Abstract
As an option for genetic disease treatment and an alternative for traditional cancer chemotherapy, gene therapy achieves significant attention. Nucleic acid delivery, however, remains a main challenge in human gene therapy. Polymer-based delivery systems offer a safer and promising route for therapeutic gene delivery. Over the past five decades, various cationic polymers have been optimized for increasingly effective nucleic acid transfer. This resulted in a chemical evolution of cationic polymers from the first-generation polycations towards bioinspired multifunctional sequence-defined polymers and nanocomposites. With the increasing of knowledge in molecular biological processes and rapid progress of macromolecular chemistry, further improvement of polymeric nucleic acid delivery systems will provide effective tool for gene-based therapy in the near future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Gene therapy is a disease treatment approach that introduces exogenous nucleic acids into specific cells for modulating the gene expression [1]. Benefiting from the advances of nucleic acid research in biology and chemistry, the therapeutic nucleic acids already expanded from plasmid DNA (pDNA) to messenger RNA (mRNA), small interfering RNA (siRNA), microRNA (miRNA), antisense oligonucleotides, or even genome editing systems [2]. For instance, systemic delivery of CRISPR/Cas9 mRNA, guide RNA and repair template DNA for therapeutic genome editing in mice was recently reported [3].
Since 1989, numerous clinical trials of gene therapy have been approved worldwide [4]. However, success in clinical trials was hampered by numerous extracellular and intracellular barriers (Fig. 1) for gene delivery vectors, such as nucleic acid packaging, serum stability, targeting to specific cells, cellular internalization, endosomal escape, efficient intracellular unpackaging, and transport to specific organelles [5–7]. Viral vectors have evolved to overcome these barriers, but coming with some fundamental drawbacks, like toxicity [8], immunogenicity [9], limited nucleic acid capacity [10] and difficult production upscale [11]. Synthetic cationic polymers came out as a new opportunity for better safety and easier large scale manufacture [6, 7] with efficient condensation of larger nucleic acids into nanoscale particles called polyplexes and effective transfection of gene into cells [5, 12]. Meanwhile, benefiting from the flexibility in chemical synthesis, a large variety of cationic polymers with different cationic backbones was designed for improved nucleic acid delivery [5, 6, 12]. It is also feasible to synthesize virus-inspired multifunctional polymeric gene delivery systems by incorporating all kinds of functional units into the polyplexes, such as targeting ligands, shielding domains, endosomolytic units and nuclear localization signals, to conquer the biological hurdles [5, 12, 13]. In summary, nucleic acid transfer by polymeric materials may have great advantages in gene therapy. Nevertheless, only few clinical trials with polymeric carriers were performed [14–25], because of their previous lower efficiency in gene delivery as compared with viral vectors. Therefore, great efforts remain to be taken to improve further polymeric gene delivery systems for effective human genetic therapy.
In this review, we outline the historical evolution of polymer-based gene delivery systems during the past 50 years. The strengths and weaknesses, as well as structure–activity relations of developed cationic polymers will be discussed in the following sections.
2 First-Generation Polycations: DNA Binding, Compaction, and Targeting
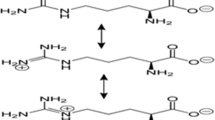
Our learning curve of polymeric gene delivery systems initiated from the first-generation polycations (Fig. 2). In 1962, Smull and Ludwig reported that cationic proteins could significantly enhance the infectivity of poliovirus RNA, which opened the door for polycation-based gene transfection [26]. In 1965, Pagano and colleagues found that diethylaminoethyl (DEAE) dextran (Fig. 2a) could strongly enhance poliovirus RNA transfection [27]. Similar findings on SV40 viral DNA were demonstrated subsequently [28]. Besides DEAE dextran, other cationic polymers, such as polyornithine, polylysine, and polyarginine (Fig. 2a), also had the potential for transferring nucleic acids into cells. Their efficiency for exogenous DNA transfection was evaluated in comparison by Farber and Butel in 1975; as a result, polyornithine presented the best performance [29]. In the early 1990s Kabanov & Kabanov designed poly(N-alkyl-4-vinylpyridinium) salts (Fig. 2a) as transfection agents [30, 31]. Our understanding of the interaction between cationic polymers and negatively charged nucleic acids was further deepened by the finding that cationic polymers not only bind nucleic acids, but also spontaneously condense them into compact structures (Fig. 2c–e) with specific shapes and sizes which resemble natural viruses [32, 33]. Most recent research using pair correlation microscopy by Hinde and Gaus showed that polymeric nanoparticles with same surface modifications, but different shapes presented different moving rates through the diverse cellular obstacles, which may be regarded as a reference for the role of polyplex shape in gene delivery [34].
First-generation polycations for gene delivery. a DEAE-dextran diethylaminoethyl-dextran; PLL poly-l-lysine; PLO poly-l-ornithine; PLR poly-l-arginine; PVP poly(N-alkyl-4-vinylpyridinium) bromide. b First targeted gene delivery polymer by Wu and Wu 1987, PLL-asialoorosomucoid (ASOR) conjugate. c Compaction of pDNA by transferrin-polylysine by Wagner 1990 and 1991 into donut-like nanoparticles (top) with 100 nm size, comparable with natural adenovirus (bottom) dimension. d Compaction of T4 DNA with poly (ethylene oxide) by Laemmli 1975. Adapted from [32] with kind permission from National Academy of Sciences and Professor U. K. Laemmli. e Compaction of T7 DNA with spermidine by Chattoraj 1978 into donut-like nanoparticles. Adapted from [33] with kind permission from Elsevier
A milestone in the progress of polymer-based gene delivery came out in 1987 with the report of receptor-mediated gene transfection by George and Catherine Wu [35, 36]. They first introduced asialoorosomucoid (ASOR), a ligand for asialoglycoprotein receptor specifically expressed on hepatic cells, into polylysine and combined this conjugate with DNA to form receptor-targeted polyplexes (Fig. 2b). They could verify asialoglycoprotein receptor-mediated DNA transfection via enhanced cellular internalization of plasmid DNA into hepatocytes both in vitro and in vivo into livers upon intravenous administration in rat. The CAT marker gene expression was transient. Gene transfer in combination with partial hepatectomy, which during liver regeneration triggers intensive hepatocyte proliferation, resulted in transgene integration and prolonged gene expression after 11 weeks [37, 38]. Using the receptor-targeted pDNA complexes, partial correction of genetic liver defects in experimental animals was possible [39, 40]. Following on this breakthrough, other receptor targeted gene delivery systems were developed as reviewed in [5, 41]. Published in 1990, Birnstiel, Wagner and colleagues investigated transferrin-protamine and transferrin-polylysine for their transferrin receptor-specific nucleic acid transfer efficiency into tumor and hematopoietic cells [42–44]. In 1993 Pam Davis and colleagues demonstrated gene transfer into respiratory cells by targeting the polymeric immunoglobin receptor [45].
Although the incorporation of ligands into polyplexes improved the gene delivery into specific cells, the efficiency of polylysine-based gene delivery was still moderate [46]. For example, Cotten and colleagues found intensive uptake of transferrin-polylysine/DNA complexes into human transferrin receptor overexpressing K562 leukemia cells, but insignificant gene expression [43]. Luthman and Magnusson had established in 1983 that the lysosomotropic anti-malaria drug chloroquine could significantly enhance DEAE-dextran mediated gene transfection [47]. Consistently, transferrin-polylysine mediated targeted DNA transfer into K562 cells was strongly enhanced by this treatment [43]. In those days the detailed mechanism of the chloroquine enhancement was unclear; hypothetical modes of action ranged from block of lysosomal acidification and thus rescue of endo-lysosomal DNA from enzymatic degradation, to dissociation of DNA complexes by chloroquine, facilitating intracellular release of DNA [48, 49].
The effectiveness of chloroquine indicated that some biological barriers hindered the highly effective transfection of polyplexes. The effectiveness of chloroquine depended on the natural endosomal acidification process mediated by the vacuolar ATP-dependent vATPase [48–50]. vATPase actively pumps protons into the early endosomes or late endosomes and lysosomes and keeps an acidic microenvironment in the early endosomes (pH 5.9–6.5) or late endosomes and lysosomes (pH 5.0) [51, 52]. Unprotonated weakly basic chloroquine can freely diffuse into the cells, but is trapped as protonated form in the acidifying organelles endosomes and lysosomes. With continuous protons uptake by vATPase, the protonated chloroquine keep gathering in the vesicles coming with continuous internalization of chloride counter ions and water, consequently leading to the osmotic swelling and membrane disruption of endosomes or lysosomes.
Realizing the bottleneck of endo-lysosomal entrapment, more active measures for cytosolic delivery were followed, similar to those which natural viruses use in their cellular infection process. In 1991 Curiel and Cotten introduced inactivated adenoviruses [53, 54] to enhance nucleic acids transfection by co-incubation with receptor-targeted gene transfection systems. Later on also rhinoviruses were used for this purpose [55]. It was assumed that the viruses promoted the nucleic acids transfer through enhancing the endosomal escape of the co-delivered polyplexes with their nature-derived endosome membrane destabilization domains. Further refinements were carried out by Wagner et al. via direct attachment of whole inactivated adenoviruses (Fig. 3) via various couplings including a biotin-streptavidin linkage [56, 57]. A series of related virus-linked systems were subsequently developed presenting high efficacy in vitro and in vivo [58–70]. The exceptionally efficient gene transfer activity of AVET (adenovirus-enhanced transferrinfection) even in case of “difficult-to-transfect” primary cells and primary tumor cell isolates, resulted in the first polymer-based human gene therapy trial in 1994 [14, 15]. In this study, GMP production of autologous melanoma vaccines transfected with IL-2 pDNA was performed for each individual melanoma stage 4 patient.
Adenovirus enhanced transferrinfection (AVET). a Chemically (8-methoxypsoralen/UV light) inactivated biotinylated adenovirus particles attached to streptavidine-polylysine/transferrin-polylysine/pDNA polyplexes. Reproduced with kind permission from Dr. Matthew Cotten. b Receptor-mediated uptake and endosomal escape of AVET. Adapted from [71] with kind permission from Elsevier
For endosomal escape, instead of whole viruses, proteins were tested. The group of Lee enhanced cytosolic delivery of pDNA by a sulfhydryl-activatable listeriolysin O/protamine conjugate [72]; Gottschalk et al. introduced a prefringolysin O into polyplexes via a biotin-streptavidin linkage [73]. The group of Wels designed recombinant multidomain proteins including receptor-binding, toxin derived endosomal escape, and pDNA binding motifs [74–76]. Influenza virus hemagglutinin subunit HA2-derived synthetic fusion peptides named INF (Fig. 4) were incorporated by Plank and Wagner into receptor-targeted polylysine/pDNA polyplexes, which could also strongly enhance gene transfer [77–81]. As part of this series, Plank developed in 1992 the first synthetic “artificial virus” based on polylysine conjugated with a synthetic INF peptide and a synthetic tetra-antennary galactose ligand for the hepatocyte asialoglycoprotein receptor [78]. As endo-lysosomal entrapment remains a substantial bottleneck also for the majority of novel nucleic acid nanoparticle types, analogous active peptide modifications are still relevant [82–84]. Also, artificial amphipathic endosomolytic peptides such as GALA and KALA, GALA-INF, EGLA, JTS1, or H5WGY have been introduced into polyplexes by the group of Szoka [85, 86] and other labs [79, 87–90]. Photochemical internalization (PCI) presents an alternative method developed by Berg and Hogset to enhance gene transfer by loading intracellular vesicles with a photosensitizer followed by photoinduced endosomal release of co-internalized polyplexes [91–93].
N-Terminal sequence of the influenza virus hemagglutinin subunit HA-2 at neutral and acidic pH. Adapted from [79] with kind permission from the American Society for Biochemistry and Molecular Biology
3 Second-Generation Polycations: Proton Sponges or Endosomolytic Polymers
The next step in development of polymeric gene delivery systems included polymers with inherent endosomal escape characteristics, to avoid the need of additional agents. Since the early 1990s, the lab of Frank Szoka developed polyamidoamine (PAMAM) dendrimers [85, 94, 95], which were found particularly effective in pDNA transfer also in the absence of chloroquine or fusion peptides. These polyamine dendrimers (Fig. 5a) are “proton sponges”, displaying at neutral pH only moderate protonation of nitrogens, which continuously increases with endosomal acidification. At demonstrated in subsequent mechanistic studies [96], this increased density of positive charge density leads to an influx of chloride and water into the endosome. As a consequence of the “proton sponge effect”, the endosome bursts presumably due to the elevated osmotic pressure and the membrane destabilizing effect of positively charged polymer domains. The classical gene delivery polymers such as polylysine or polyarginine could not meet this criterion because of their complete protonation at neutral pH, enabling only nucleic acid binding, but not endolysosomal buffering.
