different substitutents led to lower enantioselectivity (Table 6, entries 18–20).
It is worth noting that, in a reassessment investigation, some notably different results were observed by Singh and coworkers [62]. They found that recrystallization of gem-diphenyl Py-box ligand 7c from diethyl ether would provide 91% ee as opposed to the 75% ee obtained in the previous study [61] in the case of cyclohexene; the reaction time was also drastically reduced from 24 h to only 1 h. Apparently, effective purification of chiral ligand 7c is responsible for the improvement in catalytic activity. It was further found that, contrary to their later study [61], the beneficial effect of 4 Å MS was not observed and reconsideration of the results showed a decrease in enantioselectivity in the presence of MS. Interestingly, when the reaction was carried out in the presence of water (6 mol%) not only was no decline in enantioselectivity and yield observed, but the reaction time also increased from 1 h (67% yield, 91% ee) to 11 h (79% yield, 90% ee). These findings showed that, in this situation, the reaction is not moisture sensitive [62]. The experimental observations showed that allylic oxidation would be completed in a short time, providing that the Cu (I) species were prepared in situ by reduction of 7c-Cu(OTf)2 with phenylhydrazine in acetone. Besides, changing the solvent to acetonitrile increased the reaction time to several days from a few hours, and a drop in enantioselectivity (from 91 to 86% ee for cyclohexene) was also observed. The use of electron paramagnetic resonance (EPR) spectroscopy elucidated that both phenylhydrazine and phenylhydrazone, which is generated in situ from condensation of acetone with phenylhydrazine, are able to reduce species of copper (II) to copper (I), and are responsible for the rate enhancement. However, the effect of phenylhydrazine is much stronger than that of phenylhydrazone. Moreover, a mechanism was proposed in which a π–π stacking interaction between the phenyl groups at the C-5 of the gem-diphenyl Py-box ligand 7c and the incoming benzoate of perester in the transition state plays a key role in asymmetric induction [63, 64] (Fig. 2).
Based on this proposed model, in the favored transition state, a π–π interaction would control the approach of the incoming benzoate from the less sterically hindered side. The olefin approaches Cu from the opposite side, due to disfavored interaction with the R group at C-4 of the oxazoline moiety. This transition state illustrates why the S product is formed as the major product. This model was supported by replacing phenyl groups at C-5 of ligand 7c with hydrogen and benzyl groups. In these cases, due to lack of π–π interaction, asymmetric induction was declined. Consequently, it was expected that, because of stronger π–π stacking in the peresters containing electron-withdrawing substituents (such as NO2), the chiral allylic esters would be formed with higher enantioselectivity. But, contrary to expectation, experimental observations have shown a decline in reactivity as well as selectivity. Instead, electron-donating groups (like methoxy and alkyl) provided the desired chiral allylic esters with excellent enantioselectivity. These findings indicated that, when the π–π interaction is stronger, steric repulsion with the R group of oxazoline at the sterogenic center does not play a significant role and, therefore, the reaction will proceed through both pathways. On the other hand, weaker stacking by electron-donating groups is not sufficient to overcome steric repulsion. Therefore, an optimal interaction is responsible for the highest asymmetric induction. The proposed model also explained the high level of enantioselectivity of the reaction with ligand 7e, where R is sec-butyl [63, 64]. In this situation, oxidation using tert-butyl-o-methoxyperbenzoate provided the desired enantioenriched allylic esters in short reaction time with good-to-excellent enantioselectivities (Table 7) [63]. Cyclohexene, for example, gave (S)-cyclohex-2-en-1-yl 4-methoxybenzoate in excellent enantioselectivity (98% ee) and good yield (71%) in 2 h (Table 7, entry 8). In case of cycloheptene and cyclooctene ee values and yields were all high (Table 7, entries 9–16); however, cyclopentene was oxidized with good yield and ee (80%) (Table 7, entry 4).
Similarly, high ee values up to 96% were obtained for cyclooctadiene substrates with tert-butyl-p-methoxy perbenzoate in the presence of ligand 7c, although 1,5-cyclooctadien gave a mixture of products with roughly the same ee values. Regarding the mixture of cis and trans cyclododecene, both enantioselectivity and yield were low to medium (Scheme 9) [63]. It should be noted that while high level of facial selectivity was obtained for most cycloolefines, the reaction was not effective for acyclic ones. Oxidation of 1-octene with tert-butyl-p-methoxy perbenzoate gave a mixture of allylic esters with poor enantioselectivity up to 31% (Scheme 9).
Continuing their investigations, Andrus et al. [65] developed and evaluated bistolyl bis-oxazoline ligands (S, aS, S)-8 and (S, aR, S)-9 in the asymmetric allylic oxidation. Evidence revealed that the axial chirality of the ligands has a significant effect on the stereoselectivity of the resulting ester; thus, (S, aS, S)-atropisomer 8 resulted in allylic esters with higher enantioselectivity compared with (S, aR, S)-atropisomer 9 (Table 8). A detailed look at the geometry of ligands signifies that this discrepancy can be attributed to the steric control of the R groups in the stereoisomers. The R groups in the (S, aS, S)-isomer occupy a pseudo axial position, whereas in the (S, aR, S)-isomer they are in the equatorial position (Scheme 10). In the (S, aR, S)-ligands all four quadrants are almost equally congested, leading to products with lower ee values.
On the other hand, in contrast to the malonyl bisoxazolines [55] in which steric hindrance of the tert-butyl group led to great stereoselectivity, higher reactivity and also enantioselectivity were achieved by use of this type of ligands containing less hindered Ph and Bn substituents (Table 8). It seems that replacement of the less sterically demanding groups on the oxazoline by more sterically bulky tert-butyl contributes to a larger bite-angle and consequently both the reaction rate and enantioselectivity were decreased. Employing differnt t-butyl perbenzoate derivatives showed that the best results can be obtained in the presence of t-butyl p-nitro-perbenzoate (Table 8).
In an another attempt, Andrus et al. [66] examined different atropisomeric ligands naphthyloxazolinyl quinazoline 10 and oxazolinyl isoquinoline 11. Based on promising results in the previous study, the reaction was again carried out with t-butyl p-nitro-perbenzoate in the presence of Cu (I) at −20 °C in acetonitrile. Although the results were not acceptable, ligand 11 showed better outcomes compared with 10. It can be proposed that the greater steric congestion of phenyl groups in ligand 10 is responsible for this discrepancy. Similar to Singh’s findings [61] when the reaction was carried out in the presence of phenylhydrazine in conjunction with Cu(OTf)2 in acetone, an improvement in enantioselectivity and yield was observed (64% and 37%, respectively). In such a situation, the reaction time was reduced dramatically from 6 days to 6 h at room temperature too (Scheme 11).
By applying quinolinyloxazoline 12, which contains a structure similar to ligand 11 [66], Zhou et al. [67] obtained moderate enantioselectivities and yields with cyclohexene and cycloheptene but only with 6% ee for cyclopentene (Scheme 12).
