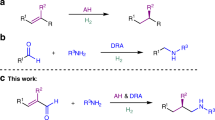
Abstract
The cascade [1,n]-hydrogen transfer/cyclization, recognized as the tert-amino effect one century ago, has received considerable interest in recent decades, and great achievements have been made. With the aid of this strategy, the inert C(sp3)–H bonds can be directly functionalized into C–C, C–N, C–O bonds under catalysis of Lewis acids, Brønsted acids, as well as organocatalysts, and even merely under thermal conditions. Hydrogen can be transferred intramolecularly from hydrogen donor to acceptor in the form of hydride, or proton, followed by cyclization to furnish the cyclic products in processes featuring high atom economy. Methylene/methine adjacent to heteroatoms, e.g., nitrogen, oxygen, sulfur, can be exploited as hydride donor as well as methylene/methine without heteroatom assistance. Miscellaneous electrophilic subunits or intermediates, e.g., alkylidene malonate, carbophilic metal activated alkyne or allene, α,β-unsaturated aldehydes/ketone, saturated aldehydes/iminium, ketenimine/carbodiimide, metal carbenoid, electron-withdrawing groups activated allene/alkyne, in situ generated carbocation, can serve as hydride acceptors. This methodology has shown preeminent power to construct 5-, 6-, or 7-membered heterocyclic as well as carbon rings. In this chapter, various hydrogen donors and acceptors are adequately discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
When talking about hydride donors, undoubtedly miscellaneous metal hydrides serving as reducing agents should be mentioned first, e.g., NaBH4, LiAlH4, Red-Al, selectride, etc. In addition, organic molecules can also play the role of hydride donors, which could be retrospected to 1853 when Cannizzaro reported base-mediated disproportionation of benzaldehyde into benzyl alcohol and benzoic acid via intermolecular hydride transfer from a deprotonated hemiacetal intermediate onto an aldehyde [1–3]. The Tishchenko reaction may be considered as the seminal example of a reaction proceeding by an intramolecular hydride shift. Evans et al. further developed this reaction to diastereoselectively construct β-hydroxy ketones, which is known as the Evans–Tishchenko reaction [4]. Similarly, by changing the redox state of the reactants in the Tishchenko reaction, the alcohol can be readily oxidized to aldehyde via the Meerwein–Pondorf–Verley (MPV) reduction [5–7], and aldehyde can be reduced to alcohol via the reverse Oppenauer oxidation, which operate by means of an identical Al3+-preorganized intramolecular 1,5-hydride shift [8–10].
Over the past decades, tremendous progress has been made in functionalization of inert C–H with the demand of green and sustainable chemistry [11–14]. The fast development of this vigorous research field arises from the recognition by the chemical community that such methodologies are able to streamline synthetic routes and facilitate the direct formation of C–C bonds and C–Z bonds (Z=O, N, B, Si, etc.) without prefunctionalization of inert C–H bonds to C–X bonds (X = halogens, OTf, etc.). In this context, a large number of innovative and efficient synthetic methodologies have been developed, thus offering chemists powerful tools for the rapid buildup of molecules with complex architectures. Among these methodologies, the transition metal-catalyzed C(sp2)–H bond activation has dominated this area and the direct functionalization of inert C(sp3)–H bonds still remains a great challenge owing to the high bond dissociation energy of C(sp3)–H bonds. Only recently, some promising catalytic processes for the selective functionalization of C(sp3)–H have been reported with noble metal salts, e.g., Pd, Rh as catalysts. Despite numerous challenges posed by direct C(sp3)–H bond activation, the cascade [1,n]-hydrogen transfer/cyclization process opens new avenues, which provides organic chemists unusual solutions to address their synthetic challenges [15–20]. This fascinating chemistry was discovered in 1895, which was initially termed as ‘tert-amino effect’ by Meth-Cohn and Suschitzky in 1972 [21]. This name refers to the tendency of substituted N,N-dialkylanilines to undergo unusually facile ring-closing reactions involving various groups at the ortho position. However, the tertiary amine group is neither necessary nor sufficient to guarantee a successful reaction for a particular substrate.
The substrate requires a hydride acceptor proximal to a C–H bond serving as hydride donor, and the reaction is initiated by a hydride shift (or related H-atom-transfer step), which formally oxidizes the carbon donor and reduces the hydride acceptor. The new C–X bond will be formed at the hydride-donor atom, after the hydride shift takes place. The defining characteristic for these reactions is the functionalization of a C–H bond concurrent with a hydride shift. The names proposed by Sames (“HT-cyclization”) and Akiyama (“Internal Redox Cascade”) would seem more appropriate if focusing more on the unique hydride-shift mechanism that draws together a diverse group of substrates, at least for intramolecular examples.