Proton sponge polymers. a Polyamidoamine (PAMAM) dendrimers, branched polyethylenimine (BPEI), linear polyethylenimine (LPEI), gluconic acid-modified polyhistidine (G-pHis) and histidylated polylysine (His-pLK). b Schematic illustration of the proton-sponge hypothesis. The protonation of polymers with proton-sponge effect mediates the influx of protons, chloride counterions and water into endolysosomes, resulting in the increased osmotic pressure and endolysosomal swelling and then, facilitated by interaction of cationized polymer with phospholipid membrane, the rupture of endolysosomes
Jean-Paul Behr and coworkers had previously demonstrated the positive transfection characteristics of the cationic lipo-spermine DOGS [97, 98]. They hypothesized a favorable endosomal buffering effect by the ionizable spermine head group, and, therefore, screened a series of commonly available cationic polymers having proton-sponge characteristics. Polyhistidine has buffer capacity, but no sufficient cationization at physiological pH for effective nucleic acid binding. The hypothesis-driven search resulted in the discovery of an abundantly available and powerful transfection polymer, polyethylenimine (PEI) (Fig. 5a) [99–102]. Owing to the high transfection efficiency both in vitro and in vivo and only moderate cytotoxicity, clinical trials in localized applications with modified PEI and derivatives were approved and performed [103]. A huge diversity of branched PEI (BPEI) and linear PEI (LPEI) with different molecular weights can be produced. As benefit from this chemical flexibility, one may tune the stability, transfection efficacy and cytotoxicity of PEI polyplexes [104–106]. Because of the necessity of nucleic acid unpackaging at the targeting organelles, it might be contra-productive to keep a high polyplex stability for the whole transfection process. Itaka and colleagues found that, compared with poly-l-lysine (PLL) formed DNA polyplex, both LPEI and BPEI based polyplexes were able to mediate endosomal escape, but LPEI polyplexes presented higher efficiency in gene transfection and expression compared with BPEI polyplexes. This was attributed to the higher stability of BPEI polyplexes with limited payload unpacking [105]. The less stable LPEI polyplexes also exhibited better nucleus delivery in non-dividing cells. The intranuclear transfer of BPEI polyplexes however correlated with the breakdown of nuclear membrane, which was more efficient for dividing cells that were in or before mitosis [107–109].
Although the detailed mechanism of proton sponge effect by PAMAM dendrimer, PEI and other similar transfection polymers remains under debate [13, 96, 106, 110–116], significant proof sustains the proton sponge hypothesis at least in slightly modified form (Fig. 5b). The effect of proton sponge strongly depended on the vATPase-mediated proton internalization into endo-lysosomes. Bafilomycin, a specific inhibitor for vATPase, hindered the transfection, while the transfection was enhanced by chloroquine that becomes protonated itself. Free PEI, unattached to polyplexes, but co-delivered with the polyplexes, promotes the endosomal escape [116]. Delayed acidification process, chloride gathering, and accidental disappearance of vesicles have been verified. Some new mechanistic models of the proton sponge are also reported [13, 114, 115]. Benjaminsen and Andresen found that the proton sponge of PEI did not notably change the pH of lysosomes, which is not contradictory, but a potent evidence for the PEI cationization. Some critical calculations presented that the osmotic pressure deriving from the proton sponge effect was not powerful enough to rupture endosomes. Furthermore, some cationic polymers with proton sponge effect do not mediate nucleic acid transfection [117]. Even introducing polyethylene glycol (PEG) molecules to the PEI polyplex surface could block the transfection activity, which is recovered by removing acid-cleavably linked PEG in endo-lysosomes [118–120]. Besides the osmotic effect of proton sponge, these reports are in agreement with the hypotheses that higher cationization of the PEI molecules in protonation-dependent way results in the direct endosomal membrane interaction and permeabilization. Such lytic effects have been found during the studies of high-density polycations, for example in erythrocyte membrane destabilization in the absence of osmotic pressure. Presumably synergistic effects exist depending both on the increasing osmotic pressure and direct destabilizing membrane contact. More recently, a thermosensitive nanoparticle swelling was applied for cytosolic siRNA delivery. siRNA pluronic/PEI2 K nanocapsules of 100 nm size at 37 °C during transfection into cells undergo subsequent swelling to a size of 400 nm upon cooling to 15 °C, which mechanically induced endosomal disruption [121].
Based on the hypothesis of the proton sponge, some buffering units with a pK a around 6 have been introduced into polymers to enhance their buffering ability, yielding polymers such as histidinylated polylysine or imidazole-containing biopolymers [122, 123] (Fig. 5a). The positive effects of these buffering units on gene delivery efficiency were reported by many studies [124–128].
Polyethylenimine, as the classical proton sponge polymer, presents many favorable properties on nucleic acid transfection, but also displays obvious disadvantages: it is not degradable and significantly toxic in a molecular weight-dependent manner [129, 130]. The cytotoxicity of PEI contains the defects on cell surface and mitochondria or nucleus membranes, inducing apoptosis and necrosis, or also the inhibition of ATP synthesis in mitochondria [131]. In addition to the direct cytotoxicity, many cationic polymers such as PEI and polylysine are recognized by the innate immune system and induce complement activation both in vitro and in vivo [132]. For instance, this led to strong anaphylactic reactions in pigs upon intravenous administration of PEI [133].
Amphiphilic cationic polymers (Fig. 6) present a second class of carriers with endosomal escape capacity. Allan Hoffman and collaborators synthesized pH-sensitive amphiphilic polymers containing hydrophobic alkyl groups and hydrophilic acidic carboxyl groups, which could enhance the disruption of lipid membranes because of the increased hydrophobicity when protonated in acidic pH. They further polymerized a functional vinyl monomer, pyridyl disulfide acrylate (PDSA) with butyl acrylate and methacrylic acid to generate the redox-sensitive and pH-dependent membrane disruption polymers to enhance cytosolic delivery of functional molecules such as antisense oligonucleotides. However, these amphiphilic polymers themselves suffered from inefficient DNA binding ability because of the lack of a cationic backbone. To promote the DNA binding ability of amphiphilic polymers, Rozema and colleagues synthesized amphiphilic cationic polyvinyl ethers. They introduced amino groups into these polymers to form stable DNA complexes, simultaneously incorporated alkyl groups containing one to four carbons to disrupt the membrane. The membrane-disruptive activity was found to be dependent on the carbon chain length. The DNA transfection efficiency of these polyvinyl ethers depended on their membrane-disruptive activity. The polyvinyl ethers containing butyl groups obtained highest nucleic acid transfection efficiency compared with the ones containing shorter alkyl groups [134–137].
Amphiphilic polymers. a PPAAc, poly(propyl acrylic acid); PEAAc, poly(ethyl acrylic acid); EA-AAc, random 1:1 copolymers of ethyl acrylate (EA) and acrylic acid (AAc); Poly(MAAc-co-BA-co-PDSA), poly(methacrylic acid-co-butyl acrylate-co-pyridyl disulfide acrylate). b Amphiphilic cationic polyvinyl ethers containing methyl, ethyl, propyl or butyl groups (from left to right)
To sum up, the first two generations of polymers provided efficient gene transfer reagents, deepening also our understanding of gene delivery. It, however, was critically required to reduce unspecific effects and the cytotoxicity of cationic polymers. Therefore, as will be outlined in the following sections, biocompatible polymers such as nature-derived polymers, biodegradable polymers, or nanoparticle-shielding polymers remained to be synthesized, ideally in pure form with maximum precision.
4 Third-Generation Polycations: Biodegradable and Biocompatible
The research of cationic polymers has been dominated by three aims: higher transfection efficiency, improved biocompatibility, and refined pharmaceutical precision. Learning from the lessons of first-generation polymers, multiple efforts were taken to improve the biocompatibility of polymer-based gene delivery systems.
Nature-derived polymers are advantageous templates for the generation of biodegradable and increasingly biocompatible polymers [138]. Chromosomal proteins such as HMG1 [139, 140] or histones [141] with or without modifications were already used for nucleic acid transfection. Cationic forms of collagen including gelatin (thermally denatured collagen, extracted under acidic conditions) and atelocollagen (derived from native collagen, with the terminal telo-peptides enzymatically removed) were also investigated as gene delivery vectors [142, 143]. Atelocollagen/siRNA polyplexes presented effective systemic siRNA delivery into bone metastatic tumor. The previously mentioned DEAE-dextrans are also nature-derived polymers based on carbohydrates.
Many researchers have reported that chitosan (poly-d-glucosamine, generated by deacetylation of chitin) (Fig. 7a) and derivatives showed great potential as nucleic acid delivery carriers [144, 145]. Further chemical modification of chitosan such as PEGylation or methylation could effectively improve the colloidal stabilization and formation of DNA polyplexes [146, 147]. Other favorable modifications contains histidinylation for facilitating endosomal escape [148], targeting ligands, or thiolation for improvement in cellular internalization [145, 149].
Cyclodextrins (CD) including alpha-, beta-, or gamma forms are degradable carbohydrate-based polymers with favorable biocompatibility. They were used to modify other cationic polymers. Beta-CD is also well known for its ability to form a CD-adamantane host–guest interaction. For example, introduction of beta-CD into LPEI and BPEI (Fig. 7b) decreased the toxicity of polyplexes, but kept the transfection efficiency high [150]. Using the host–guest interaction between beta-CD and adamantane, adamantly-PEG was inserted into PEI polyplexes, and the resulting PEGylated PEI polyplexes were used for systemic nucleic acid delivery to the liver [151]. Ping and Li recently reported low molecular weight PEI-CD based pDNA polyplexes containing an adamantly-modified peptide ligand for fibroblast growth factor receptors (FGFRs) and adamantly disulfide-connected PEG molecules for shielding [152].
Hwang and Davis synthesized linear cationic β-cyclodextrin-based polymers (β-CDPs) via amidine group cross-linkages. The CD modified polycations presented better biocompatibility than the one lacking CD modifications [153]. Furthermore, the CD units on these polycations was applied to introduce the receptor-targeting serum protein transferrin through CD-adamantane host–guest interaction [154]. Davis and colleagues further improved this concept into PEG-shielded, transferrin receptor-targeted therapeutic siRNA polyplexes [155]. With these polyplexes (Fig. 8) they evidenced for the first time that intravenous administration of siRNA can mediate gene silencing in metastatic tumors of humans as published in 2010 [22]. Polycationic CDs could be also synthesized by direct incorporation of heptakis-pyridylamino moieties [156]. To tune the cationic modification degree of polymers, Ooya and Harashima synthesized biocleavable polycationic polyrotaxanes with monocationic CDs cascaded on degradable linear polymer chains, which unraveled enhanced gene transfection efficiency [157].
Synthetic biodegradable polymers are another direction to increase the biocompatibility of polyplexes. We already learned that polycations such as polylysine and PEI present highly molecular weight dependent cytotoxicity. For instance, PEI with a low molecular weight (MW) of 800 exhibits low cellular toxicity but also low transfection efficiency, whereas linear PEI 22 K or branched PEI 25 K are more powerful, but have remarkable cytotoxicity, and PEI 800 K has high cytotoxicity and moderate transfection activity. To combine the advantages of low molecular weight polymers (low cytotoxicity) and high molecular weight polymers (high transfection efficiency), biodegradable polymers were designed and generated by assembling low molecular weight polymers into high molecular weight conjugates by bioreversible linkages.
One direction has been to synthesize various biodegradable cationic conjugates with low molecular weight oligoethylenimine (OEI) via bioreversible linkages [158], for example, ketals [159, 160], imines [161], disulfides [162, 163], ester bonds [164, 165], polyglutamic acid amide, and other amides [166–169] (Fig. 9). Introduction of bioreversible linkages such as hydrolyzable ester bonds in intra- or extracellular locations, endosomally degradable imine or acetal bonds, or bioreducible disulfide bonds in cytosol into cationic polymer backbone obviously increased biocompatibility and maintained or even promoted nucleic acid transfection efficiency.
Another direction is to synthesize new polymers with cleavable backbones. Sung Wan Kim and collaborators performed pioneering work published in 2000 by replacing the amide bonds in polylysine with ester bonds; consequently, nontoxic and biodegradable analog poly(alpha-(4-aminobutyl)-l-glycolic acid) (Fig. 10a) was generated [170]. Although the transfection efficiency was enhanced up to twofold compared with polylysine, it remained moderate, probably due to the lack of effective endosomal escape ability. More effective hyperbranched network-type poly(amino esters) named as n-PAE (Fig. 10b) and poly(beta-amino esters) were synthesized through Michael addition of amines to acrylate esters [171, 172]. n-PAE presented comparable transfection efficiency and endosomal escape ability to PEI, but a far higher biocompatibility [171]. Linear beta-amino ester cationic polymers were also optimized by reacting 4-aminobutanol with 1,4-butanediol diacrylate or 1-6-hexanediol diacrylate and exhibited effective gene transfection [172]. To find better gene delivery polymers, the laboratory of Robert Langer set a next conceptual milestone around 2001. Based on a combinatorial Michael addition approach, they added a variety of primary amines or secondary diamines to diverse bisacrylates (Fig. 10c) and obtained a library including 2350 biodegradable cationic polymers. With a large-scale screen on transfection efficiency, some interesting candidates were identified [173–175]. This approach was further applied to other library chemistries. For example, introduction of aliphatic hydrocarbon-associated epoxides, combined with a high throughput screen, resulted in a series of hydrophobic oligocationic polymers termed lipidoids, which were used for siRNA transfection [176, 177]. Also in polylactides, the ester bonds present a biodegradable character. Cheng and colleagues synthesized cationic polymeric nanocapsules from tertiary amine- and allyl-functionalized polylactide and a bis-(thiol) crosslinker, applying thiol-ene chemistry. They used these carriers for pDNA and siRNA delivery [178–180]. The group of Kataoka introduced small protonatable oligoamines onto the degradable polymeric amide backbone of poly(aspartate) via the amidation of the side chain of polyaspartic acid (Fig. 10d) [114, 181–183].
Biodegradable cationic poly(amino esters). a Poly(α-(4-aminobutyl)-l-glycolic acid). b Hyperbranched network-type poly(amino ester) (n-PAE). c Combinatorial design and generation of β-amino polyesters by Michael addition. d Poly(aspartic acid) amidated with different oligoamines containing diethylenetriamine (DET), triethylenetetramine (TET), or tetraethylenepentamine (TEP)
Michael addition, similarly to studies by Langer and colleagues, was also used by the groups of Engbersen, Kim and Feijen [184, 185] for the synthesis of disulfide bond-based biodegradable polymers (Fig. 11a). As amine reactants, small oligoethylenimines such as triethylene tetramine were reacted with cystamine bis-acrylamide, generating effective and biodegradable PEI analogs. With a different synthetic route, Lu and collaborators first generated defined bioreducible oligomers (Fig. 11b) including triethylene tetramine, histidines, and disulfide-forming cysteines by solid-phase-assisted synthesis followed by oxidative disulfide formation [186].