In a notable study using malonyl-derived bisoxazolines, Andrus’s group demonstrated that excellent enantiocontrol can be obtained when the reaction conditions and the ligand structure are finely tuned for each specific substrate [68]. They succeeded in achieving enantioenriched allylic esters with the highest ee, which had not been reported until then. For each cycloolefin in optimal condition, Cu(CH3CN)4PF6 and t-butyl p-nitroperbenzoate in acetonitrile at −20 °C, the efficiency of different malonyl-derived bisoxazoline ligands were evaluated. In such circumstances, cyclohexenyl ester was produced in 96% ee by using gem-dimethyl diphenyl ligand 1a, while gem-diethyl diphenyl ligand 13 was suitable for oxidation of cyclopentene with a remarkable 99% ee (Table 9, entries 5, 6). Oxidation of cycloheptene with gem-dimethyl di-i-Pr 1b resulted in very high enantioselectivity (99%); 1,5-cyclooctadiene, in contrast, exhibited excellent enantioselectivity (94%) in the presence of gem-dimethyl di-t-butyl 1c (Table 9, entries 12,18). It should be noted that, in all the cases, (S)-allylic esters were produced from the S, S ligands and, in contrast to the high levels of asymmetric induction, the yields were not satisfactory even in extended times. These observations can be interpreted in accordance with the geometry of bisoxazoline-Cu complexes. Different studies [69, 70] have shown that gem-dimethyl bisoxazoline Cu-complexes prefer a distorted square planer geometry. It was envisioned that both t-Bu in 1c occupy pseudo-axial positions, whereas in 1a, one of the phenyl groups tends to be in pseudo-axial and the other in more relaxed pseudo-equatorial position. Lower yields achieving by cyclopentene (49% vs 52%) and cyclohexene (44% vs 61%) in the presence of 1a can be attributed to the disfavored interaction between phenyl at the pseudo-equatorial position and incoming benzoate. However, this interaction presumably is favored for higher degree of asymmetric induction in cyclopentene (82% vs 79%) and cyclohexene (96% vs 84%). Similarly, due to the stronger interaction of bulky t-Bu in 1c with cycloheptene and cycloocadiene, the corresponding esters were obtained in high enantioselectivities (95% and 94%), but very poor yields (3% and 13%) (Table 9, entries 13 and 18).
Due to good stereocontrol for a range of substrates with the malonyl-derived bisoxazoline ligands bearing phenyl groups at the stereogenic centers, and also more flexibility in comparison with the corresponding t-butyl derivatives, Andrus supposed that by replacing phenyl groups with naphthyl, the yields would be enhanced without affecting enantioselectivities [71]. In this situation, (S)-cyclohex-2-en-1-yl 4-nitrobenzoate was obtained with 75% and 40% yields and 85% and 80% ee values in the presence of naphthyl-substituted malonyl-derived bisoxazolines 1e and 1f, prepared from naphthyl amino alcohols by the Sharpless aminohydroxylation of vinylnaphtalenes [72], respectively (Table 10, entries 1 and 2). It seems that the greater flexibility of ligand 1f compared with ligand 1e, is responsible for this difference. It was also found that, in contrast to earlier studies with ligands 1 and 13 [68], perester was totally consumed by using the copper complex of ligand 1e. Besides, tridentate Pybox 2b did not contribute to reasonable results (Table 10, entry 3). It is worth noting that the low solubility of employed ligands in the preferred acetonitrile solvent, which was inevitably replaced by chloroform and methylene chloride or a mixture of the two, could be responsible for a decrease in both reactivity and enantioselectivity (Table 10).
By employing (1S, 2R)-1-aminoindan-2-ol, Clark and co-workers [73] introduced a different series of C2-symmetric bisoxazoline ligands 14–16, which are capable of inducing acceptable ee values even at a temprature higher than the previous studies (40 °C). Under these conditions, the yield and reactivity were increased and the reaction completed in almost 1 day. Cyclohexene, for example, was converted to the corresponding (S)-allylic ester after 24 h with 74% ee and 65% yield. As expected, lowering the reaction temperature to 0 °C improved the level of asymmetric induction and decreased both the yield and rate of reaction (21 days) (Table 11, entry 7). In the case of cyclopentene, BOX ligand 1a showed higher enantioselectivity than ligands 14. Moreover, using this type of ligand has not contributed to reasonable results for cyclooctene. Contrary to the above-mentioned studies by Katsuki [58] and Singh [60], enantioselectivity was not affected significantly in the presence of activated 4 Å MS, although an increase in yield was observed (Table 11, entry 6 vs 8). In contrast to Pfaltz’s report [54], ligand BOX 1a showed better enantioselectivities than 1c. It should be noted that, in the presence of ligands 15b and 16, the induced configuration of the resulting allylic esters is R (Table 11, entries 13–15).
When exo-methylene cycloolefins were employed as substrates, ligand 14c showed the best results for methylenecyclohexane (26% ee), while the best outcomes in the case of methylenecyclopentane were obtained by ligand 14d (43% ee). However, the absolute configuration of these products has not been reported (Scheme 13) [73].
In another study similar to Katsuki [59], Clark evaluated enantioselective symmetrizing–desymmetrizing allylic oxidation of a range of bridged bicyclic olefins 17a–d using copper complexes of the bisoxazoline ligands as catalysts [74]. They succeed in obtaining the corresponding endo chiral allylic esters 21 through achiral meso allylic radicals in moderate-to-good yield and good selectivity (Table 12). Generally, derivatives of Pybox ligands 2c, 18–20 provide better stereocontrol for substrate 17a in comparison with malonyl bisoxazoline ligands 1a and 1c, although it was found that the enantioselectivities and the yields were not very influenced by the substituents at the ligands. In case of substrate 17b, BOX ligand 1a showed more preferable results. Under these conditions, substrates containing oxygen (17c–d) were unreacted and bicyclic diene 17e was decomposed (Table 12). It is interesting to note that enhancement of enantioselectivity (up to 70% ee) and yield (up to 84%) was observed when the reaction was conducted under microwave irradiation or in a sealed tube at high temperature (60 °C).
In a different study, this group introduced peroxycarbamate as an efficient type of oxidant instead of peroxyester [75]. In comparison with peroxyesters under similar conditions, peroxycarbamates 22a–d in conjunction with Cu-(R, R)-1a complex were capable of converting cyclohexene and cyclopentene into chiral esters with good enantioselectivity in a smooth process and in reasonable times (Table 13). Peroxycarbamate 22e, containing a sulfonamide group, was able to direct the reaction towards allylic amination reaction instead of allylic oxidation, in moderate ee but low yield (Table 13, entries 9 and 10). More precisely, in the case of peroxycarbamate 22e, the tendency to decarboxylation is more facile compared with peroxycarbamates 22a–d. Such behavior indicates that, by adjusting the conditions, asymmetric allylic oxidation or amination reaction can occur.
Good enantioselectivities, yields and also high regioselectivities for cyclic and acyclic olefins under mild conditions as well as reasonable reaction time, without any additives, were obtained by Zhou [76, 77], who has introduced a new class of bisoxazolines 23 and 24 containing a chiral spiro bisindane backbone. In the case of cyclopentene and cyclohexene, room temperature was found to be the optimum temperature. Decreasing the reaction temperature to 0 °C or increasing it to 40 °C did not much affect the ee (Table 14, entries 7 and 8). Further studies have shown that the best results are achieved when the reaction is performed in the presence of 6 mol% of ligand 23b and 5 mol% of Cu(CH3CN)4PF6 in acetone. Additionally, in contrast to previous reports, substituents in the perester did not have a marked effect on the ee. Although the chemical yields were improved by para substituents, in the presence of t-butyl o-nitro-perbenzoate, the yield dropped to 16% (Table 14, entries 12–15 vs entry 3) [76].