This cascade process has been recognized as an efficient and powerful method for selective activation and direct functionalization of inactive C(sp3)–H bonds. It represents an intriguing sequential C(sp3)–H activation/C–C, C–N or C–O bonds formation process and proves to be a versatile protocol to construct 5-, 6-, or 7–membered hetero/carbon spiro or fused cycles, such as tetrahydroquinolines [22], chromans [23–25], spiroethers [26–30], and tetrahydropyrans [31–36], which are common moieties in biologically important natural products and pharmaceuticals.
A series of significant review papers have been published on this chemistry [15–18] and we aim to cover the progress in this field since 2006. Some reputable groups such as Sames, Seidel, Akiyama, Vidal, Liu, and Gagosz showed preeminent applications of this methodology to build various hetero/carbon spirocycles and fused rings. In the following, these elegant findings will be categorized according to the types of hydride donors (i.e., tert-amino effect, C(sp3)–H bonds α to ethereal oxygen and sulfur, benzylic C(sp3)–H bond, non-benzylic C(sp3)–H bond) and the types of hydrogen acceptors (benzylidene malonate, transition metal activated alkyne or allene, enal or enone, aldehyde or imine, ketenimine/carbodiimide). This review will provide elementary insight into these cascade reactions concerning the mechanism, the reactivity of hydrogen donor and acceptor, migration modes of hydrogen, etc.
2 Mechanistic Insight into [1,5]-Hydrogen Transfer
[1,5]-Hydrogen transfer is selected as a model reaction for discussing the mechanism as it is the most common mode of hydrogen migration.
2.1 Possible Reaction Pathways
The exact nature of the hydrogen transfer is still a matter of debate in the scientific community. Some argue that hydrogen is transferred via a sigmatropic shift, whereas others believe that the migration of hydrogen to the acceptor occurs in the form of a hydride anion [37–42]. A plausible mechanism of this transformation is depicted in Scheme 1. Initially, 1 and zwitterion B form a resonance hybrid. Subsequently B undergoes [1,5]-hydrogen transfer in the form of a sigmatropic hydrogen, resulting in zwitterion A. A consecutive intramolecular nucleophilic attack affords the final cyclic product 2 [43–45].
This cascade process can also be rationalized in an alternative way (Scheme 2). The zwitterion A is generated from 1 via [1,5]-hydrogen transfer from the carbon α to heteroatom X to the electrophilic acceptor in the form of hydride anion, followed by an intramolecular 6-endo-trig cyclization (or intramolecular nucleophilic attack), giving rise to the heterocycle 2 [46–48]. DFT calculations show that [1,5]-hydride (or hydrogen) transfer is the rate-determining step and the energy barrier of the subsequent cyclization step is very low [49–52]. Generally speaking, the second theoretical explanation is more reasonable because the aromatic ring is dispensable and the cascade HT-cyclization can proceed smoothly within many aliphatic substrates.
The final cyclization can also proceed in the manner of a 6π-electrocyclic ring closure (6π-ERC) (Scheme 3 ). After a [1,5]-hydride transfer, the conjugated 1,3,5-hexatriene intermediate I can be produced, which undergoes subsequent 6π-ERC to give the cyclized product 4. Thus, the formation of unstable zwitterionic intermediate A (Schemes 1, 2) with charge separation is avoided [52–56].
2.2 Reactivity of Different Hydrogen Donors
Heteroatoms, e.g., nitrogen, oxygen, and sulfur, adjacent to hydrogen donors (methylene or methine) can facilitate the hydride migration, so do aryl groups and alkyl groups. Theoretically, the heteroatoms play three roles. Firstly, heteroatoms with high electronegativities will polarize the C–X bond, causing the weakening of the C–H bond. Secondly, the hyperconjugation effect of σ *C–H orbital with a neighboring atom lone pair or π-orbital promotes the hydride shift (Fig. 1) [57–59]. This effect not only weakens the C(sp3)–H bond but also increases the negative charge density of the hydrogen atom.