In parallel to the development of novel nature-derived or biodegradable polymers, the increasing knowledge of the relevant transfection mechanisms resulted in the modification of existing effective polymers. For example, PEI has limited transfection efficiency for siRNA. Simple modifications of 10% nitrogens of a 25 kDa branched PEI by succinylation [187], or 20% nitrogens with tyrosine residues [188], or pyridylthiourea grafting [189] made this PEI molecule a potent siRNA delivery polymer. Introduction of uncharged hydrophilic moieties such as PEG molecules [118, 190–192], carbohydrates [150, 193], poly[N-(2-hydroxypropyl) methacrylamide] (pHPMA) [194], hyaluronic acid [195, 196], hydroxyl ethyl starch [197] or polysarcosine [198] strongly decreased the toxicity and increased the biocompatibility of the synthesized polycations. PEG-pLys based DNA polyplexes were the first ones used in epithelia of cystic fibrosis patients in human clinical trials [17, 199].
As thought before, PEG reduced non-specific cellular interactions in a stealth effect by hindering protein adsorption. Recently, Schöttler and Wurm found that besides reducing protein adsorption, PEG and poly(ethyl ethylene phosphate) (PEEP) could also regulate the composition of protein corona by enriching clusterin proteins around nanocarriers, which are distinct proteins required to reduce non-specific cellular internalization, and PEEP presented higher clusterin enrichment than PEG [200]. This finding may contribute to the future design of shielding polymers, for example, the screen of more potent shielding polymers by identify their functions on clusterin enrichment.
In general, several directions have been explored to identify more biocompatible polymers, for example, development of nature-derived polycations, generating and optimizing new degradable polymers, and modifications of well-established polymers with shielding units.
5 Pharmaceutical Precise Polymers
One always must keep in mind that the final purpose of our design and optimization of polymers is for clinical application. Therefore, pharmaceutical precision of polymers is equally significant to transfection efficiency and biocompatibility. The pharmaceutical precision requests the generated polymers to be defined and reproducible in size, topology (dendritic, branched, hyperbranched, linear, comb), number, and coupling sequence and sites of subunits.
All these elements obviously play a vital role in polymer transfection efficiency. Therefore, in addition to pharmaceutical precision, synthesis of chemically precise polymers also has great significance for the investigation of structure–activity relations. Several of the previously mentioned polymer generations suffer from the lack of chemical precision: polymer conjugates frequently stand for heterogenous mixtures in macromolecule sizes, topologies, and chemical conjugation sites.
Dendrimers are one class of well-defined polymers with globular morphology. They are synthesized by precise step-wise coupling of branching sites to a molecular core, resulting in a duplication of surface chains after each coupling. Commonly used dendrimers include polyamidoamine (PAMAM), dendritic polylysine, polypropylenimine (PPI) (Fig. 12a), and their derivatives [85, 201–204]. The transfection efficiency and cellular toxicity of dendrimers are basically generation-dependent. Several efforts were made to increase the biocompatibility. For example, incorporation of transferrin, a protein ligand, into dendrimers, improved the biocompatibility and sustained the transfer efficiency of in vivo nucleic acid delivery [205]. Haag and collaborators applied well-tolerated dendritic oligo-glycerol cores modified with oligoamine shells for effective siRNA delivery [206]. Russ and colleagues used a low molecular weight branched PEI or a PPI dendrimer core modified with various oligoamines such as oligoethylenimine via biodegradable hexanediol diacrylate ester linkages. This pseudo-dendrimer presented a more defined structure than randomly cross-linked polymer preparations, higher biocompatibility, and efficient in vivo DNA transfection and expression [207–211].
Defined polymers. a Polypropylenimine (PPI) dendrimer. b Artificial oligoamino acid building blocks (glutaryl-triethylene-tetramine, glutaryl-tetraethylene-pentamine, succinyl-tetraethylene-pentamine, succinyl-pentaethylene-hexamine, presented in fmoc/tBoc-protected form). c Schematic illustration of the linear assembly of Stp building blocks
Nature provides us great hints how to pursue precisely sequence-defined macromolecular architecture. Natural viruses with diverse functional microdomains have been created based on defined protein sequences translated from the genetic sequence information stored in the viral nucleic acids. The functional domains in viruses were optimized by natural selection and evolution. With the great progress of macromolecular chemistry based on solid-phase-assisted synthesis, generation of oligonucleotide and peptide sequences is routine. To apply this analogous sequence-based generation and evolution process to artificial polymer optimization, some requirements need to be met. For example, the identification of novel functional building blocks for specific delivery processes, which could be artificial moieties or nature-derived amino acids or lipids. These building blocks must be assembled in defined macromolecular sequences, and the sequences evaluated by relevant bioassays for gene delivery. The obtained structure–activity relationships can be used for further improvements of novel polymers with several rounds of evolutional selection. Many investigators have already carried out several elements of such a “chemical evolution” process.
Such sequence-defined structure–activity relationships were first investigated for peptide-based transfection reagents, such as the requirements for sequence and numbers of gene binding amino acids including arginine, lysine, or ornithine in defined branched or linear peptide structures, the effect on polyplex stability of cysteines, improvements of nucleic acid delivery with endosomal-buffering histidines [126, 186, 212–214]. Laura Hartmann and Hans Börner applied solid-phase-assisted peptide generation by assembling completely artificial building blocks such as protected di-acids and diamines into poly(aminoamides), which can be used for DNA polyplex formation [215, 216]. Based on this concept, Schaffert and colleagues designed novel artificial amino acid building blocks (Fig. 12b). These oligoamino acids protected by tBoc and Fmoc contain several repeats of the effective aminoethylene motif mediating the proton sponge effect in PEI [217, 218]. These novel oligoamino acid building blocks can be assembled into a large variety of sequences and topologies using classical peptide synthesis methodology [219, 220].
The simple questions on structure–activity relations can be explained by the precise chemical design. For instance, linear assembly of Stp building blocks (succinyl tetraethylene pentamine including three protonatable nitrogens per unit) were implemented to evaluate the effect of increasing molecular weight on linear oligo(ethanamino) amides (Fig. 12c) [221]. Exceeding a critical length of more than 10 Stp repeats, containing 31 protonatable nitrogens, the linear oligo (ethanamino) amides can efficiently form polyplexes with DNA and mediate nucleic acid transfection. With an optimized length of 30 Stp repeats, containing 91 protonatable nitrogens, the transfection efficiency was enhanced up to sixfold compared to a standard linear PEI of 22 kDa, which includes about 500 (±200) protonatable nitrogens. Importantly, this oligomer containing 30 Stp repeats presented better biocompatibility, with a 10-fold lower cytotoxicity than linear PEI.
6 Polymer-Containing Nanocomposites
For nucleic acid delivery, in addition to pure polymer-based polyplexes also lipid-based lipoplexes [222], combined lipopolyplexes, and various nanocomposites have been designed as reviewed in the following.
For example, polymer-based transfection has been enhanced by physical targeting using a magnetic field. In this process, termed “magnetofection” [223, 224], cationic magnetic nanoparticles are associated with the pDNA or other nucleic acid cargo, which then is further compacted by cationic polymers or other delivery carriers. An appropriately placed magnetic field results in faster and more intensive accumulation of nucleic acids in the target cells. Magnetofection has shown enhanced gene transfer activity in numerous applications, including a clinical phase I study in cats with fibrosarcoma [225]. Immunostimulatory gene therapy with two intratumoral GM-CSF pDNA magnetofection treatments resulted in enhanced fraction of recurrence-free patients and did not show any treatment related toxicity.
Kostarelos and colleagues have introduced single-walled or multi-walled carbon nanotubes (CNT) as nanocarriers for nucleic acid delivery, as reviewed in [226]. Cationic groups have either been directly incorporated by chemical modifications, by polymer conjugation, or indirectly by physical attachment. CNTs may provide the nanocomposite a defined structure and shape. Also, polymer-modifed graphene oxide (GO) has been extensively investigated as carrier for pDNA and siRNA [227–230]. Zhang and colleagues demonstrated that PEGylated reduced GO (rGO) presented higher ssRNA loading and delivery capacity than the PEG-GO analog [231]. PEI-PEG-rGO and PEI-PEG-GO were developed as pDNA transfection agents or a nanohybrid theranostic for simultaneous doxorubicin (via MMP2-cleavable linker) delivery and gene transfer, respectively [230, 232]. Using the NIR optimal absorbance, photothermally enhanced pDNA or siRNA delivery was obtained [227, 232]. First systems toward therapeutic in vivo applications in myocardiac tissue [229] or neurons [233] have already been established.
Kim et al. [234] combined polymer complexes with gold nanoparticles (AuNPs). Unimolecular siRNA polyion complexes (uPICs) were generated by charge-matched polyionic complexation. A single siRNA (~40 negative charges was complexed with a single copolymer molecule of poly(ethylene glycol)-b-poly(l-lysine) with the DPPLL = ~40 (~positive charges) and a thiol group at the ω-end of PEG (PEG-PLL-SH). Subsequently, the formed uPICs were coupled to 20 nm AuNP via thiol-gold coordinated bonding, resulting in a siRNA uPIC-AuNP nanoarchitecture with a homogeneous size of 38 nm. Upon intravenous administration these siRNA complexes displayed enhancing blood circulation time, efficiently accumulated in a subcutaneous HeLa-Luc cervical cancer and achieved significant luciferase gene silencing in the tumor mouse model. Analogous subsequent work introduced cRGD for active targeting of tumor and tumor endothelial integrins, resulting in therapeutic antitumoral effects [235].
Bein and colleagues generated core–shell mesoporous silica nanoparticles (MSNs) with increased pore size for high-capacity loading with siRNA. To inhibit premature release of siRNA from loaded MSNs, the pores were capped with a multifunctional lipo-oligomer which also enhanced endosomal escape of internalized MSNs. This process resulted in highly efficient marker gene silencing [236].
The Kataoka lab developed an inorganic/organic hybrid micelle system for siRNA delivery. They combined siRNA with a calcium phosphate (CaP) core and poly(ethylene glycol)-block-charge-conversional polymer (PEG-CCP) shell. With this system, effective silencing in spontaneous pancreatic tumors in transgenic mice after intravenous injection was demonstrated [237].
7 Virus-Inspired Multifunctional Dynamic Polymers
As mentioned above, for effective gene transfection into cells, numerous extracellular and intracellular barriers must be overcome, such as nucleic acid packaging, serum stability, targeting to specific cells, cellular internalization, endosomal escape, efficient unpackaging, and subsequent transport into specific organelles (Fig. 1). Significant advances were made by the design and synthesis of block copolymers with two or more functional delivery domains. For example, PEG-PEI copolymers can condense pDNA by electrostatic interaction, partly shield the polyplexes with PEG molecules, promote cellular association and internalization via residual cationic charges, and to some degree enhance endosomal escape through the additional protonation of PEI. However, this type of polymer cannot meet all requirements for effective gene delivery, because PEG shielding reduces cell association and endosomal escape via positive surface charges. In fact, some delivery goals work against each other, for instance, optimization of polyplex stability is opposed to intracellular nucleic acid release, and optimized shielding is opposed to the optimal endosomal escape [118].
Inspired by natural viruses with distinct structural and functional domains to promote the step-wise transfection, first “synthetic virus” prototypes were designed [77, 78, 114, 118, 238–242]. This research direction presents a significant conceptual progress over the former design of standard polyplexes. For example, introduction of various tailor-made functional transfection domains into the nanoparticle at different sites (shell or core), with temporally and spatially programmed activation of the functional subdomains at the targeting cellular compartments is conceived. The second point, spatio-temporal control, demands the introduction of chemical sensing moieties, which could mediate cleavage of chemical bonds and/or changes of conformations and physical characteristics responding to the distinct biological microenvironment. Based on this concept, dynamic and programmed nanoparticles should be generated [242].
A large number of targeting ligands has been applied in polymer-based gene delivery. They were incorporated into the polyplexes by several methods, for example, as separate polymers co-integrated into prepared polyplexes [243], by coupling to a preformed polyplex [190], or by direction incorporation into a core-forming polymer [244]. A library of possible targeting ligands may include large proteins such as antibodies and transferrin, or small peptides, carbohydrates, vitamins, and chemical ligands. Targeted polyplexes have been successfully applied in vivo to target various organs and tissues, such as brain, lung, liver, and tumors [5, 41]. Transferrin, as one of the most frequently used targeting ligand, has been already applied in clinical investigations. In the 1990s, within the very first polymer-based ex vivo human gene therapy, transferrin was used as targeting ligand in an interleukin-2 gene-modified cancer vaccine, due to its effective targeting to transferrin receptor on primary melanoma cells [14, 15]. Recently, transferrin was applied to target cationized cyclodextrin/PEGylated siRNA polyplexes to tumors in an in vivo human clinical trial [20, 22].
Usually, targeting ligands, especially smaller ligands, were introduced by direct conjugation with shielding domains such as PEG. PEGylation can also counteract undesired biological reaction of cationic polymers. For example, the interaction of polylysine and other polycations with the innate immune system, and the activation of the complement system can be greatly reduced [132]. Moreover, cationic nanoparticles without shielding domains could ionically interact and aggregate with blood cells and serum, resulting in opsonization by a serum corona and the loss of targeting ability or triggering in vivo toxicity.
Polyplexes with shielding domains against unspecific interactions may suffer from inefficient transfection into target cells. In this regard, PEG domains with acid-induced detachment abilities were introduced to PEI polyplexes and can significantly enhance the in vitro and in vivo nucleic acid transfection efficiency, when compared with analogous polyplexes with stable shielding [118]. One can also hypothesize that a stable PEG shielding effect will reduce the interactions between cationic polymers and the endo-lysosomal compartment membrane, whereas this problem is solved with removable PEG molecules (Fig. 13a, b). Commonly used acid-sensitive bonds [119] between PEG or other shielding domains and cationic polymers include acetals [160, 245, 246], pyridyl-hydrazones [118, 120], and dialkylmaleic acid monoamides [135, 247].