As shown in the previous studies by Andrus [55] and Singh [63], allylic oxidation of acyclic olefins presents a major challenge, as enantioselectivities and regioselectivities are generally low. It was found that Cu (I)-ligand 23b complex can be considered as a suitable catalyst for achieving satisfactory results [77]. With this catalyst, not only were the corresponding allylic esters obtained in good enantioselectivities (up to 67%) and yields (up to 64%), the regioselectivities (the ratio of branched I to linear esters II) were also improved (> 20:1 in most cases) (Table 15). Conducting the reaction at a temperature of 30 °C, in most cases produced almost exclusively branched allylic esters with good-to-moderate ee values and yields; whereas changing the temperature led to a drop in the enantioselectivities and yields (Table 15, entries 1–3).
Most studies have indicated that, in addition to oxazoline backbones, the use of additives such as 4 Å MS and phenylhydrazine has a major effect on both enantioselectivity and yield. In 2013, our research group developed the synthesis of bistolyl bisoxazoline ligands (S, aS, S)-8 and (S, aR, S)-9 in gram scale from inexpensive and commercially available 3-methyl benzoic acid along with introducing a range of additives in asymmetric allylic oxidation [30] (Scheme 14). Similar to Andrus’ report [65], it was found that higher asymmetric induction can be obtained by applying a (S, aS, S)-8a bisoxazoline-bearing phenyl group at stereogenic centers in conjunction with Cu(CH3CN)4PF6. However, as expected, the effect of the additives on yields and enantioselectivity was noticeable. In the case of cyclohexene, for instance, at −10 °C in conjunction with 4 Å MS and phenylhydrazine, cyclohexyl benzoate was obtained with 80% ee and 85% yield, versus 73% ee and 78% yield reported by Andrus. Lowering the temperature to more than −10 °C had no significant effect on the ee. However, evaluation of a range of additives such as activated silica gel, mesoporous silica SBA-15 and MCM-41, nanocrystalline MgO, CuO, and TiO2, for the first time, has shown that in the presence of SBA-15 mesoporous silica both yields and enantioselectivities can be increased to 96% and 93%, respectively, in a shorter time (36 h vs 120 h, compared with Andrus’s results). This upward trend was also observed with cyclopentene (90%, 81% ee) and seven-, and eight-membered cycloalkenes (Table 16). It should be noted that in contrast to Singh reports [62,63,64], oxidation of 1,5-cyclooctadiene led to only one regioisomer with higher yield (up to 95%) and ee (up to 97%). This behavior can be attributed to the catalyst complex cavity, which can be fitted to a twist-boat conformation of 1,5-cyclooctadiene determined by the substituents on the bisoxazoline ligand [78,79,80].
Although this type of ligand can be effective for achieving excellent yields and ee, only the (S, aS, S)-stereoisomer is able to induce high enantioselectivity. Moreover, due to similar polarity of both atropisomers, separation of diastereomeric ligands 8 and 9 is a tedious and time-consuming process. These limitations have been ingeniously solved by employing ligands 24. It has been demonstrated that if both methyl groups are replaced with hydrogen atoms, rotation around the biphenyl axis will be possible, resulting in an equilibrium between atropisomers at ambient temperature [81,82,83]. In this case, by a chelation-induced process [84], a metal complex of only one diastereomer can be formed. Accordingly, a series of bisoxazoline ligands 24 with an axis-unfixed biphenyl backbone were synthesized from inexpensive and available starting material 2-amino benzoic acid [31]. As expected, both diastereomeric atropisomers were in equilibrium in the solution phase. Notably, complexation of the equilibrium mixture with Cu(CH3CN)4PF6 led to the formation of only (S, aS, S) diastereomer (Scheme 15). This complex produced chiral allylic esters with high enantioselectivities and yields, similar to our former study [30]. Moreover, a study on the effect of the above-mentioned additives [30], especially SBA-15 silica, again led to improved results. The best results were obtained in the presence of bisoxazoline containing benzyl groups 24b at the stereogenic centers at −10 °C in CH3CN. 1,5-Cyclooctadiene was also oxidized in the highest yield (up to 99%) and up to 95% ee, as in the previous study [30] (Table 17). It is noteworthy that in both studies, similar to Andrus’ results [65], the synergistic effect between configurations of the oxazoline rings and the backbone can enhance asymmetric induction.
Although both types of ligands 8 and 24 present high yields and very satisfactory levels of asymmetric induction, their preparation and separation suffer from laborious procedures. Thus, we attempted to design and synthesis a simple and efficient class of chiral amido-oxazolines 26a-d from aminooxazolines 25, which are generated from various amino alcohols and cyanogen bromide, and para-substituted benzoic acids such as OMe, NH2, Br, and NO2 [85]. It was observed that two equivalents of these ligands can coordinate with one equivalent of copper to form a C2-symmetric complex as a catalyst (Scheme 16). Use of these ligands in allylic oxidation showed that the best results can be obtained by using 26a-CuOTf complex in acetone at 0 °C (Table 18, entries 1 and 5). Under these conditions good ee values and yields were also observed for large cycloalkenes (Table 18, entries 9 and 13), although in the case of 1,5-cyclooctadiene, ligand 26b bearing a benzyl group gave better enantioselectivity and yield than others (Table 18, entry 18). Consistent with the previous observation, use of t-butyl p-nitro-perbenzoate resulted in enantioenriched allylic esters with higher stereoselectivites and yields in a shorter time than other p-substituted peresters. In addition, as expected, inorganic additives such as MS (4 Å), MCM-41, SBA-15, and HZSM-5 showed an increase in yields and enantioselectivities and also accelerated the reaction rate. However, HZSM-5 exhibited superior results compared with the other porous additives (Table 18). A key feature of this study, compared with other publications, was the decrease in the ratio of olefin to perester from 5–10:1 to 3:1 without affecting the obtained results. It should be noted that this protocol can be considered as an efficient procedure in the asymmetric allylic oxidation of cyclooefins, which gave chiral esters with high enantioselectivities (up to 94%) and yields (up to 95%).
3.1.2 Other Studies
In addition to the above-mentioned comprehensive researches, some short investigations have been reported, describing a number of notable oxazoline ligands.
In most allylic oxidations, perester is added directly to the reaction mixture, but in a different strategy by Feringa and Zondervan [86], perester was generated in situ from a mixture of propionic acid and t-butyl hydroperoxide. However, the enantioselectivities with thiophenic and phenolic oxazoline ligands 27 and 28, which were used in their study, were very low (Scheme 17).