Thirdly, the carbocationic intermediate generated upon hydride migration, with which iminium, oxocarbenium, and thiocarbenium can form resonance hybrids, would be stabilized by adjacent heteroatoms via p–p conjugation. Consequently, the rate of C(sp3)–H bond cleavage is closely associated with the stability of cationic intermediate, thus any factor that can stabilize this intermediate will dramatically promote this process, while the groups destabilizing it will retard the proximal C(sp3)–H bond cleavage. In contrast to iminium cation, which has considerable stability, oxocarbenium- and thiocarbenium ions are less stable and more difficult to generate, not to mention benzylic and tert-alkyl carbocation (Fig. 2). DFT calculations and experimental results show that the thiocarbenium ion exhibits a little bit higher stability than the oxocarbenium species [60]. The cationic intermediate can also be stabilized by the aromatic or alkyl substituents on the hydrogen donors via π–p conjugation or σ–p hyperconjugation, respectively. Primary C–H bond is rarely exploited as hydrogen donor except in Zhang’s and Chatani’s reports [40, 61, 62]. In addition to the above-mentioned hydride donors, acetalic and dithioacetalic C–H bonds can also work as the hydride donors [52, 57–59].
2.3 Activation Mode of Hydride Acceptors
Almost all the electrophilic groups or electrophilic intermediates can be employed as the hydride acceptors, e.g., alkylidene malonates, carbophilic transition metals activated alkynes or allenes, enal/enones, aldehyde/ketones, imines, ketenimine/carbodiimides, metal carbenoids, alkynes carrying electron-withdrawing groups, as well as in situ-generated carbocations (Fig. 3).
The feasibility of hydrogen transfer strongly depends on the natures of hydrogen acceptors and donors. It can be imagined that there is competition between the transient cationic subunits and the electrophilic hydride acceptors for the hydride after cleavage of the inert C(sp3)–H bond (Fig. 3). If the hydride acceptor is electrophilic enough, it can “snatch” hydride to give the zwitterion A, followed by an intramolecular nucleophilic attack to give the cyclized product 2 (Scheme 2); whereas if not, the cationic subunit will “retrieve” hydride and no reaction will occur. Therefore, two strategies are applicable to facilitate the cascade process, i.e., increasing the electrophilicity of the hydride acceptor or increasing the stability of the cationic subunit generated in situ upon hydride migration. Remarkably, hydrogen can be transferred not only in the form of hydride anion but also in the form of protons and hydrogen atoms [63]. If the hydrogen acceptor is a relatively strong nucleophile, hydrogen will be abstracted by the acceptor in the form of a proton [53, 62, 64–68], and if the hydrogen acceptor is a free radical, the C–H of hydrogen donator will be homolyzed to give the hydrogen atom, which is then transferred to the hydrogen acceptor [63]. The hydrogen can be transferred not only in [1,5]-manner but also in the manners of [1,4]-[69, 70] [1,6]-[39, 66, 71–77], and [1,9]-manner [78]. If the hydride donor and acceptor are active enough, the hydride migration may occur through space, giving rise to a zwitterionic intermediate. Only if the nucleophile and electrophile in the zwitterionic intermediate are located in proper geometric positions, the subsequent intramolecular nucleophilic attack will occur, resulting in the formation of 5- [72, 73], 6-, or 7- [41, 79, 80] membered products. If no nucleophile is available or cyclization was blocked by steric hindrance, hydride will merely serve as reductant [81–84] or unwanted side products will be produced [74].
3 C(sp3)–H Bond Adjacent to Tert-Amino Moieties as Hydride Donor (tert-Amino Effect)
The term “tert-amino effect” is used to describe ring-closure of N, N-dialkyl-substituted anilines with an unsaturated electrophilic ortho substituent to afford fused tetrahydroquinolines [22] or other N-heterocycles [15, 19, 20, 46, 85–87]. The tert-amino effect has been widely utilized in the synthesis of pyridine, pyrimidine, and pyridazine derivatives, which has been well reviewed by Mátyus et al. [15].
3.1 Electrophilic Benzylidene Malonates as the Hydride Acceptors
Hurd et al. elegantly elaborated this methodology in the key step of total synthesis of PNU-286607 (Scheme 4) [88]. The benzylidene intermediate 5 was prepared in situ and [1,5]-hydride migration readily proceeded under thermal conditions to give zwitterionic intermediate 6. Via trans–cis isomerization of methyl group, the zwitterion 6 was converted to thermodynamically more favorable zwitterion 7, and a subsequent intramolecular equatorial attack of the enolate on the iminium subunit furnished cis (−)-PNU-286607 in 74 % yield and >99:1 er.