Dynamic polymers. a PEGylated polymers with endosomal pH-cleavable pyridylhydrazone bonds. b PEGylated polymers with endosomal pH and cytosol redox cleavable bonds. c PLL conjugates with disulfide attached siRNA and pH-reversible masked lytic peptide melittin. d Block copolymer VIPER (virus-inspired polymer for endosomal release) with a polycationic dimethylaminoethyl (DMA) block and a pH-sensitive diisopropylaminoethyl (DPA) block. Acid-labile bonds are labeled in red and bioreducible bonds in blue
A virus-like polyplex should have chemically dynamic characteristics [248]. Xu and Shen designed a ter-polymer (PCL/PDEA/PEG) to package pDNA into the core of virion-mimicking nanocapsules [249]. They used the pH-responsive building block poly[2-(N,N-diethyl)aminoethyl methacrylate] (PDEA), which is protonated and cationic at pH below 6. Poly(ε-caprolactone) (PCL) was used as a hydrophobic, capsule–forming module and PEG as a capsule-shielding module. At pH 5, the ter-polymer cationic micelles undergo, with pDNA, a pH-sensitive hierarchical self-assembly into a pDNA containing nanocapsule, which retains pDNA internally also at physiological pH 7. Upon endocytosis, at endosomal pH the PDEA block binds and protects pDNA against degradation, whereas the PCL ester block probably hydrolyzes in endo-lysosomes. After endosomal escape, at neutral cytosolic pH the PDEA unit becomes uncharged again, and pDNA is released from this block. Upon intravenous administration in SKOV3 tumor-bearing mice, pEGFP nanocapsules mediate efficient eGFP gene transfer in the distant subcutaneous tumors [249].
In the 2007 work of Wolff and Rozema, the acidic microenvironment in the endo-lysosomes not only helps to remove the hydrophilic shielding molecules from polyplexes, but also makes the hydrophobic lytic polymer units exposed to the endosomal vehicle membrane, and then facilitates the endosomal escape of polyplexes into the hepatocyte cytosol [135, 250]. Similarly, Meyer and collaborators [251, 252] reported around 2008 that with PEGylation and introduction of endosomally activated endosomolytic melittin peptides they could convert inactive polylysine-based polyplexes into highly efficient siRNA transfection reagents (Fig. 13c). Pun and colleagues recently generated a novel block copolymer called VIPER (virus-inspired polymer for endosomal release) [253] (Fig. 13d). VIPER contains a cationic hydrophilic block (DMA) to condense nucleic acids and a pH-sensitive block (DPA), which is hydrophobic at physiological pH, but turns out to be hydrophilic in the acidic endosomal environment. This known sharp phase transition of the DPA block around pH 6 [254] can destabilize the VIPER self-assembled micellar structures, resulting in the display of melittin peptide buried in the hydrophobic core and enhanced endosomal release through disrupting endolysosomal membrane, further mediating efficient in vivo DNA gene transfer to solid tumors [253]. The DPA building block was also recently applied by Haijun Yu and Yaping Li as component of pH-sensitive micelleplexes, used for anti-PD L1 siRNA-enhanced photodynamic cancer immunotherapy [255].
Moreover, also the Warburg effect-induced acidic microenvironment [256] around the tumor (pH 6.2–6.9) [257] has been applied for an extracellularly acid-sensitive PEG deshielding. Sun and Wang [258] synthesized an acid-sensitive bridged copolymer for in vivo siRNA delivery, where the PEG molecules will be extracellularly detached from the copolymers response to the acidic pH of the tumor microenvironment, resulting in the exposure of cationic nona-arginine (R9) and enhanced cellular uptake, finally mediating efficient gene silencing and inhibition of tumor growth with better biocompatibility.
In addition to the acidic pH in the endo-lysosomes, the bioreductive microenvironment of the cytosol can also be used to activate the transfection functions [72, 259–261]. For example, disulfide bonds within the polyplexes have been shown to increase extracellular nanoparticle stability. Reductive cleavage in the cytosol by glutathione can trigger beneficial intracellular nucleic acid unpackaging [262, 263]. Sterically accessible disulfide bonds were found also to be extracellularly reduced [264, 265] in a process that is mediated by protein disulfide isomerases (PDI) on the cell surface. Therefore, it is possible to detach the disulfide-coupled PEG molecules via the bioreducing cleavage on the cell membrane [152, 266–268].
Although our understanding of intracellular biological processes significantly increased, one must keep in mind that great hurdles persist, for example, the inefficient delivery of DNA into the cellular nucleus, and longer persistence of gene expression. Appropriate nuclear localization functional domains were evaluated for their effect on gene transfection, however still need to be verified [269–271]. In addition, the nanoparticle size in relation to the nuclear pore needs to be considered. Recent work demonstrated that only sub-50 nm PEI polyplexes locate to the inner side of the nuclear pore without damaging the nuclear envelope [272].
Incorporation of various functional units, such as targeting ligands, shielding domains, endosomolytic units, and nuclear localization signals as mentioned above may help to conquer the diverse biological barriers, but also makes polymers become very sophisticated virus-like gene carriers [239]. To fulfill all these required functions, macromolecular chemistry suffers from a new challenge: orthogonal conjugation chemistries with defined conjugation sites and precise cationic polymers with low or no polydispersity must be available. Actually, polymer chemists frequently conjugate functional domains into polymers in a rather random way, making defined and reproducible production of polymer conjugates with more than three functional domains practically impossible. However, as reviewed above, with solid phase-assisted synthesis technology, based on the previous knowledge of conventional polymers, it is feasible to synthesize more sophisticated multifunctional and precise sequence-defined nucleic acid carriers. For example, targeting ligands such as peptide sequences [128, 273], methotrexate [274], or folic acid [83] were attached to precise PEG shielding domains and sequence-defined oligoamine domains with different topologies such as two-arm or four-arm architectures (Fig. 14). In addition to the direct conjugation of targeting and shielding functional units into oligomers during the same solid phase-assisted generation process, it is also feasible to couple them to cationic oligomer core via site-specific native chemical peptide ligation reaction [275].
Multifunctional sequence-defined oligomer. Oligomer contains a dual-functional methotrexate ligand for targeting and therapy, PEG for shielding, artificial oligoamino acid Stp for nucleic acid binding and endosomal escape, terminal cysteines for bioreversible polyplex stabilization, and histidines for endosomal escape. The functions of the individual units are indicated in color: two colors present a dual function
Various further modifications on oligomers can improve the nucleic acid transfection efficiency. For example, introduction of terminal cysteines can enhance the bioreversible stability of polyplexes [83, 218, 276], incorporation of tyrosine residues improved the siRNA transfection efficiency of novel oligomers [277], analogously as has been reported for the conversion of PEI into efficient siRNA transfection polymers [188]. Also, histidines, enhancing endosomal escape, and transfection efficiency of diverse polymers [125] were incorporated into novel cationic oligomers with or without shielding domains and targeting ligands and could promote their endosomal escape [128]. Meanwhile, with incorporation of specific numbers of the aminoethylene unit, Lächelt and colleagues [128] could finely tune the proton sponge effect of the oligoamine microdomains. The microdomain with even protonatable nitrogens showed preferential buffering at pH 5–6 in endosomes, whereas with odd protonatable nitrogens, the buffering ability was around pH 7. These findings were well consistent with prior reports by Kataoka and colleagues. They designed degradable cationic polymers via the amidation of the side chain in polyaspartic acid with various small defined oligoamines [114, 182].
Synthesis of precise sequence-defined oligomers provides a great progress for the chemical evolution of polymeric gene delivery systems. However, screening and identification of promising carrier candidates and further optimization of polyplexes present a new challenge. One selection strategy first applied in 2001 by Langer and collaborators [173] was the high-throughput semi-automated screening method. With this strategy, they could screen libraries of a variety of novel polymers on selected cell lines and identify the most interesting candidates [175, 176]. It will be important to identify the most relevant criteria for in vivo effectiveness and develop corresponding screening assays. Very significant in this respect, Whitehead and colleagues recently reported the identification of four criteria, which are necessary to predict the in vivo activities of siRNA lipidoid nanoparticles [278].
8 Conclusions and Perspectives
From the first-generation cationic polymers to the multifunctional sequence-defined gene delivery system, the evolution of polymers experiences a period of 50 years. Within this time span, several breakthroughs in conception and chemistry were obtained (Table 1) deriving from our better understanding on the biological process of gene delivery and the great progress in macromolecular synthetic chemistry. The efficacy of polymeric gene delivery vectors, however, is still moderate, and clinical applications of these vectors remain limited. Therefore, further chemical improvement of polymeric carriers is necessary. It is also predictable that the rapid advances in nanotechnology and microscopy will further deepen our understanding of nanoscale particles for nucleic acid delivery. With the continuous efforts, it is reasonable to believe that in near future polymeric gene delivery system will play a significant role in human nucleic acid and gene therapy.
References
Mulligan RC (1993) The basic science of gene therapy. Science 260:926–932
Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG (2014) Non-viral vectors for gene-based therapy. Nat Rev Genet 15:541–555
Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y, Wu Q, Park A, Yang J, Suresh S, Bizhanova A, Gupta A, Bolukbasi MF, Walsh S, Bogorad RL, Gao G, Weng Z, Dong Y, Koteliansky V, Wolfe SA, Langer R, Xue W, Anderson DG (2016) Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol 34:328–333
Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J (2013) Gene therapy clinical trials worldwide to 2012—an update. J Gene Med 15:65–77
Lachelt U, Wagner E (2015) Nucleic acid therapeutics using polyplexes: a journey of 50 years (and beyond). Chem Rev 115:11043–11078
Mintzer MA, Simanek EE (2009) Nonviral vectors for gene delivery. Chem Rev 109:259–302
Pack DW, Hoffman AS, Pun S, Stayton PS (2005) Design and development of polymers for gene delivery. Nat Rev Drug Discov 4:581–593
Verma IM, Somia N (1997) Gene therapy—promises, problems and prospects. Nature 389:239–242
Bessis N, GarciaCozar FJ, Boissier MC (2004) Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther 11:S10–S17
Thomas CE, Ehrhardt A, Kay MA (2003) Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 4:346–358
Bouard D, Alazard-Dany D, Cosset FL (2009) Viral vectors: from virology to transgene expression. Br J Pharmacol 157:153–165
Wagner E (2014) Polymers for nucleic acid transfer—an overview. Adv Genet 88:231–261
Wagner E (2012) Polymers for siRNA delivery: inspired by viruses to be targeted, dynamic, and precise. Accounts Chem Res 45:1005–1013
Stingl G, Brocker EB, Mertelsmann R, Wolff K, Schreiber S, Kampgen E, Schneeberger A, Dummer W, Brennscheid U, Veelken H, Birnstiel ML, Zatloukal K, Schmidt W, Maass G, Wagner E, Baschle M, Giese M, Kempe ER, Weber HA, Voigt T (1996) Phase I study to the immunotherapy of metastatic malignant melanoma by a cancer vaccine consisting of autologous cancer cells transfected with the human IL-2 gene. Hum Gene Ther 7:551–563