A diverse range of compounds containing allylic and benzylic C–H bond has been studied by Schulz et al. with different oxidizing agents like perester, t-BuOOH, and t-BuOOH/acetic acid in the presence of Pybox 2c-Cu(I)OTf complex [87]. The corresponding allylic esters and peroxides were obtained with poor-to-moderate selectivities and yields. The asymmetric peroxidation of methyl cyclohexene, for example, led to chiral regioisomeric allylic peroxides with enantioselectivities in the range of 0–32%. It was also observed that diminishing the amount of catalyst from 17 mol% to around 1.5 mol% led to a drastic decrease in the level of asymmetric induction. However, this drop in selectivity was almost compensated for when MS were added. It seems that deactivation of the catalyst by water, generated from decomposition of tert-butyl hydroperoxide, is responsible for this drop. A similar phenomenon was observed in Katsuki’s studies with tetradentated tris-oxazoline ligands 4 [58, 59]. Additionally, allylic oxidation of the substituted cyclohexenes, especially 1-methyl cyclohexene, by using Pybox 2c-Cu(I), resulted in all three isomeric esters with higher enantioselectivities than those reported for BOX ligands [54], but with poor conversion. Besides, employing cyclohexene under conditions reported by Feringa and Zondervan [86] (a mixture of t-BuOOH/acetic acid), revealed competition between allylic acetoxylation and peroxidation (Scheme 18).
Using a series of chiral dinuclear bisoxazoline complexes, Fahrni [88] has shown that variation of the copper: ligand ratio strongly affects asymmetric induction, presumably due to changes in the structure of active catalyst species. However, the results obtained with this class of ligands were disappointing. For instance, under optimum conditions, cyclohexenyl benzoate was obtained around 38% ee and moderate yield (Table 19, entry 9). It is worth noting that analysis of the side products of the reaction in the presence of ligands 30 revealed formation of an imide product in low yield and enantioselectivity, which is formed through a sequence involving reaction of acetonitrile as solvent with the intermediate Cu(II)-benzoate complex (Scheme 19, Table 19, entries 5–7).
The extra ratio of olefin to perester 5–10 to 1 equivalent can be considered as a major drawback of allylic oxidation. This limitation has been partially solved by Christ and Sorokin [89]. Stepwise addition of t-butyl peroxybenzoate to the reaction mixture at a 1:1 molar ratio of cyclohexene to perester led to (S)-cyclohex-2-en-1-yl benzoate with good ee (68%) and excellent yield (99%) (Scheme 20).
Manas [90] tried to increase enantioselectivity by employing malonyl gem-dimethyl bisoxazoline 1g copper (I) complex bearing bulky and rigid adamantyl moiety, but this strategy was not so successful in comparison with the standard t-Bu-BOX (1c) [54]. The ee obtained in case of cyclopentene was up to 82% (Scheme 21).
Desimoni and Sinou [91] have evaluated the effect of introducing a further sterogenic center at C-5 of the oxazoline rings of malonyl gem-dimethyl and pyridine bisoxazoline ligands 34–37. It was observed that the malonyl ligands showed better yields and selectivity than the Pybox ligands. Oxidation of five- to seven-membered cycloalkenes in the presence of Cu (I)-complexes of these type of ligands led to allylic esters up to 84% ee and 80% yield, i.e., no better than the simple and more accessible standard BOX ligands 1 [54] (Table 20). It should be noted that the sense of the asymmetric induction is determined by the absolute configuration at C-4, while the additional stereogenic centers at C-5 induce a small-to-modest match/mismatch effect.
Additional steric congestion has been introduced by attaching various groups to the bridging carbon of the bisoxazolines. Gade and his colleagues have reported a series of bisxazoline-derivatives with one or two sidearms attached to the bridge carbon atoms [92]. Under optimum conditions in the presence of 5 mol% of Cu (II)-ligands 38–42 in combination with tert-butyl p-nitroperbenzoate at 20 °C, cyclohex-2-en-1-yl 4-nitrobenzoate was obtained with respectable levels of asymmetric induction (ee up to 85%) and moderate yield (52%) (Table 21, entry 8). It was shown that the ee is increased considerably by introducing two sidearms on the bisoxazolines. It seems that, as a result of coordination of 2-pyridyl groups to the Cu at the bridging C-atom of ligand 42a, the level of asymmetric induction and yield are better than ligand 42b with 3-pyridyl groups (Table 21, entries 5 vs 6 and 10 vs 11).
Malkov and Kocovsky [93] introduced pinene-derived pyridyl oxazolines 43, containing additional chiral elements in the pinene backbone, for allylic oxidation of five- to seven-membered cycloolefins. The use of this type of ligand in conjunction with copper (II) and phenyl hydrazine resulted in the corresponding chiral allylic esters in modest enantioselectivities and yields. In the best case, up to 67% ee was obtained in the oxidation of cycloheptene employing chiral ligand 43a in acetone at room temperature. It should be noted that changes in the stereochemistry of the ligand at the oxazoline moiety did change the configuration of the chiral allylic esters (Table 22, entries 12–14), reflecting the fact that enantiodiscrimination is influenced mainly by the chirality of the oxazoline ring rather than by the pinene unit.
In general, a drop in asymmetric induction is observed when the reaction temperature is raised. Pfaltz and coworkers [94] could partially overcome this drawback by designing boron-bridged bisoxazolines (Borabox) 44 prepared from chiral oxzolines and haloboranes. Higher electron density at the metal center of Borabox complexes, and a larger bite angle due to the longer bond between the boron atom and the oxazoline rings, are the main features of this type of ligand. It was observed that both enantioselectivity and yield were affected only moderately by the reaction temperature, and good-to-high enantiomeric excesses were obtained within a temperature range from –15 to + 80 °C (Table 23). Cyclopentene showed lower temperature dependence of the asymmetric induction compared with cyclohexene. At high temperature (+80 °C), cyclopentene and cyclohexene were oxidized with 76% and 65% enantioselectivities and good yields in 1 h and 11 h, respectively (Table 23, entries 6 and 9).
3.2 Heterogeneous catalysts
Due to potential benefits of heterogeneous over homogeneous catalyst systems such as easy handling as well as simple separation and also combination of these features with several reused cycles without considerable loss of catalyst activity, some attempts have been made to prepare chiral heterogeneous oxazoline-based catalysts during the last two decades [32, 33, 95,96,97]. Although only a small number of such heterogeneous catalysts have been applied in asymmetric allylic oxidation to date, remarkable results have been obtained and in some cases these catalysts can compete with homogeneous analogs.
The first study with heterogeneous catalyst was carried out in 2004 by Sinou and Bayardon [98], who prepared fluorous bis(oxazolines) with fluorine content between 52.7 and 58.7% by alkylation of the methylene bridge of bis(oxazolines) 45–51. Allylic oxidation of cycloalkenes at room temperature catalyzed by Cu (Ι)-ligand 45 and 46 complexes under biphasic conditions (FC72/CH3CN) gave the corresponding allylic benzoates in enantioselectivities of up to 77% ee and yields of up to 86% after 7 days (Table 24). These results were quite close to those obtained with analogous non-flourous bis(oxazolines) under similar conditions (Table 24, entry 7). Although the recycling of CuPF6-ligands 45 and 46 was unsatisfactory (Table 24, entries 9 and 14), allylic oxidation at 50 °C in a mixture of CHCl3/CH3CN allowed easy separation of the catalyst system. It should be noted that reusing the heterogeneous catalyst in another reaction, resulted in a considerable decrease in the enantioselectivity (Table 24, entry 4 vs 5). Increasing the fluorine content to 56.9% and 59.3% by using bis (oxazolines) 50 and 51 derived from amino acids (S)-serine and (S)-tyrosine gave (S)-cyclohex-2-en-1-yl benzoate in lower yield and enantioselectivity [99]. It was found that conducting the reaction in a mono-phasic solvent system (CHCl3/CH3CN) provided better results compared with the biphasic system FC72/CH3CN (Table 24, entries 23 vs 20–21). In fact, in comparison with ligand 50, when the reaction was carried out with ligand 51 at 50 °C, the rate was accelerated (2 days vs 7 days) and a slight increase in the ee and a drop in yield was observed (Table 24, entry 20 vs 21). Moreover, recovery and reuse of the catalyst did not show any change in enantioselectivy, while the yield was diminished slightly (Table 24, entry 22).