Schreiber S, Kampgen E, Wagner E, Pirkhammer D, Trcka J, Korschan H, Lindemann A, Dorffner R, Kittler H, Kasteliz F, Kupcu Z, Sinski A, Zatloukal K, Buschle M, Schmidt W, Birnstiel M, Kempe RE, Voigt T, Weber HA, Pehamberger H, Mertelsmann R, Brocker EB, Wolff K, Stingl G (1999) Immunotherapy of metastatic malignant melanoma by a vaccine consisting of autologous interleukin 2-transfected cancer cells: outcome of a phase I study. Hum Gene Ther 10:983–993
Ohana P, Gofrit O, Ayesh S, Al-Sharef W, Mizrahi A, Birman T, Schneider T, Matouk I, de Groot N, Tavdy E, Sidi AA, Hochberg A (2004) Regulatory sequences of the H19 gene in DNA based therapy of bladder cancer. Gene Ther Mol Biol 8:181–192
Konstan MW, Davis PB, Wagener JS, Hilliard KA, Stern RC, Milgram LJ, Kowalczyk TH, Hyatt SL, Fink TL, Gedeon CR, Oette SM, Payne JM, Muhammad O, Ziady AG, Moen RC, Cooper MJ (2004) Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther 15:1255–1269
Sidi AA, Ohana P, Benjamin S, Shalev M, Ransom JH, Lamm D, Hochberg A, Leibovitch I (2008) Phase I/II marker lesion study of intravesical BC-819 DNA plasmid in H19 over expressing superficial bladder cancer refractory to bacillus Calmette-Guerin. J Urology 180:2379–2383
Fewell JG, Matar MM, Rice JS, Brunhoeber E, Slobodkin G, Pence C, Worker M, Lewis DH, Anwer K (2009) Treatment of disseminated ovarian cancer using nonviral interleukin-12 gene therapy delivered intraperitoneally. J Gene Med 11:718–728
Davis ME (2009) The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm 6:659–668
Anwer K, Barnes MN, Fewell J, Lewis DH, Alvarez RD (2010) Phase-I clinical trial of IL-12 plasmid/lipopolymer complexes for the treatment of recurrent ovarian cancer. Gene Ther 17:360–369
Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A (2010) Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464:1067–1070
Lisziewicz J, Bakare N, Calarota SA, Banhegyi D, Szlavik J, Ujhelyi E, Toke ER, Molnar L, Lisziewicz Z, Autran B, Lori F (2012) Single DermaVir immunization: dose-dependent expansion of precursor/memory T cells against all HIV antigens in HIV-1 infected individuals. PLoS One 7:e35416
Gofrit ON, Benjamin S, Halachmi S, Leibovitch I, Dotan Z, Lamm DL, Ehrlich N, Yutkin V, Ben-Am M, Hochberg A (2014) DNA based therapy with diphtheria toxin-A BC-819: a Phase 2b marker lesion trial in patients with intermediate risk nonmuscle invasive bladder cancer. J Urology 191:1697–1702
Rodriguez B, Asmuth DM, Matining RM, Spritzler J, Jacobson JM, Mailliard RB, Li XD, Martinez AI, Tenorio AR, Lori F, Lisziewicz J, Yesmin S, Rinaldo CR, Pollard RB (2013) Safety, tolerability, and immunogenicity of repeated doses of dermavir, a candidate therapeutic HIV vaccine, in HIV-infected patients receiving combination antiretroviral therapy: results of the ACTG 5176 trial. J Acquir Immune Defic Syndr 64:351–359
Smull CE, Ludwig EH (1962) Enhancement of the plaque forming capacity of poliovirus ribonucleic acid with basic proteins. J Bacteriol 84:1035–1040
Vaheri A, Pagano JS (1965) Infectious poliovirus RNA: a sensitive method of assay 2. Virology 27:434–436
McCutchan JH, Pagano JS (1968) Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer I 41:351–357
Farber FE, Melnick JL, Butel JS (1975) Optimal conditions for uptake of exogenous DNA by Chinese hamster lung cells deficient in hypoxanthine-guanine phosphoribosyltransferase. Biochim Biophys Acta 390:298–311
Kabanov AV, Astafyeva IV, Chikindas ML, Rosenblat GF, Kiselev VI, Severin ES, Kabanov VA (1991) DNA interpolyelectrolyte complexes as a tool for efficient cell transformation. Biopolymers 31:1437–1443
Kabanov AV, Astafieva IV, Maksimova IV, Lukanidin EM, Georgiev GP, Kabanov VA (1993) Efficient transformation of mammalian cells using DNA interpolyelectrolyte complexes with carbon chain polycations. Bioconjugate Chem 4:448–454
Laemmli UK (1975) Characterization of DNA condensates induced by poly(ethylene oxide) and polylysine. Proc Natl Acad Sci 72:4288–4292
Chattoraj DK, Gosule LC, Schellman A (1978) DNA condensation with polyamines. II. Electron microscopic studies 95. J Mol Biol 121:327–337
Hinde E, Thammasiraphop K, Duong HTT, Yeow J, Karagoz B, Boyer C, Gooding JJ, Gaus K (2016) Pair correlation microscopy reveals the role of nanoparticle shape in intracellular transport and site of drug release. Nat Nanotechnol. doi:10.1038/nnano.2016.160
Wu GY, Wu CH (1987) Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J Biol Chem 262:4429–4432
Wu GY, Wu CH (1988) Receptor-mediated gene delivery and expression in vivo 738. J Biol Chem 262:14621–14624
Wu CH, Wilson JM, Wu GY (1989) Targeting genes: delivery and persistent expression of a foreign gene driven by mammalian regulatory elements in vivo 939. J Biol Chem 264:16985–16987
Chowdhury NR, Wu CH, Wu GY, Yerneni PC, Bommineni VR, Chowdhury JR (1993) Fate of DNA targeted to the liver by asialoglycoprotein receptor-mediated endocytosis in vivo. Prolonged persistence in cytoplasmic vesicles after partial hepatectomy 111. J Biol Chem 268:11265–11271
Wu GY, Wilson JM, Shalaby F, Grossman M, Shafritz DA, Wu CH (1991) Receptor-mediated gene delivery in vivo. Partial correction of genetic analbuminemia in Nagase rats. J Biol Chem 266:14338–14342
Wilson JM, Grossman M, Wu CH, Chowdhury NR, Wu GY, Chowdhury JR (1992) Hepatocyte-directed gene transfer in vivo leads to transient improvement of hypercholesterolemia in low density lipoprotein receptor-deficient rabbits 720. J Biol Chem 267:963–967
Ogris M, Wagner E (2011) To be targeted: is the magic bullet concept a viable option for synthetic nucleic acid therapeutics? Hum Gene Ther 22:799–807
Wagner E, Zenke M, Cotten M, Beug H, Birnstiel ML (1990) Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci 87:3410–3414
Cotten M, Langle-Rouault F, Kirlappos H, Wagner E, Mechtler K, Zenke M, Beug H, Birnstiel ML (1990) Transferrin-polycation-mediated introduction of DNA into human leukemic cells: stimulation by agents that affect the survival of transfected DNA or modulate transferrin receptor levels. Proc Natl Acad Sci 87:4033–4037
Zenke M, Steinlein P, Wagner E, Cotten M, Beug H, Birnstiel ML (1990) Receptor-mediated endocytosis of transferrin-polycation conjugates: an efficient way to introduce DNA into hematopoietic cells. Proc Natl Acad Sci 87:3655–3659
Ferkol T, Kaetzel CS, Davis PB (1993) Gene transfer into respiratory epithelial cells by targeting the polymeric immunoglobulin receptor 831. J Clin Invest 92:2394–2400
Wagner E, Ogris M, Zauner W (1998) Polylysine-based transfection systems utilizing receptor-mediated delivery. Adv Drug Deliver Rev 30:97–113
Luthman H, Magnusson G (1983) High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res 11:1295–1308
Erbacher P, Roche AC, Monsigny M, Midoux P (1996) Putative role of chloroquine in gene transfer into a human hepatoma cell line by DNA/lactosylated polylysine complexes. Exp Cell Res 225:186–194
Cheng JJ, Zeidan R, Mishra S, Liu A, Pun SH, Kulkarni RP, Jensen GS, Bellocq NC, Davis ME (2006) Structure—function correlation of chloroquine and analogues as transgene expression enhancers in nonviral gene delivery. J Med Chem 49:6522–6531
Mellman I, Fuchs R, Helenius A (1986) Acidification of the endocytic and exocytic pathways. Annu Rev Biochem 55:663–700
Cain CC, Sipe DM, Murphy RF (1989) Regulation of endocytic pH by the Na+, K+-ATPase in living cells. Proc Natl Acad Sci 86:544–548
Maxfield FR, McGraw TE (2004) Endocytic recycling. Nat Rev Mol Cell Bio 5:121–132
Curiel DT, Agarwal S, Wagner E, Cotten M (1991) Adenovirus enhancement of transferrin-polylysine-mediated gene delivery. Proc Natl Acad Sci 88:8850–8854
Cotten M, Wagner E, Zatloukal K, Birnstiel ML (1993) Chicken adenovirus (CELO virus) particles augment receptor-mediated DNA delivery to mammalian cells and yield exceptional levels of stable transformants. J Virol 67:3777–3785
Zauner W, Blaas D, Kuechler E, Wagner E (1995) Rhinovirus-mediated endosomal release of transfection complexes. J Virol 69:1085–1092
Curiel DT, Wagner E, Cotten M, Birnstiel ML, Agarwal S, Li CM, Loechel S, Hu PC (1992) High-efficiency gene transfer mediated by adenovirus coupled to DNA-polylysine complexes. Hum Gene Ther 3:147–154
Wagner E, Zatloukal K, Cotten M, Kirlappos H, Mechtler K, Curiel DT, Birnstiel ML (1992) Coupling of adenovirus to transferrin-polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc Natl Acad Sci 89:6099–6103
Cotten M, Wagner E, Zatloukal K, Phillips S, Curiel DT, Birnstiel ML (1992) High-efficiency receptor-mediated delivery of small and large (48 kilobase gene constructs using the endosome-disruption activity of defective or chemically inactivated adenovirus particles. Proc Natl Acad Sci 89:6094–6098
Zatloukal K, Wagner E, Cotten M, Phillips S, Plank C, Steinlein P, Curiel DT, Birnstiel ML (1992) Transferrinfection: a highly efficient way to express gene constructs in eukaryotic cells. Ann NY Acad Sci 660:136–153
Gao L, Wagner E, Cotten M, Agarwal S, Harris C, Romer M, Miller L, Hu PC, Curiel D (1993) Direct in vivo gene transfer to airway epithelium employing adenovirus-polylysine-DNA complexes. Hum Gene Ther 4:17–24
Cristiano RJ, Smith LC, Kay MA, Brinkley BR, Woo SL (1993) Hepatic gene therapy: efficient gene delivery and expression in primary hepatocytes utilizing a conjugated adenovirus-DNA complex 139. Proc Natl Acad Sci 90:11548–11552
Cotten M, Saltik M, Kursa M, Wagner E, Maass G, Birnstiel ML (1994) Psoralen treatment of adenovirus particles eliminates virus replication and transcription while maintaining the endosomolytic activity of the virus capsid. Virology 205:254–261
Curiel TJ, Cook DR, Bogedain C, Jilg W, Harrison GS, Cotten M, Curiel DT, Wagner E (1994) Efficient foreign gene expression in Epstein-Barr virus-transformed human B-cells. Virology 198:577–585
Frank S, Krasznai K, Durovic S, Lobentanz EM, Dieplinger H, Wagner E, Zatloukal K, Cotten M, Utermann G, Kostner GM (1994) High-level expression of various apolipoprotein(a) isoforms by “transferrinfection”: the role of kringle IV sequences in the extracellular association with low-density lipoprotein. Biochemistry 33:12329–12339
Zatloukal K, Cotten M, Berger M, Schmidt W, Wagner E, Birnstiel ML (1994) In vivo production of human factor VII in mice after intrasplenic implantation of primary fibroblasts transfected by receptor-mediated, adenovirus-augmented gene delivery. Proc Natl Acad Sci 91:5148–5152
Zatloukal K, Schneeberger A, Berger M, Koszik F, Schmidt W, Wagner E, Cotten M, Buschle M, Maass G, Stingl G (1994) Genetic modification of cells by receptor-mediated adenovirus-augmented gene delivery: a new approach for immunotherapy of cancer. Verh Deut G 78:171–176
Buschle M, Cotten M, Kirlappos H, Mechtler K, Schaffner G, Zauner W, Birnstiel ML, Wagner E (1995) Receptor-mediated gene transfer into human T lymphocytes via binding of DNA/CD3 antibody particles to the CD3 T cell receptor complex. Hum Gene Ther 6:753–761
Cristiano RJ, Roth JA (1996) Epidermal growth factor mediated DNA delivery into lung cancer cells via the epidermal growth factor receptor. Cancer Gene Ther 3:4–10
Gagnoux-Palacios L, Vailly J, Durand-Clement M, Wagner E, Ortonne JP, Meneguzzi G (1996) Functional Re-expression of laminin-5 in laminin-gamma2-deficient human keratinocytes modifies cell morphology, motility, and adhesion. J Biol Chem 271:18437–18444
Nguyen DM, Wiehle SA, Koch PE, Branch C, Yen N, Roth JA, Cristiano RJ (1997) Delivery of the p53 tumor suppressor gene into lung cancer cells by an adenovirus/DNA complex 884. Cancer Gene Ther 4:191–198
Wagner E, Curiel D, Cotten M (1994) Delivery of drugs, proteins and genes into cells using transferrin as a ligand for receptor-mediated endocytosis. Adv Drug Deliver Rev 14:113–136
Saito G, Amidon GL, Lee KD (2003) Enhanced cytosolic delivery of plasmid DNA by a sulfhydryl-activatable listeriolysin O/protamine conjugate utilizing cellular reducing potential. Gene Ther 10:72–83
Gottschalk S, Tweten RK, Smith LC, Woo SL (1995) Efficient gene delivery and expression in mammalian cells using DNA coupled with perfringolysin O 244. Gene Ther 2:498–503
Fominaya J, Wels W (1996) Target cell-specific DNA transfer mediated by a chimeric multidomain protein. Novel non-viral gene delivery system 835. J Biol Chem 271:10560–10568
Fominaya J, Uherek C, Wels W (1998) A chimeric fusion protein containing transforming growth factor-alpha mediates gene transfer via binding to the EGF receptor. Gene Ther 5:521–530
Uherek C, Fominaya J, Wels W (1998) A modular DNA carrier protein based on the structure of diphtheria toxin mediates target cell-specific gene delivery. J Biol Chem 273:8835–8841
Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML (1992) Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc Natl Acad Sci 89:7934–7938
Plank C, Zatloukal K, Cotten M, Mechtler K, Wagner E (1992) Gene transfer into hepatocytes using asialoglycoprotein receptor mediated endocytosis of DNA complexed with an artificial tetra-antennary galactose ligand. Bioconjugate Chem 3:533–539
Plank C, Oberhauser B, Mechtler K, Koch C, Wagner E (1994) The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J Biol Chem 269:12918–12924
Plank C, Zauner W, Wagner E (1998) Application of membrane-active peptides for drug and gene delivery across cellular membranes. Adv Drug Deliver Rev 34:21–35
Wagner E (1998) Effects of membrane-active agents in gene delivery. J Control Release 53:155–158
Wagner E (1999) Application of membrane-active peptides for nonviral gene delivery. Adv Drug Deliver Rev 38:279–289
Dohmen C, Edinger D, Frohlich T, Schreiner L, Lachelt U, Troiber C, Radler J, Hadwiger P, Vornlocher HP, Wagner E (2012) Nanosized multifunctional polyplexes for receptor-mediated siRNA delivery. ACS Nano 6:5198–5208
Zhang W, Muller K, Kessel E, Reinhard S, He D, Klein PM, Hohn M, Rodl W, Kempter S, Wagner E (2016) Targeted siRNA delivery using a lipo-oligoaminoamide nanocore with an influenza peptide and transferrin shell. Adv Healthc Mater 5:1493–1504
Haensler J, Szoka FC Jr (1993) Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjugate Chem 4:372–379
Wyman TB, Nicol F, Zelphati O, Scaria PV, Plank C, Szoka FC Jr (1997) Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry 36:3008–3017
Gottschalk S, Sparrow JT, Hauer J, Mims MP, Leland FE, Woo SL, Smith LC (1996) A novel DNA-peptide complex for efficient gene transfer and expression in mammalian cells 844. Gene Ther 3:48–57
Mechtler K, Wagner E (1997) Gene transfer mediated by influenza virus peptides: the role of peptide sequence. New J Chem 21:105–111
Midoux P, Kichler A, Boutin V, Maurizot JC, Monsigny M (1998) Membrane permeabilization and efficient gene transfer by a peptide containing several histidines 878. Bioconjugate Chem 9:260–267
Kichler A, Leborgne C, Marz J, Danos O, Bechinger B (2003) Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells. Proc Natl Acad Sci 100:1564–1568
Berg K, Weyergang A, Prasmickaite L, Bonsted A, Hogset A, Strand MT, Wagner E, Selbo PK (2010) Photochemical internalization (PCI): a technology for drug delivery. Methods Mol Biol 635:133–145
de Bruin KG, Fella C, Ogris M, Wagner E, Ruthardt N, Brauchle C (2008) Dynamics of photoinduced endosomal release of polyplexes. J Control Release 130:175–182
Kloeckner J, Prasmickaite L, Hogset A, Berg K, Wagner E (2004) Photochemically enhanced gene delivery of EGF receptor-targeted DNA polyplexes. J Drug Target 12:205–213
Tang MX, Redemann CT, Szoka FC Jr (1996) In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjugate Chem 7:703–714
Tang MX, Szoka FC (1997) The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes 2. Gene Ther 4:823–832
Sonawane ND, Szoka FC Jr, Verkman AS (2003) Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem 278:44826–44831
Behr JP, Demeneix B, Loeffler JP, Perez-Mutul J (1989) Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA 6. Proc Natl Acad Sci 86:6982–6986
Remy JS, Sirlin C, Vierling P, Behr JP (1994) Gene transfer with a series of lipophilic DNA-binding molecules 547. Bioconjugate Chem 5:647–654
Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci 92:7297–7301
Behr JP (1997) The proton sponge: a trick to enter cells the viruses did not exploit. Chimia 51:34–36
Coll JL, Chollet P, Brambilla E, Desplanques D, Behr JP, Favrot M (1999) In vivo delivery to tumors of DNA complexed with linear polyethylenimine. Hum Gene Ther 10:1659–1666
Zou SM, Erbacher P, Remy JS, Behr JP (2000) Systemic linear polyethylenimine (L-PEI)-mediated gene delivery in the mouse. J Gene Med 2:128–134
Neuberg P, Kichler A (2014) Recent developments in nucleic acid delivery with polyethylenimines. Adv Genet 88:263–288
Godbey WT, Ku KK, Hirasaki GJ, Mikos AG (1999) Improved packing of poly(ethylenimine)/DNA complexes increases transfection efficiency. Gene Ther 6:1380–1388
Itaka K, Harada A, Yamasaki Y, Nakamura K, Kawaguchi H, Kataoka K (2004) In situ single cell observation by fluorescence resonance energy transfer reveals fast intra-cytoplasmic delivery and easy release of plasmid DNA complexed with linear polyethylenimine. J Gene Med 6:76–84
Wightman L, Kircheis R, Rossler V, Carotta S, Ruzicka R, Kursa M, Wagner E (2001) Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J Gene Med 3:362–372
Brunner S, Sauer T, Carotta S, Cotten M, Saltik M, Wagner E (2000) Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther 7:401–407
Brunner S, Furtbauer E, Sauer T, Kursa M, Wagner E (2002) Overcoming the nuclear barrier: cell cycle independent nonviral gene transfer with linear polyethylenimine or electroporation. Mol Ther 5:80–86
Grandinetti G, Reineke TM (2012) Exploring the mechanism of plasmid DNA nuclear internalization with polymer-based vehicles. Mol Pharm 9:2256–2267
Godbey WT, Wu KK, Mikos AG (1999) Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc Natl Acad Sci 96:5177–5181
Kichler A, Leborgne C, Coeytaux E, Danos O (2001) Polyethylenimine-mediated gene delivery: a mechanistic study. J Gene Med 3:135–144
Akinc A, Langer R (2002) Measuring the pH environment of DNA delivered using nonviral vectors: implications for lysosomal trafficking. Biotechnol Bioeng 78:503–508
Akinc A, Thomas M, Klibanov AM, Langer R (2005) Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med 7:657–663
Miyata K, Nishiyama N, Kataoka K (2012) Rational design of smart supramolecular assemblies for gene delivery: chemical challenges in the creation of artificial viruses. Chem Soc Rev 41:2562–2574
Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL (2013) The possible “proton sponge “ effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther 21:149–157
Boeckle S, von Gersdorff K, van der Piepen S, Culmsee C, Wagner E, Ogris M (2004) Purification of polyethylenimine polyplexes highlights the role of free polycations in gene transfer. J Gene Med 6:1102–1111
Funhoff AM, van Nostrum CF, Koning GA, Schuurmans-Nieuwenbroek NME, Crommelin DJA, Hennink WE (2004) Endosomal escape of polymeric gene delivery complexes is not always enhanced by polymers buffering at low pH. Biomacromolecules 5:32–39
Walker GF, Fella C, Pelisek J, Fahrmeir J, Boeckle S, Ogris M, Wagner E (2005) Toward synthetic viruses: endosomal pH-triggered deshielding of targeted polyplexes greatly enhances gene transfer in vitro and in vivo. Mol Ther 11:418–425
Meyer M, Wagner E (2006) pH-responsive shielding of non-viral gene vectors. Expert Opin Drug Del 3:563–571
Fella C, Walker GF, Ogris M, Wagner E (2008) Amine-reactive pyridylhydrazone-based PEG reagents for pH-reversible PEI polyplex shielding. Eur J Pharm Sci 34:309–320
Lee SH, Choi SH, Kim SH, Park TG (2008) Thermally sensitive cationic polymer nanocapsules for specific cytosolic delivery and efficient gene silencing of siRNA: swelling induced physical disruption of endosome by cold shock. J Control Release 125:25–32
Midoux P, Monsigny M (1999) Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjugate Chem 10:406–411
Pack DW, Putnam D, Langer R (2000) Design of imidazole-containing endosomolytic biopolymers for gene delivery. Biotechnol Bioeng 67:217–223
Pichon C, Goncalves C, Midoux P (2001) Histidine-rich peptides and polymers for nucleic acids delivery. Adv Drug Deliver Rev 53:75–94
Bertrand E, Goncalves C, Billiet L, Gomez JP, Pichon C, Cheradame H, Midoux P, Guegan P (2011) Histidinylated linear PEI: a new efficient non-toxic polymer for gene transfer. Chem Commun 47:12547–12549
Chen QR, Zhang L, Stass SA, Mixson AJ (2001) Branched co-polymers of histidine and lysine are efficient carriers of plasmids. Nucleic Acids Res 29:1334–1340
Leng Q, Mixson AJ (2016) The neuropilin-1 receptor mediates enhanced tumor delivery of H2K polyplexes. J Gene Med 18:134–144
Lachelt U, Kos P, Mickler FM, Herrmann A, Salcher EE, Rodl W, Badgujar N, Brauchle C, Wagner E (2014) Fine-tuning of proton sponges by precise diaminoethanes and histidines in pDNA polyplexes. Nanomedicine 10:35–44
Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A (2005) A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther 11:990–995
Grandinetti G, Ingle NP, Reineke TM (2011) Interaction of poly(ethylenimine)-DNA polyplexes with mitochondria: implications for a mechanism of cytotoxicity. Mol Pharm 8:1709–1719
Hall A, Larsen AK, Parhamifar L, Meyle KD, Wu LP, Moghimi SM (2013) High resolution respirometry analysis of polyethylenimine-mediated mitochondrial energy crisis and cellular stress: mitochondrial proton leak and inhibition of the electron transport system. Biochim Biophys Acta 1827:1213–1225
Plank C, Mechtler K, Szoka FC Jr, Wagner E (1996) Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery. Hum Gene Ther 7:1437–1446
Merkel OM, Urbanics R, Bedocs P, Rozsnyay Z, Rosivall L, Toth M, Kissel T, Szebeni J (2011) In vitro and in vivo complement activation and related anaphylactic effects associated with polyethylenimine and polyethylenimine-graft-poly(ethylene glycol) block copolymers. Biomaterials 32:4936–4942
Wakefield DH, Klein JJ, Wolff JA, Rozema DB (2005) Membrane activity and transfection ability of amphipathic polycations as a function of alkyl group size. Bioconjugate Chem 16:1204–1208
Rozema DB, Lewis DL, Wakefield DH, Wong SC, Klein JJ, Roesch PL, Bertin SL, Reppen TW, Chu Q, Blokhin AV, Hagstrom JE, Wolff JA (2007) Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci 104:12982–12987
Murthy N, Robichaud JR, Tirrell DA, Stayton PS, Hoffman AS (1999) The design and synthesis of polymers for eukaryotic membrane disruption. J Control Release 61:137–143
Bulmus V, Woodward M, Lin L, Murthy N, Stayton P, Hoffman A (2003) A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J Control Release 93:105–120
Dang JM, Leong KW (2006) Natural polymers for gene delivery and tissue engineering. Adv Drug Deliver Rev 58:487–499
Zaitsev SV, Haberland A, Otto A, Vorob’ev VI, Haller H, Bottger M (1997) H1 and HMG17 extracted from calf thymus nuclei are efficient DNA carriers in gene transfer. Gene Ther 4:586–592
Bottger M, Vogel F, Platzer M, Kiessling U, Grade K, Strauss M (1988) Condensation of vector DNA by the chromosomal protein HMG1 results in efficient transfection. Biochim Biophys Acta 950:221–228
Chen J, Stickles RJ, Daichendt KA (1994) Galactosylated histone-mediated gene transfer and expression. Hum Gene Ther 5:429–435
Takeshita F, Minakuchi Y, Nagahara S, Honma K, Sasaki H, Hirai K, Teratani T, Namatame N, Yamamoto Y, Hanai K, Kato T, Sano A, Ochiya T (2005) Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc Natl Acad Sci 102:12177–12182
Young S, Wong M, Tabata Y, Mikos AG (2005) Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release 109:256–274
Erbacher P, Zou S, Bettinger T, Steffan AM, Remy JS (1998) Chitosan-based vector/DNA complexes for gene delivery: biophysical characteristics and transfection ability 826. Pharm Res 15:1332–1339
Park IK, Kim TH, Park YH, Shin BA, Choi ES, Chowdhury EH, Akaike T, Cho CS (2001) Galactosylated chitosan-graft-poly(ethylene glycol) as hepatocyte-targeting DNA carrier. J Control Release 76:349–362
Thanou M, Florea BI, Geldof M, Junginger HE, Borchard G (2002) Quaternized chitosan oligomers as novel gene delivery vectors in epithelial cell lines. Biomaterials 23:153–159
Germershaus O, Mao S, Sitterberg J, Bakowsky U, Kissel T (2008) Gene delivery using chitosan, trimethyl chitosan or polyethylenglycol-graft-trimethyl chitosan block copolymers: establishment of structure-activity relationships in vitro. J Control Release 125:145–154
Chang KL, Higuchi Y, Kawakami S, Yamashita F, Hashida M (2010) Efficient gene transfection by histidine-modified chitosan through enhancement of endosomal escape. Bioconjugate Chem 21:1087–1095
Lee D, Zhang W, Shirley SA, Kong X, Hellermann GR, Lockey RF, Mohapatra SS (2007) Thiolated chitosan/DNA nanocomplexes exhibit enhanced and sustained gene delivery. Pharm Res 24:157–167
Pun SH, Bellocq NC, Liu A, Jensen G, Machemer T, Quijano E, Schluep T, Wen S, Engler H, Heidel J, Davis ME (2004) Cyclodextrin-modified polyethylenimine polymers for gene delivery. Bioconjugate Chem 15:831–840
Pun SH, Davis ME (2002) Development of a nonviral gene delivery vehicle for systemic application. Bioconjugate Chem 13:630–639
Ping Y, Hu Q, Tang G, Li J (2013) FGFR-targeted gene delivery mediated by supramolecular assembly between beta-cyclodextrin-crosslinked PEI and redox-sensitive PEG. Biomaterials 34:6482–6494