In an interesting approach, Reiser and Garcia [100] introduced a recoverable and reusable catalyst based on ditopic aza-bisoxazoline ligand (DaX 54) and Cu salts. This ligand is capable of coordinating with two copper ions, leading to the formation of linear polymers, where each Cu salt is linked to two bisoxazoline units. In contrast with conventional heterogeneous catalysts, these coordination polymers are disassembled by coordinating solvents such as acetone and acetonitrile and then converted to soluble homogeneous aza-bisoxazoline-copper complexes. However, the initial insoluble solid coordination polymer could be reassembled if the solvent was replaced with a non-coordinating solvent like n-hexane. Evaluation of this ingenious catalytic system in allylic oxidation of five- to seven-membered cycloolefins in the presence of copper (II) and phenylhydrazine at room temperature gave the chiral allylic products in good-to-high yield (up to 92%) and enantioselectivity (up to 90%) with outperforming analogous homogeneous catalysts derived from bis(oxazolines) and aza-bis(oxazolines) 52 and 53 (Table 25). N-methylated azabis(oxazoline) 53a showed better results than the corresponding unsubstituted ligand 52a (Table 25, entries 1–7). Besides, in agreement with most studies, the use of phenylhydrazine, as a reducing agent, for reducing Cu (II) to catalytically active Cu (I) is crucial (Table 25, entries 4, 5). The bulky tert-butyl substituted ligand 53b did not result in reasonable enantiomeric excess (Entry 6). Another notable feature of the self-supported ligand 54-Cu catalytic system is that it can be recycled easily by filtration and reused several times without considerable loss of reactivity and enantioselectivity. It was also observed that recharging the reaction with a small amount of the chiral ligand (0.5 mol %) after each recovery, maintained both the yield and the enantioselectivity relatively constant, whereas recharging with 0.5 mol % Cu(OTf)2 contributed to an increase in the yield and a small decrease in ee.
In continuation of our studies with mesoporous additives that had a notable effect on the enantioselectivity, our research group designed and synthesized a series of chiral 4-oxazolinylaniline ligands 55 on a gram scale from 4-amino benzoic acid in four steps and then covalently grafted them on ordered silica mesoporous MCM-41 (Scheme 22). Evaluating these heterogeneous catalysts showed that the most promising results were obtained with catalyst 56a in conjunction with Cu(CH3CN)4PF6 in acetonitrile at −10 °C and in the presence of phenylhydrazine (Table 26, entries 1, 5, 9, 13, 17). Although only a slight decrease in the enantioselectivity and yield was observed in the absence of phenylhydrazine, the reactivity was diminished significantly. The corresponding chiral allylic esters were obtained in moderate-to-good enantioselectivities (up to 80%) and high yields (up to 95%). 1,5-Cyclooctadiene was also oxidized with the highest enantioselectivity and yield (Table 26, entry 17), in line with our previous studies. Importantly, the catalyst could be recovered easily and reused five times without notable decrease in enantioselectivity, yield, and reactivity [32].
In our most recent study, amino oxazoline ligands 25 were immobilized covalently on mesoporous SBA-15 (Scheme 23) [101]. Consistent with our previous studies and the findings of Katsuki [58], the best results were achieved with phenyl- or benzyl-substituted oxazolines (57a or 57b), which can be attributed to the interaction between allyl radical intermediates and aryl substituents in the transition state. In contrast to most catalyst systems, reactions at room temperature provided remarkably high enantioselectivities. Although lowering the temperature led to a small increase in ee, both the reactivity and yield were markedly diminished (Table 27, entries 1 vs 6 and 7). Allylic oxidation by using t-butyl p-nitroperbenzoate and t-butyl o-iodoperbenzoate in conjunction with Cu(CH3CN)4PF6 in acetonitrile led to almost the same ee values and yields, which were higher than those obtained with other peroxybenzoates containing electron-donating or -withdrawing substituents at the ortho or para position (Table 27, entries 1 vs 8–13). This catalyst system gave higher yields (up to 95%) and enantioselectivities (up to 96%) for cyclic alkenes in comparison with the other reported heterogeneous catalysts (Table 27, entries 1 and 13–19). In the case of acyclic substrates such as 1-hexene, 1-octene, and allyl benzene, the reaction proceeded with poor yields of up to 23% and enantioselectivities of up to 18% (Table 27, entries 20–22). Moreover, the results have shown that the homogeneous N-Me-amino oxazoline (N-Me-25b) can induce chirality better than amino oxazoline 25b. Nevertheless, the enantioselectivity of heterogeneous catalysts 57b was higher than that of homogeneous analogues (Table 27, entries 1 vs 23, 24). This heterogeneous catalyst system can be recovered easily and applied to several consecutive catalytic runs without affecting the results significantly.
4 Conclusions
This review focused on the development of asymmetric allylic oxidation of olefıns in the presence of copper-oxazoline complexes. Although accomplished studies have been limited so far mostly to simple substrates, some general conclusions can be drawn. In the case of unsubstituted cyclic olefins, although accessible standard BOX at low temperature (−20 °C) functions as an effective ligand, the yields are only moderate and the rate of the reaction is slow. Use of Cu(CH3CN)4PF6 and CuOTf, and sometimes Cu(OTf)2, in acetonitrile and acetone was found to give the best results. Excellent enantioselectivity can be obtained using t-butyl p-nitroperbenzoate as an oxidant. Addition of a small amount of phenylhydrazine as a reducing agent, in most cases, leads to the acceleration of this slow reaction. Moreover, porous additives such as MS, SBA-15, and HZSM-5 have a beneficial effect on the enantioselectivities, yields and also on the rate of reaction. The high ratio of olefin to perester is a drawback that can be reduced from 10–5:1 to 3:1 by employing homogeneous 26-CuOTf catalysts. Only a few studies on heterogeneous ligands have been reported to date, but the results have been very promising. Simple aminooxazolines immobilized on SBA-15 (ligand 57) gave the corresponding enantioenriched allylic esters in high ees and yields at room temperature in shorter times than analogous homogeneous catalysts. It is noteworthy that this heterogeneous system can be recovered and reused easily without significant loss of catalytic activity. Despite considerable progress in this field, low regioselectivity remains as a major challenge for unsymmetric cyclic substrates and acyclic olefins. Moderate enantioselectivity and yield with high regioselectivity have been obtained for acyclic olefins only by spiro ligand 24. So far, there is still a lack of a reliable mechanistic model that could serve as a basis for the design of new catalysts. Thus, further studies will be needed to emphasize mechanistic aspects in order to obtain a clearer picture of the catalyst-substrate interactions and other factors that play a role in the reaction, such as additives, solvent, etc.