Hwang SJ, Bellocq NC, Davis ME (2001) Effects of structure of beta-cyclodextrin-containing polymers on gene delivery. Bioconjugate Chem 12:280–290
Bellocq NC, Pun SH, Jensen GS, Davis ME (2003) Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjugate Chem 14:1122–1132
Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ (2005) Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing’s sarcoma. Cancer Res 65:8984–8992
Cryan SA, Holohan A, Donohue R, Darcy R, O’Driscoll CM (2004) Cell transfection with polycationic cyclodextrin vectors. Eur J Pharm Sci 21:625–633
Ooya T, Choi HS, Yamashita A, Yui N, Sugaya Y, Kano A, Maruyama A, Akita H, Ito R, Kogure K, Harashima H (2006) Biocleavable polyrotaxane-plasmid DNA polyplex for enhanced gene delivery. J Am Chem Soc 128:3852–3853
Wagner E, Kloeckner J (2006) Gene delivery using polymer therapeutics. Adv Polym Sci 192:135–173
Knorr V, Russ V, Allmendinger L, Ogris M, Wagner E (2008) Acetal linked oligoethylenimines for use as pH-sensitive gene carriers. Bioconjugate Chem 19:1625–1634
Knorr V, Ogris M, Wagner E (2008) An acid sensitive ketal-based polyethylene glycol-oligoethylenimine copolymer mediates improved transfection efficiency at reduced toxicity. Pharm Res 25:2937–2945
Kim YH, Park JH, Lee M, Park TG, Kim SW (2005) Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release 103:209–219
Gosselin MA, Guo W, Lee RJ (2001) Efficient gene transfer using reversibly cross-linked low molecular weight polyethylenimine. Bioconjugate Chem 12:989–994
Yu H, Russ V, Wagner E (2009) Influence of the molecular weight of bioreducible oligoethylenimine conjugates on the polyplex transfection properties. AAPS J 11:445–455
Forrest ML, Koerber JT, Pack DW (2003) A degradable polyethylenimine derivative with low toxicity for highly efficient gene delivery. Bioconjugate Chem 14:934–940
Kloeckner J, Wagner E, Ogris M (2006) Degradable gene carriers based on oligomerized polyamines. Eur J Pharm Sci 29:414–425
Chen L, Tian H, Chen J, Chen X, Huang Y, Jing X (2010) Multi-armed poly(l-glutamic acid)-graft-oligoethylenimine copolymers as efficient nonviral gene delivery vectors. J Gene Med 12:64–76
Petersen H, Merdan T, Kunath K, Fischer D, Kissel T (2002) Poly(ethylenimine-co-l-lactamide-co-succinamide): a biodegradable polyethylenimine derivative with an advantageous pH-dependent hydrolytic degradation for gene delivery. Bioconjugate Chem 13:812–821
Kloeckner J, Bruzzano S, Ogris M, Wagner E (2006) Gene carriers based on hexanediol diacrylate linked oligoethylenimine: effect of chemical structure of polymer on biological properties. Bioconjugate Chem 17:1339–1345
Tarcha PJ, Pelisek J, Merdan T, Waters J, Cheung K, von Gersdorff K, Culmsee C, Wagner E (2007) Synthesis and characterization of chemically condensed oligoethylenimine containing beta-aminopropionamide linkages for siRNA delivery. Biomaterials 28:3731–3740
Lim YB, Han SO, Kong HU, Lee Y, Park JS, Jeong B, Kim SW (2000) Biodegradable polyester, poly[alpha-(4-aminobutyl)-l-glycolic acid], as a non-toxic gene carrier. Pharm Res 17:811–816
Lim YB, Kim SM, Suh H, Park JS (2002) Biodegradable, endosome disruptive, and cationic network-type polymer as a highly efficient and nontoxic gene delivery carrier. Bioconjugate Chem 13:952–957
Akinc A, Anderson DG, Lynn DM, Langer R (2003) Synthesis of poly(beta-amino ester)s optimized for highly effective gene delivery. Bioconjugate Chem 14:979–988
Lynn DM, Anderson DG, Putnam D, Langer R (2001) Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J Am Chem Soc 123:8155–8156
Akinc A, Lynn DM, Anderson DG, Langer R (2003) Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J Am Chem Soc 125:5316–5323
Anderson DG, Lynn DM, Langer R (2003) Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew Chem Int Edit 42:3153–3158
Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Narayanannair JK, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DW, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG (2008) A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol 26:561–569
Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DW, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG (2010) Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci 107:1864–1869
Chen C-K, Law W-C, Aalinkeel R, Nair B, Kopwitthaya A, Mahajan SD, Reynolds JL, Zou J, Schwartz SA, Prasad PN, Cheng C (2012) Well-defined degradable cationic polylactide as nanocarrier for the delivery of siRNA to silence angiogenesis in prostate cancer. Adv Healthc Mater 1:751–761
Chen CK, Jones CH, Mistriotis P, Yu Y, Ma XN, Ravikrishnan A, Jiang M, Andreadis ST, Pfeifer BA, Cheng C (2013) Poly(ethylene glycol)-block-cationic polylactide nanocomplexes of differing charge density for gene delivery. Biomaterials 34:9688–9699
Chen CK, Law WC, Aalinkeel R, Yu Y, Nair B, Wu JC, Mahajan S, Reynolds JL, Li YK, Lai CK, Tzanakakis ES, Schwartz SA, Prasad PN, Cheng C (2014) Biodegradable cationic polymeric nanocapsules for overcoming multidrug resistance and enabling drug-gene co-delivery to cancer cells. Nanoscale 6:1567–1572
Miyata K, Oba M, Nakanishi M, Fukushima S, Yamasaki Y, Koyama H, Nishiyama N, Kataoka K (2008) Polyplexes from poly(aspartamide) bearing 1,2-diaminoethane side chains induce pH-selective, endosomal membrane destabilization with amplified transfection and negligible cytotoxicity. J Am Chem Soc 130:16287–16294
Uchida H, Miyata K, Oba M, Ishii T, Suma T, Itaka K, Nishiyama N, Kataoka K (2011) Odd-even effect of repeating aminoethylene units in the side chain of N-substituted polyaspartamides on gene transfection profiles. J Am Chem Soc 133:15524–15532
Uchida H, Itaka K, Nomoto T, Ishii T, Suma T, Ikegami M, Miyata K, Oba M, Nishiyama N, Kataoka K (2014) Modulated protonation of side chain aminoethylene repeats in N-substituted polyaspartamides promotes mRNA transfection. J Am Chem Soc 136:12396–12405
Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong Z, Lin C, Engbersen JF, Feijen J (2006) Reducible poly(amido ethylenimine)s designed for triggered intracellular gene delivery. Bioconjugate Chem 17:1233–1240
Hoon JJ, Christensen LV, Yockman JW, Zhong Z, Engbersen JF, Jong KW, Feijen J, Wan KS (2007) Reducible poly(amido ethylenimine) directed to enhance RNA interference. Biomaterials 28:1912–1917
Wang XL, Jensen R, Lu ZR (2007) A novel environment-sensitive biodegradable polydisulfide with protonatable pendants for nucleic acid delivery. J Control Release 120:250–258
Zintchenko A, Philipp A, Dehshahri A, Wagner E (2008) Simple modifications of branched PEI lead to highly efficient siRNA carriers with low toxicity. Bioconjugate Chem 19:1448–1455
Creusat G, Rinaldi AS, Weiss E, Elbaghdadi R, Remy JS, Mulherkar R, Zuber G (2010) Proton sponge trick for pH-sensitive disassembly of polyethylenimine-based siRNA delivery systems. Bioconjugate Chem 21:994–1002
Creusat G, Thomann JS, Maglott A, Pons B, Dontenwill M, Guerin E, Frisch B, Zuber G (2012) Pyridylthiourea-grafted polyethylenimine offers an effective assistance to siRNA-mediated gene silencing in vitro and in vivo. J Control Release 157:418–426
Blessing T, Kursa M, Holzhauser R, Kircheis R, Wagner E (2001) Different strategies for formation of pegylated EGF-conjugated PEI/DNA complexes for targeted gene delivery. Bioconjugate Chem 12:529–537
Merdan T, Kunath K, Petersen H, Bakowsky U, Voigt KH, Kopecek J, Kissel T (2005) PEGylation of poly(ethylene imine) affects stability of complexes with plasmid DNA under in vivo conditions in a dose-dependent manner after intravenous injection into mice. Bioconjugate Chem 16:785–792
Miteva M, Kirkbride KC, Kilchrist KV, Werfel TA, Li HM, Nelson CE, Gupta MK, Giorgio TD, Duvall CL (2015) Tuning PEGylation of mixed micelles to overcome intracellular and systemic siRNA delivery barriers. Biomaterials 38:97–107
Reineke TM, Davis ME (2003) Structural effects of carbohydrate-containing polycations on gene delivery. 2. Charge center type. Bioconjugate Chem 14:255–261
Carlisle RC, Etrych T, Briggs SS, Preece JA, Ulbrich K, Seymour LW (2004) Polymer-coated polyethylenimine/DNA complexes designed for triggered activation by intracellular reduction. J Gene Med 6:337–344
Ito T, Yoshihara C, Hamada K, Koyama Y (2010) DNA/polyethyleneimine/hyaluronic acid small complex particles and tumor suppression in mice. Biomaterials 31:2912–2918
Hornof M, de la FM, Hallikainen M, Tammi RH, Urtti A (2008) Low molecular weight hyaluronan shielding of DNA/PEI polyplexes facilitates CD44 receptor mediated uptake in human corneal epithelial cells. J Gene Med 10:70–80
Noga M, Edinger D, Rodl W, Wagner E, Winter G, Besheer A (2012) Controlled shielding and deshielding of gene delivery polyplexes using hydroxyethyl starch (HES) and alpha-amylase. J Control Release 159:92–103
Heller P, Birke A, Huesmann D, Weber B, Fischer K, Reske-Kunz A, Bros M, Barz M (2014) Introducing PeptoPlexes: polylysine-block-polysarcosine based polyplexes for transfection of HEK 293T cells. Macromol Biosci 14:1380–1395
Davis PB, Cooper MJ (2007) Vectors for airway gene delivery. AAPS J 9:E11–E17
Schottler S, Becker G, Winzen S, Steinbach T, Mohr K, Landfester K, Mailander V, Wurm FR (2016) Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol 11:372–377
Kukowska-Latallo JF, Raczka E, Quintana A, Chen C, Rymaszewski M, Baker JR Jr (2000) Intravascular and endobronchial DNA delivery to murine lung tissue using a novel, nonviral vector 999. Hum Gene Ther 11:1385–1395
Okuda T, Sugiyama A, Niidome T, Aoyagi H (2004) Characters of dendritic poly(-lysine) analogues with the terminal lysines replaced with arginines and histidines as gene carriers in vitro. Biomaterials 25:537–544
Schatzlein AG, Zinselmeyer BH, Elouzi A, Dufes C, Chim YTA, Roberts CJ, Davies MC, Munro A, Gray AI, Uchegbu IF (2005) Preferential liver gene expression with polypropylenimine dendrimers. J Control Release 101:247–258
Kadlecova Z, Rajendra Y, Matasci M, Baldi L, Hacker DL, Wurm FM, Klok HA (2013) DNA delivery with hyperbranched polylysine: a comparative study with linear and dendritic polylysine. J Control Release 169:276–288
Koppu S, Oh YJ, Edrada-Ebel R, Blatchford DR, Tetley L, Tate RJ, Dufes C (2010) Tumor regression after systemic administration of a novel tumor-targeted gene delivery system carrying a therapeutic plasmid DNA. J Control Release 143:215–221
Fischer W, Calderon M, Schulz A, Andreou I, Weber M, Haag R (2010) Dendritic polyglycerols with oligoamine shells show low toxicity and high siRNA transfection efficiency in vitro. Bioconjugate Chem 21:1744–1752
Frohlich T, Edinger D, Russ V, Wagner E (2012) Stabilization of polyplexes via polymer crosslinking for efficient siRNA delivery. Eur J Pharm Sci 47:914–920
Russ V, Frohlich T, Li Y, Halama A, Ogris M, Wagner E (2010) Improved in vivo gene transfer into tumor tissue by stabilization of pseudodendritic oligoethylenimine-based polyplexes. J Gene Med 12:180–193
Klutz K, Russ V, Willhauck MJ, Wunderlich N, Zach C, Gildehaus FJ, Goke B, Wagner E, Ogris M, Spitzweg C (2009) Targeted radioiodine therapy of neuroblastoma tumors following systemic nonviral delivery of the sodium iodide symporter gene. Clin Cancer Res 15:6079–6086
Russ V, Elfberg H, Thoma C, Kloeckner J, Ogris M, Wagner E (2008) Novel degradable oligoethylenimine acrylate ester-based pseudodendrimers for in vitro and in vivo gene transfer. Gene Ther 15:18–29
Russ V, Gunther M, Halama A, Ogris M, Wagner E (2008) Oligoethylenimine-grafted polypropylenimine dendrimers as degradable and biocompatible synthetic vectors for gene delivery. J Control Release 132:131–140
Leng Q, Scaria P, Zhu J, Ambulos N, Campbell P, Mixson AJ (2005) Highly branched HK peptides are effective carriers of siRNA. J Gene Med 7:977–986
Stevenson M, Ramos-Perez V, Singh S, Soliman M, Preece JA, Briggs SS, Read ML, Seymour LW (2008) Delivery of siRNA mediated by histidine-containing reducible polycations. J Control Release 130:46–56
Leng Q, Mixson AJ (2005) Small interfering RNA targeting Raf-1 inhibits tumor growth in vitro and in vivo. Cancer Gene Ther 12:682–690
Hartmann L, Börner HG (2009) Precision polymers: monodisperse, monomer-sequence-defined segments to target future demands of polymers in medicine. Adv Mater 21:3425–3431
Hartmann L, Krause E, Antonietti M, Borner HG (2006) Solid-phase supported polymer synthesis of sequence-defined, multifunctional poly(amidoamines). Biomacromolecules 7:1239–1244
Schaffert D, Badgujar N, Wagner E (2011) Novel Fmoc-polyamino acids for solid-phase synthesis of defined polyamidoamines. Org Lett 13:1586–1589
Salcher EE, Kos P, Frohlich T, Badgujar N, Scheible M, Wagner E (2012) Sequence-defined four-arm oligo(ethanamino)amides for pDNA and siRNA delivery: impact of building blocks on efficacy. J Control Release 164:380–386
Schaffert D, Troiber C, Wagner E (2012) New sequence-defined polyaminoamides with tailored endosomolytic properties for plasmid DNA delivery. Bioconjugate Chem 23:1157–1165
Schaffert D, Troiber C, Salcher EE, Frohlich T, Martin I, Badgujar N, Dohmen C, Edinger D, Klager R, Maiwald G, Farkasova K, Seeber S, Jahn-Hofmann K, Hadwiger P, Wagner E (2011) Solid-phase synthesis of sequence-defined T-, i-, and U-shape polymers for pDNA and siRNA delivery. Angew Chem Int Edit 50:8986–8989
Scholz C, Kos P, Leclercq L, Jin X, Cottet H, Wagner E (2014) Correlation of length of linear oligo(ethanamino) amides with gene transfer and cytotoxicity. ChemMedChem 9:2104–2110
Felgner PL, Barenholz Y, Behr JP, Cheng SH, Cullis P, Huang L, Jessee JA, Seymour L, Szoka F, Thierry AR, Wagner E, Wu G (1997) Nomenclature for synthetic gene delivery systems. Hum Gene Ther 8:511–512
Plank C, Zelphati O, Mykhaylyk O (2011) Magnetically enhanced nucleic acid delivery. Ten years of magnetofection-progress and prospects. Adv Drug Deliver Rev 63:1300–1331
Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Kruger A, Gansbacher B, Plank C (2002) Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther 9:102–109
Huttinger C, Hirschberger J, Jahnke A, Kostlin R, Brill T, Plank C, Kuchenhoff H, Krieger S, Schillinger U (2008) Neoadjuvant gene delivery of feline granulocyte-macrophage colony-stimulating factor using magnetofection for the treatment of feline fibrosarcomas: a phase I trial. J Gene Med 10:655–667
Bates K, Kostarelos K (2013) Carbon nanotubes as vectors for gene therapy: past achievements, present challenges and future goals. Adv Drug Deliver Rev 65:2023–2033
Feng L, Yang X, Shi X, Tan X, Peng R, Wang J, Liu Z (2013) Polyethylene glycol and polyethylenimine dual-functionalized nano-graphene oxide for photothermally enhanced gene delivery. Small 9:1989–1997
Kim H, Namgung R, Singha K, Oh IK, Kim WJ (2011) Graphene oxide-polyethylenimine nanoconstruct as a gene delivery vector and bioimaging tool. Bioconjugate Chem 22:2558–2567
Paul A, Hasan A, Kindi HA, Gaharwar AK, Rao VT, Nikkhah M, Shin SR, Krafft D, Dokmeci MR, Shum-Tim D, Khademhosseini A (2014) Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano 8:8050–8062
Qin SY, Feng J, Rong L, Jia HZ, Chen S, Liu XJ, Luo GF, Zhuo RX, Zhang XZ (2014) Theranostic GO-based nanohybrid for tumor induced imaging and potential combinational tumor therapy. Small 10:599–608
Zhang LM, Wang ZL, Lu ZX, Shen H, Huang J, Zhao QH, Liu M, He NY, Zhang ZJ (2013) PEGylated reduced graphene oxide as a superior ssRNA delivery system. J Mater Chem B 1:749–755
Kim H, Kim WJ (2014) Photothermally controlled gene delivery by reduced graphene oxide-polyethylenimine nanocomposite. Small 10:117–126
Hsieh TY, Huang WC, Kang YD, Chu CY, Liao WL, Chen YY, Chen SY (2016) Neurotensin-conjugated reduced graphene oxide with multi-stage near-infrared-triggered synergic targeted neuron gene transfection in vitro and in vivo for neurodegenerative disease therapy. Adv Healthc Mater 5:3016–3026
Kim HJ, Takemoto H, Yi Y, Zheng M, Maeda Y, Chaya H, Hayashi K, Mi P, Pittella F, Christie RJ, Toh K, Matsumoto Y, Nishiyama N, Miyata K, Kataoka K (2014) Precise engineering of siRNA delivery vehicles to tumors using polyion complexes and gold nanoparticles. ACS Nano 8:8979–8991
Yi Y, Kim HJ, Mi P, Zheng M, Takemoto H, Toh K, Kim BS, Hayashi K, Naito M, Matsumoto Y, Miyata K, Kataoka K (2016) Targeted systemic delivery of siRNA to cervical cancer model using cyclic RGD-installed unimer polyion complex-assembled gold nanoparticles. J Control Release 244:247–256
Moller K, Muller K, Engelke H, Brauchle C, Wagner E, Bein T (2016) Highly efficient siRNA delivery from core-shell mesoporous silica nanoparticles with multifunctional polymer caps. Nanoscale 8:4007–4019
Pittella F, Cabral H, Maeda Y, Mi P, Watanabe S, Takemoto H, Kim HJ, Nishiyama N, Miyata K, Kataoka K (2014) Systemic siRNA delivery to a spontaneous pancreatic tumor model in transgenic mice by PEGylated calcium phosphate hybrid micelles. J Control Release 178:18–24
Zuber G, Dauty E, Nothisen M, Belguise P, Behr JP (2001) Towards synthetic viruses. Adv Drug Deliver Rev 52:245–253
Wagner E (2004) Strategies to improve DNA polyplexes for in vivo gene transfer: will “artificial viruses” be the answer? Pharm Res 21:8–14
Boeckle S, Wagner E (2006) Optimizing targeted gene delivery: chemical modification of viral vectors and synthesis of artificial virus vector systems. AAPS J 8:E731–E742
Murthy N, Campbell J, Fausto N, Hoffman AS, Stayton PS (2003) Bioinspired pH-responsive polymers for the intracellular delivery of biomolecular drugs. Bioconjugate Chem 14:412–419
Wagner E (2007) Programmed drug delivery: nanosystems for tumor targeting. Expert Opin Biol Th 7:587–593
Kursa M, Walker GF, Roessler V, Ogris M, Roedl W, Kircheis R, Wagner E (2003) Novel shielded transferrin-polyethylene glycol-polyethylenimine/DNA complexes for systemic tumor-targeted gene transfer. Bioconjugate Chem 14:222–231
Kircheis R, Kichler A, Wallner G, Kursa M, Ogris M, Felzmann T, Buchberger M, Wagner E (1997) Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. Gene Ther 4:409–418
Murthy N, Campbell J, Fausto N, Hoffman AS, Stayton PS (2003) Design and synthesis of pH-responsive polymeric carriers that target uptake and enhance the intracellular delivery of oligonucleotides. J Control Release 89:365–374
Knorr V, Allmendinger L, Walker GF, Paintner FF, Wagner E (2007) An acetal-based PEGylation reagent for pH-sensitive shielding of DNA polyplexes. Bioconjugate Chem 18:1218–1225
Beckert L, Kostka L, Kessel E, Krhac Levacic A, Kostkova H, Etrych T, Lachelt U, Wagner E (2016) Acid-labile pHPMA modification of four-arm oligoaminoamide pDNA polyplexes balances shielding and gene transfer activity in vitro and in vivo. Eur J Pharm Biopharm 105:85–96
Wagner E (2008) Converging paths of viral and non-viral vector engineering. Mol Ther 16:1–2
Xu PS, Li SY, Li Q, Van Kirk EA, Ren J, Murdoch WJ, Zhang ZJ, Radosz M, Shen YQ (2008) Virion-mimicking nanocapsules from pH-controlled hierarchical self-assembly for gene delivery. Angew Chem Int Edit 47:1260–1264
Wolff JA, Rozema DB (2008) Breaking the bonds: non-viral vectors become chemically dynamic. Mol Ther 16:8–15
Meyer M, Philipp A, Oskuee R, Schmidt C, Wagner E (2008) Breathing life into polycations: functionalization with pH-responsive endosomolytic peptides and polyethylene glycol enables siRNA delivery. J Am Chem Soc 130:3272–3273
Meyer M, Dohmen C, Philipp A, Kiener D, Maiwald G, Scheu C, Ogris M, Wagner E (2009) Synthesis and biological evaluation of a bioresponsive and endosomolytic siRNA-polymer conjugate. Mol Pharm 6:752–762
Cheng Y, Yumul RC, Pun SH (2016) Virus-inspired polymer for efficient in vitro and in vivo gene delivery. Angew Chem Int Edit 55:12013–12017
Butun V, Armes SP, Billingham NC (2001) Synthesis and aqueous solution properties of near-monodisperse tertiary amine methacrylate homopolymers and diblock copolymers. Polymer 42:5993–6008
Wang D, Wang T, Liu J, Yu H, Jiao S, Feng B, Zhou F, Fu Y, Yin Q, Zhang P, Zhang Z, Zhou Z, Li Y (2016) Acid-activatable versatile micelleplexes for PD-L1 blockade-enhanced cancer photodynamic immunotherapy. Nano Lett 16:5503–5513
Alfarouk KO, Muddathir AK, Shayoub MEA (2011) Tumor Acidity as Evolutionary Spite. Cancers 3:408–414
Cardone RA, Casavola V, Reshkin SJ (2005) The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer 5:786–795
Sun CY, Shen S, Xu CF, Li HJ, Liu Y, Cao ZT, Yang XZ, Xia JX, Wang J (2015) Tumor acidity-sensitive polymeric vector for active targeted siRNA delivery. J Am Chem Soc 137:15217–15224
Choi S, Lee KD (2008) Enhanced gene delivery using disulfide-crosslinked low molecular weight polyethylenimine with listeriolysin o-polyethylenimine disulfide conjugate. J Control Release 131:70–76
Klein PM, Wagner E (2014) Bioreducible polycations as shuttles for therapeutic nucleic acid and protein transfection. Antioxid Redox Sign 21:804–817
Klein PM, Reinhard S, Lee DJ, Muller K, Ponader D, Hartmann L, Wagner E (2016) Precise redox-sensitive cleavage sites for improved bioactivity of siRNA lipopolyplexes. Nanoscale 8:18098–18104
McKenzie DL, Kwok KY, Rice KG (2000) A potent new class of reductively activated peptide gene delivery agents. J Biol Chem 275:9970–9977
Read ML, Bremner KH, Oupicky D, Green NK, Searle PF, Seymour LW (2003) Vectors based on reducible polycations facilitate intracellular release of nucleic acids. J Gene Med 5:232–245
Chen X, Bai Y, Zaro JL, Shen WC (2010) Design of an in vivo cleavable disulfide linker in recombinant fusion proteins. Biotechniques 49:513–518
Brulisauer L, Kathriner N, Prenrecaj M, Gauthier MA, Leroux JC (2012) Tracking the bioreduction of disulfide-containing cationic dendrimers. Angew Chem Int Edit 51:12454–12458
Cerritelli S, Velluto D, Hubbell JA (2007) PEG-SS-PPS: reduction-sensitive disulfide block copolymer vesicles for intracellular drug delivery. Biomacromolecules 8:1966–1972
Sun H, Guo B, Cheng R, Meng F, Liu H, Zhong Z (2009) Biodegradable micelles with sheddable poly(ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials 30:6358–6366
Zhu CH, Zheng M, Meng FH, Mickler FM, Ruthardt N, Zhu XL, Zhong ZY (2012) Reversibly shielded DNA polyplexes based on bioreducible PDMAEMA-SS-PEG-SS-PDMAEMA triblock copolymers mediate markedly enhanced nonviral gene transfection. Biomacromolecules 13:769–778
Zanta MA, Belguise VP, Behr JP (1999) Gene delivery: a single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc Natl Acad Sci 96:91–96
van der Aa MA, Koning GA, d’Oliveira C, Oosting RS, Wilschut KJ, Hennink WE, Crommelin DJ (2005) An NLS peptide covalently linked to linear DNA does not enhance transfection efficiency of cationic polymer based gene delivery systems. J Gene Med 7:208–217
Remaut K, Symens N, Lucas B, Demeester J, De Smedt SC (2014) Cell division responsive peptides for optimized plasmid DNA delivery: the mitotic window of opportunity? J Control Release 179:1–9
Andersen H, Parhamifar L, Hunter AC, Shahin V, Moghimi SM (2016) AFM visualization of sub-50 nm polyplex disposition to the nuclear pore complex without compromising the integrity of the nuclear envelope. J Control Release 244:24–29
Martin I, Dohmen C, Mas-Moruno C, Troiber C, Kos P, Schaffert D, Lachelt U, Teixido M, Günther M, Kessler H, Giralt E, Wagner E (2012) Solid-phase-assisted synthesis of targeting peptide-PEG-oligo(ethane amino)amides for receptor-mediated gene delivery. Org Biomol Chem 10:3258–3268
Lachelt U, Wittmann V, Muller K, Edinger D, Kos P, Hohn M, Wagner E (2014) Synthetic polyglutamylation of dual-functional MTX ligands for enhanced combined cytotoxicity of poly(I:C) nanoplexes. Mol Pharm 11:2631–2639
Zhang CY, Kos P, Muller K, Schrimpf W, Troiber C, Lachelt U, Scholz C, Lamb DC, Wagner E (2014) Native chemical ligation for conversion of sequence-defined oligomers into targeted pDNA and siRNA carriers. J Control Release 180:42–50
Fröhlich T, Edinger D, Kläger R, Troiber C, Salcher E, Badgujar N, Martin I, Schaffert D, Cengizeroglu A, Hadwiger P, Vornlocher HP, Wagner E (2012) Structure-activity relationships of siRNA carriers based on sequence-defined oligo (ethane amino) amides. J Control Release 160:532–541
Troiber C, Edinger D, Kos P, Schreiner L, Klager R, Herrmann A, Wagner E (2013) Stabilizing effect of tyrosine trimers on pDNA and siRNA polyplexes. Biomaterials 34:1624–1633
Whitehead KA, Dorkin JR, Vegas AJ, Chang PH, Veiseh O, Matthews J, Fenton OS, Zhang Y, Olejnik KT, Yesilyurt V, Chen D, Barros S, Klebanov B, Novobrantseva T, Langer R, Anderson DG (2014) Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat Commun 5:4277
Acknowledgements
Peng Zhang appreciates receiving China Scholarship Council fellowships as support to his PhD studies at Ludwig-Maximilians-Universität München, Germany. We also acknowledge the financial support by the DFG Excellence Cluster Nanosystems Initiative Munich (NIM) and SinoGermanCenter Grant GZ995.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection “Polymeric Gene Delivery Systems”; edited by Yiyun Cheng.
Communicated by: Yiyun Cheng.
Rights and permissions
About this article
Cite this article
Zhang, P., Wagner, E. History of Polymeric Gene Delivery Systems. Top Curr Chem (Z) 375, 26 (2017). https://doi.org/10.1007/s41061-017-0112-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-017-0112-0