References
Kharasch M, Sosnovsky G (1958) The reactions of t-butyl perbenzoate and olefins a stereospecific reaction. J Am Chem Soc 80:756–756
Kharasch M, Sosnovsky G (1958) Structure of peroxides derived from dyclohexanone and hydrogen peroxide. J Org Chem 23:1322–1326
Kharasch M, Sosnovsky G, Yang N (1959) Reactions of t-butyl peresters. I. The reaction of peresters with olefins. J Am Chem Soc 81:5819–5824
Boumizane K, Herzog-Cance M, Jones D, Pascal J, Potier J, Roziere J (1991) Synthesis, vibrational spectroscopy and EXAFS analysis of some divalent and trivalent trifluoromethanesulphonato complexes. Polyhedron 10:2757–2769
Andrus MB, Lashley JC (2002) Copper catalyzed allylic oxidation with peresters. Tetrahedron 58:845–866
Eames J, Watkinson M (2001) Catalytic allylic oxidation of alkenes using an asymmetric Kharasch–Sosnovsky reaction. Angew Chem Int Ed 40:3567–3571
García-Cabeza AL, Moreno-Dorado FJ, Ortega Aguera MJ, Guerra Martínez FM (2016) Copper-catalyzed oxidation of alkenes and heterocycles. Synthesis 48:2323–2342
Black JR, Levason W, Webster M (1995) Tetrakis (acetonitrile-N) copper (I) hexafluorophosphate (V) acetonitrile solvate. Acta Cryst C51:623–625
Kubas G, Monzyk B, Crumblis A (1990) Tetrakis (acetonitrile) copper (1+) hexafluorophosphate (1−). Inorg Synth 28:68–70
Denney DB, Napier R, Cammarata A (1965) A convenient method for the preparation of some optically active allylic alcohols. J Org Chem 30:3151–3153
Levina A, Muzart J (1995) A convenient one-step catalytic method for obtaining optically active 2-cyclopentenyl benzoate from cyclopentene. Synth Commun 25:1789–1794
Muzart J (1991) Enantioselective copper-catalyzed allylic acetoxylation of cyclohexene. J Mol Catal 64:381–384
Levina A, Hénin F, Muzart J (1995) On the stability of the copper-(S)-proline catalyst in the enantioselective allylic acyloxylation of alkenes. J Organomet Chem 494:165–168
Södergren MJ, Andersson PG (1996) Chiral, bicyclic proline derivatives and their application as ligands for copper in the catalytic asymmetric allylic oxidation of olefins. Tetrahedron Lett 37:7577–7580
Rispens MT, Zondervan C, Feringa BL (1995) Catalytic enantioselective allylic oxidation. Tetrahedron Asymmetry 6:661–664
Hoang VD, Reddy PA, Kim T-J (2008) Asymmetric allylic oxidation catalyzed by copper (I) complexes of chiral (iminophosphoranyl) ferrocenes. Organometallics 27:1026–1027
Chelucci G, Loriga G, Murineddu G, Pinna GA (2002) Synthesis and application in asymmetric copper (I)-catalyzed allylic oxidation of a new chiral 1, 10-phenanthroline derived from pinene. Tetrahedron Lett 43:3601–3604
Lee W-S, Kwong H-L, Chan H-L, Choi W-W, Ng L-Y (2001) Chiral bipyridine–copper (I) complex-catalyzed enantioselective allylic oxidation of cyclic alkenes. Tetrahedron Asymmetry 12:1007–1013
Boyd DR, Sharma ND, Sbircea L, Murphy D, Belhocine T, Malone JF, James SL, Allen CC, Hamilton JT (2008) Azaarene cis-dihydrodiol-derived 2, 2′-bipyridine ligands for asymmetric allylic oxidation and cyclopropanation. Chem Commun 20:5535–5537
Tan Q, Hayashi M (2008) Novel N, N-bidentate ligands for enantioselective copper (I)-catalyzed allylic oxidation of cyclic olefins. Adv Synth Catal 350:2639–2644
Teng P-F, Tsang C-S, Yeung H-L, Wong W-L, Kwong H-L, Williams ID (2006) New chiral bidentate ligands containing thiazolyl and pyridyl donors for copper-catalyzed asymmetric allylic oxidation of cyclohexene. J Organomet Chem 691:2237–2244
Solinas M, Sechi B, Chelucci G (2014) Screening of N, N-bidentate and N, N, N-tridentate pyridine-based ligands in the catalytic allylic oxidation of cyclic olefins. Appl Organomet Chem 28:831–834
Faraji L, Samadi S, Jadidi K, Notash B (2014) Synthesis of novel chiral diamino alcohols and their application in copper-catalyzed asymmetric allylic oxidation of cycloolefins. Bull Korean Chem Soc 35:1989–1995
Fache F, Piva O (2003) Synthesis and applications of the first polyfluorous proline derivative. Tetrahedron Asymmetry 14:139–143
Fache F, Piva O (2002) Rapid and reusable copper catalytic system for allylic oxidation of olefins in hexafluoroisopropanol as solvent. Synlett 2002:2035–2036
Le Bras J, Muzart J (2003) Amino acid/copper-catalyzed enantioselective allylic benzoyloxylation of olefins in water promoted by diethylene glycol. Tetrahedron Asymmetry 14:1911–1915
Le Bras J, Muzart J (2002) Selective copper-catalyzed allylic oxidations using a 1/1 ratio of cycloalkene and tert-butylperbenzoate. J Mol Catal A Chem 185:113–117
Desimoni G, Faita G, Jørgensen KA (2006) C2-symmetric chiral bis (oxazoline) ligands in asymmetric catalysis. Chem Rev 106:3561–3651
Desimoni G, Faita G, Jørgensen KA (2011) Update 1 of: C2-symmetric chiral bis (oxazoline) ligands in asymmetric catalysis. Chem Rev 111:PR284–PR437
Samadi S, Nazari S, Arvinnezhad H, Jadidi K, Notash B (2013) A significant improvement in enantioselectivity, yield, and reactivity for the copper-bi-o-tolyl bisoxazoline-catalyzed asymmetric allylic oxidation of cyclic olefins using recoverable SBA-15 mesoporous silica material. Tetrahedron 69:6679–6686
Samadi S, Jadidi K, Notash B (2013) Chiral bisoxazoline ligands with a biphenyl backbone: development and application in catalytic asymmetric allylic oxidation of cycloolefins. Tetrahedron Asymmetry 24:269–277
Samadi S, Jadidi K, Khanmohammadi B, Tavakoli N (2016) Heterogenization of chiral mono oxazoline ligands by grafting onto mesoporous silica MCM-41 and their application in copper-catalyzed asymmetric allylic oxidation of cyclic olefins. J Catal 340:344–353
Braunstein P, Naud F (2001) Hemilability of hybrid ligands and the coordination chemistry of oxazoline-based systems. Angew Chem Int Ed 40:680–699
Gómez M, Muller G, Rocamora M (1999) Coordination chemistry of oxazoline ligands. Coord Chem Rev 193:769–835
Kangani CO, Kelley DE, Day BW (2006) One pot direct synthesis of oxazolines, benzoxazoles, and oxadiazoles from carboxylic acids using the Deoxo-Fluor reagent. Tetrahedron Lett 47:6497–6499
Wipf P, Venkatraman S (1997) From aziridines to oxazolines and thiazolines: the heterocyclic route to thiangazole. Synlett 1:1–10
Wuts PG, Northuis JM, Kwan TA (2000) The synthesis of oxazolines using the Vilsmeier reagent. J Org Chem 65:9223–9225
Cryder JL, Killgore AJ, Moore C, Golen JA, Rheingold AL, Daley CJ (2010) Novel metal complexes containing a chiral trinitrogen isoindoline-based pincer ligand: in situ synthesis and structural characterization. Dalton Trans 39:10671–10677
Fraile JM, García JI, Herrerías CI, Mayoral JA, Reiser O, Socuéllamos A, Werner H (2004) The role of binding constants in the efficiency of chiral catalysts immobilized by electrostatic interactions: the case of azabis (oxazoline)–copper complexes. Chem Eur J 10:2997–3005
Gissibl A, Finn M, Reiser O (2005) Cu(II)-Aza (bisoxazoline)-catalyzed asymmetric benzoylations. Org Lett 7:2325–2328
Lang K, Park J, Hong S (2010) Development of bifunctional aza-bis (oxazoline) copper catalysts for enantioselective henry reaction. J Org Chem 75:6424–6435
Pilsl LK, Ertl T, Reiser O (2017) Enantioselective three-step synthesis of homo-β-proline: a donor–acceptor cyclopropane as key intermediate. Org Lett 19:2754–2757
Kochi J (1962) The mechanism of the copper salt catalysed reactions of peroxides. Tetrahedron 18:483–497
Kochi J, Subramanian R (1965) Kinetics of electron-transfer oxidation of alkyl radicals by copper (II) complexes. J Am Chem Soc 87:4855–4866
Kochi JK (1962) Copper salt-catalyzed reaction of butenes with peresters. J Am Chem Soc 84:774–784
Kochi JK, Krusic PJ (1968) Isomerization and electron spin resonance of allylic radicals. J Am Chem Soc 90:7157–7159
Walling C, Thaler W (1961) Positive halogen compounds. III. allylic chlorination with t-butyl hypochlorite the stereochemistry of allylic radicals1. J Am Chem Soc 83:3877–3884
Walling C, Zavitsas AA (1963) The copper-catalyzed reaction of peresters with hydrocarbons. J Am Chem Soc 85:2084–2090
Beckwith AL, Zavitsas AA (1986) Allylic oxidations by peroxy esters catalyzed by copper salts. The potential for stereoselective syntheses. J Am Chem Soc 108:8230–8234
Mayoral JA, Rodríguez-Rodríguez S, Salvatella L (2008) Theoretical insights into enantioselective catalysis: the mechanism of the Kharasch–Sosnovsky reaction. Chem Eur J 14:9274–9285
Smith K, Hupp CD, Allen KL, Slough GA (2005) Catalytic allylic amination versus allylic oxidation: a mechanistic dichotomy. Organometallics 24:1747–1755
Zhu N, Qian B, Xiong H, Bao H (2017) Copper-catalyzed regioselective allylic oxidation of olefins via C–H activation. Tetrahedron Lett 58:4125–4128
Wang X-S, Zhao H, Li Y-H, Xiong R-G, You X-Z (2005) Olefin-copper (I) complexes and their properties. Top Catal 35:43–61
Gokhale AS, Minidis AB, Pfaltz A (1995) Enantioselective allylic oxidation catalyzed by chiral bisoxazoline-copper complexes. Tetrahedron Lett 36:1831–1834
Andrus MB, Argade AB, Chen X, Pamment MG (1995) The asymmetric Kharasch reaction. catalytic enantioselective allylic acyloxylation of olefins with chiral copper (I) complexes and tert-butyl perbenzoate. Tetrahedron Lett 36:2945–2948
Andrus MB, Chen X (1997) Catalytic enantioselective allylic oxidation of olefins with copper (I) catalysts and new perester oxidants. Tetrahedron 53:16229–16240
Kawasaki K, Tsumura S, Katsuki T (1995) Enantioselective allylic oxidation using biomimetic tris (oxazolines)-copper (II) complex. Synlett 20:1245–1246
Kawasaki K, Katsuki T (1997) Enantioselective allylic oxidation of cycloalkenes by using Cu (II)-tris (oxazoline) complex as a catalyst. Tetrahedron 53:6337–6350
Kohmura Y, Katsuki T (2000) Asymmetric allylic oxidation of cycloalkenes using a tridentate tris (oxazoline) ligand as a chiral auxiliary. Tetrahedron Lett 41:3941–3945
DattaGupta A, Singh VK (1996) Catalytic enantioselective allylic oxidation of olefins with copper complexes of chiral nonracemic bis (oxazolinyl) pyridine type ligands. Tetrahedron Lett 37:2633–2636
Sekar G, DattaGupta A, Singh VK (1998) Asymmetric Kharasch reaction: catalytic enantioselective allylic oxidation of olefins using chiral pyridine bis (diphenyloxazoline)—copper complexes and tert-butyl perbenzoate. J Org Chem 63:2961–2967
Ginotra SK, Singh VK (2006) Enantioselective oxidation of olefins catalyzed by chiral copper bis (oxazolinyl) pyridine complexes: a reassessment. Tetrahedron 62:3573–3581
Ginotra SK, Singh VK (2006) Studies on enantioselective allylic oxidation of olefins using peresters catalyzed by Cu (I)-complexes of chiral pybox ligands. Org Biomol Chem 4:4370–4374
Singh PK, Singh VK (2010) Enantioselective reactions catalyzed by chiral pyridine 2, 6-bis (5’, 5’-diphenyloxazoline)-metal complexes. Pure Appl Chem 82:1845–1853
Andrus MB, Asgari D (2000) Asymmetric allylic oxidation with biarylbisoxazoline-copper (I) catalysis. Tetrahedron 56:5775–5780
Andrus MB, Sekhar BS (2001) Synthesis and preliminary use of naphthyl quinazoline and isoquinoline oxazolines for asymmetric allylic oxidation. J Heterocycl Chem 38:1265–1271
Li ZP, Wu XY, Zhou QL, Chan WL (2001) Enantioselective allylic oxidation of olefins using chiral quinolinyl-oxazoline copper complex catalysts. Chin J Chem 19:40–44
Andrus MB, Zhou Z (2002) Highly enantioselective copper-bisoxazoline-catalyzed allylic oxidation of cyclic olefins with tert-butyl p-nitroperbenzoate. J Am Chem Soc 124:8806–8807
Thorhauge J, Roberson M, Hazell RG, Jørgensen KA (2002) On the intermediates in chiral bis (oxazoline) copper (II)-catalyzed enantioselective reactions-experimental and theoretical investigations. Chem Eur J 8:1888–1898
Johnson JS, Evans DA (2000) Chiral bis (oxazoline) copper (II) complexes: versatile catalysts for enantioselective cycloaddition, aldol, Michael, and carbonyl ene reactions. Acc Chem Res 33:325–335
Zhou Z, Andrus MB (2012) Naphthyl-substituted bisoxazoline and pyridylbisoxazoline–copper (I) catalysts for asymmetric allylic oxidation. Tetrahedron Lett 53:4518–4521
Reddy KL, Sharpless KB (1998) From styrenes to enantiopure α-arylglycines in two steps. J Am Chem Soc 120:1207–1217
Clark JS, Tolhurst KF, Taylor M, Swallow S (1998) Enantioselective allylic acyloxylation catalysed by copper–oxazoline complexes. J Chem Soc Perkin Trans 1:1167–1170
Clark JS, Clarke M-R, Clough J, Blake AJ, Wilson C (2004) Asymmetric allylic oxidation of bridged-bicyclic alkenes using a copper-catalysed symmetrising–desymmetrising Kharasch-Sosnovsky reaction. Tetrahedron Lett 45:9447–9450
Clark JS, Roche C (2005) Tuneable asymmetric copper-catalysed allylic amination and oxidation reactions. Chem Commun 20:5175–5177
Liu B, Zhu S-F, Wang L-X, Zhou Q-L (2006) Preparation and application of bisoxazoline ligands with a chiral spirobiindane skeleton for asymmetric cyclopropanation and allylic oxidation. Tetrahedron Asymmetry 17:634–641
Zhang B, Zhu S-F, Zhou Q-L (2013) Copper-catalyzed enantioselective allylic oxidation of acyclic olefins. Tetrahedron Lett 54:2665–2668
Anet F, Kozerski L (1973) Determination of conformational barriers in 1, 5-cyclooctadiene by proton and carbon-13 nuclear magnetic resonance. J Am Chem Soc 95:3407–3408
Anet FA, Easton NR Jr, Yavari I (1979) Carbon-13 nuclear magnetic resonance spectra and conformations of cis, cis-1, 5-cyclooctadiene monoepoxide and cis, syn, cis-1, 5-cyclooctadiene diepoxide. Org Magn Reson 12:299–301
Sauriol-Lord F, St-Jacques M (1975) Stereodynamic investigation of dibenzo-1, 5-cyclooctadiene and 5, 6, 11, 12-tetrahydrodibenzo [b, f][1, 4] diazocine derivatives. Can J Chem 53:3768–3776
Beck AK, Dahinden R, Kühnle FN (1996) Tartrate-derived ligands for the enantioselective LiAlH4 reduction of ketones: a comparison of TADDOLates and BINOLates. ACS Symp Ser Am Chem Soc 641:52–69
Meyers A, Himmelsbach RJ (1985) An enantioselective synthesis of 2, 2’, 6-trisubstituted biphenyls. J Am Chem Soc 107:682–685
Meyers A, Meier A, Rawson DJ (1992) A highly stereoselective synthesis of axially chiral biaryls. Application to the synthesis of a potential chiral catalysts. Tetrahedron Lett 33:853–856
Imai Y, Zhang W, Kida T, Nakatsuji Y, Ikeda I (2000) Novel chiral bisoxazoline ligands with a biphenyl backbone: preparation, complexation, and application in asymmetric catalytic reactions. J Org Chem 65:3326–3333
Samadi S, Jadidi K, Samadi M, Ashouri A, Notash B (2019) Designing chiral amido-oxazolines as new chelating ligands devoted to direct Cu-catalyzed oxidation of allylic C-H bonds in cyclic olefins. Tetrahedron 75:862–867
Zondervan C, Feringa BL (1996) Remarkable reversal of the non-linear effect in the catalytic enantioselective allylic oxidation of cyclohexene using copper proline complexes and t-butyl hydroperoxide. Tetrahedron Asymmetry 7:1895–1898
Schulz M, Kluge R, Gelalcha FG (1998) Asymmetric peroxidation of prochiral allylic and benzylic compounds with tert-butyl hydroperoxide and chiral bisoxazoline–copper complexes. Tetrahedron Asymmetry 9:4341–4360
Fahrni CJ (1998) Allylic oxidation catalyzed by chiral dinuclear copper complexes. Tetrahedron 54:5465–5470
Alvarez LX, Christ ML, Sorokin AB (2007) Selective oxidation of alkenes and alkynes catalyzed by copper complexes. Appl Catal A Gen 325:303–308
Clariana J, Comelles J, Moreno-Mañas M, Vallribera A (2002) 2, 2′-isopropylidenebis [(4R)-(1-adamantyl)-2-oxazoline](Adam-Box). A new enantiopure C2-symmetrical ligand: enantioselective cyclopropanations, Diels-Alder reactions, and allylic oxidations. Tetrahedron Asymmetry 13:1551–1554
Bayardon J, Sinou D, Guala M, Desimoni G (2004) Applications of enantiopure 4, 5-diphenyl substituted box and pybox ligands in asymmetric catalysis. Tetrahedron Asymmetry 15:3195–3200
Seitz M, Capacchione C, Bellemin-Laponnaz S, Wadepohl H, Ward BD, Gade LH (2006) Bisoxazolines with one and two sidearms: stereodirecting ligands for copper-catalysed asymmetric allylic oxidations of alkenes. Dalton Trans 20:193–202
Malkov AV, Stewart-Liddon AJ, Teplý F, Kobr L, Muir KW, Haigh D, Kočovský P (2008) New pinene-derived pyridines as bidentate chiral ligands. Tetrahedron 64:4011–4025
Köhler V, Mazet C, Toussaint A, Kulicke K, Häussinger D, Neuburger M, Schaffner S, Kaiser S, Pfaltz A (2008) Chiral boron-bridged bisoxazoline (borabox) ligands: structures and reactivities of Pd and Cu complexes. Chem Eur J 14:8530–8539
De Vos DE, Dams M, Sels BF, Jacobs PA (2002) Ordered mesoporous and microporous molecular sieves functionalized with transition metal complexes as catalysts for selective organic transformations. Chem Rev 102:3615–3640
Li C, Zhang H, Jiang D, Yang Q (2007) Chiral catalysis in nanopores of mesoporous materials. Chem Commun 20:547–558
Fan Q-H, Li Y-M, Chan AS (2002) Recoverable catalysts for asymmetric organic synthesis. Chem Rev 102:3385–3466
Bayardon J, Sinou D (2004) Enantiopure fluorous bis (oxazolines): synthesis and applications in catalytic asymmetric reactions. J Org Chem 69:3121–3128
Bayardon J, Sinou D (2005) Synthesis of two new chiral fluorous bis (oxazolines) and their applications as ligands in catalytic asymmetric reactions. Tetrahedron Asymmetry 16:2965–2972
Aldea L, Delso I, Hager M, Glos M, García JI, Mayoral JA, Reiser O (2012) A reusable enantioselective catalytic system for the Kharasch–Sosnovsky allylic oxidation of alkenes based on a ditopic azabis (oxazoline) ligand. Tetrahedron 68:3417–3422
Samadi S, Ashouri A, Samadi M (2020) Synthesis of chiral allylic esters by using the new recyclable chiral heterogeneous oxazoline-based catalysts. ACS Omega 5:22367–22378
Acknowledgements
We are grateful to the University of Kurdistan Research Councils for the partial support of this work. The authors gratefully thank Prof. Andreas Pfaltz (University of Basel) for his helpful suggestions, consultations, and all his kindness. We would also wish to offer our deepest gratitude to Dr. Khosrow Jadidi (Shahid Beheshti University) for his efforts in the development and promotion of asymmetric synthesis in Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Samadi, S., Arvinnezhad, H., Nazari, S. et al. Enantioselective Allylic C–H Bond Oxidation of Olefins Using Copper Complexes of Chiral Oxazoline Based Ligands. Top Curr Chem (Z) 380, 20 (2022). https://doi.org/10.1007/s41061-022-00375-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-022-00375-9