For a long time, harsh thermal conditions were always needed to overcome the high energy barrier of [1,5]-hydride transfer, which severely limited the application of this strategy. Seidel et al. employed Ga(OTf)3 to catalyze the cascade process of 8, via which tetrahydroquinolines 9 could be furnished in 90 % yield within 15 min at room temperature (Scheme 5) [89]. Meanwhile, the chiral bisoxazoline magnesium complex 10 was employed to catalyze the asymmetric version of this reaction, furnishing the enantio-enriched product 9 in 74 % yield and 30 % ee, which presented the first report of enantioselective cascade [1,5]-HT/cyclization.
Akiyama et al. disclosed a chiral phosphoric acid 13-catalyzed asymmetric cascade [1,5]-HT/cyclization of 11, which afforded tetrahydroquinolines 12 with good to excellent enantioselectivity (Scheme 6) [90]. The benzylidene malonate subunit forms hydrogen bonds with the proton of phosphoric acid 13, which not only increased the electrophilicity of hydride acceptor but also governed the asymmetric step. Presumably, the stereoselectivity is mostly controlled at the hydride shift step and the enantiotopic hydrogen is selectively activated by chiral phosphoric acid 13.
One more asymmetric version of cascade [1,5]-hydride transfer/cyclization was reported by Feng et al. using his well-known chiral N, N′-dioxide-Co(II) complex 14 as catalyst. The optically active tetrahydroquinolines 15 were obtained in excellent yields and high enantioselectivities (Scheme 7) [91]. Theoretically, the oxygen atoms of N, N′-dioxide, amide, and the benzylidene malonate are coordinated to cobalt(II) in a hexadentate manner, hence the carbanion prefers to attack the Re face rather than the Si face of the iminium because the latter is strongly shielded by the nearby anthracenyl ring, furnishing the (S)-configured products.
Luo et al. exploited a binary catalytic system, which involved Mg(BF4)2 and chiral phosphoric acid 18 to facilitate the cascade reaction of 16, furnishing the enantio-enriched products 17 in high yields and enantioselectivities (Scheme 8) [49, 50]. Both Ha and Hb on the isoquinoline methylene carbon atom may participate in [1,5]-hydride transfer, requiring two different helical conformations I and II. Due to the suprafacial constraint, however, I is more favorable than II owing to its space tolerance. Therefore, the selective activation in complex I initiates enantiotopic [1,5]-Hb transfer, leading to the chiral helical zwitterionic intermediate. Finally, the C–C bond can be formed spontaneously with preserved stereochemistry after a small conformational change.
Matyus et al. described a cascade Knovenagel/1,5-hydride transfer/cyclization reactions of 4-aryl-2-phenyl-1,4-benzoxazepine derivatives 19, which furnished fused O,N-heterocycles 20 containing tetrahydro-1,4-benzoxazepine and tetrahydroquinoline moieties with high yields and diastereoselectivity (Scheme 9) [92]. Basically, under thermal conditions, the benzylidene intermediate I generated in situ underwent sequential 1,5-hydride shift and intramolecular 6-endo cyclization readily to furnished 20.
3.2 Electrophilic Activated Alkyne as the Hydride Acceptor
Electron-deficient alkyne can also serve as an ideal hydride acceptor. Barluenga et al. described a [1,5]-hydride transfer/cyclization process of alkynyl Fischer carbene complexes 21, which afforded 1,2-dihydroquinolynyl carbene complexes 22 (Scheme 10) [51]. The alkyne moiety in 21 activated by electrophilic Fischer carbene was a good hydride acceptor. Theoretically, migration of hydride from the benzylic methylene to the highly electrophilic β carbon of the triple bond generates zwitterionic intermediate I and a subsequent cyclization leads to the new carbene complex 22, which can be further elaborated [51, 93]. The presence of strong electron-withdrawing chromium pentacarbonyl moiety is crucial to trigger the energy-demanding [1,5]-hydride transfer. When alkynyl carbene complex 23 was heated with four equivalents of 1-hexyne 24, 5,6-dihydrophenantridine derivative 25 could be afforded.
The alkynes activated by alkynophilic metals, e.g., platinum and ruthenium, are considerably electrophilic, which can be employed as good hydride acceptors for the cascade [1,5]-HT/cyclization process. Chatani et al. reported a cycloisomerization of 9-carbazolyl substituted 1-alkyl-2-ethynylbenzene 26 catalyzed by alkynophilic metal salts PtCl2 and [RuCl2(CO)3]2 under mild conditions, which produced substituted indene 27 (Scheme 11) [40]. Basically, the metal-vinylidene complex I is formed initially via π-activation of alkyne moiety, then benzylic hydride is delivered in [1,5]-manner to the most electrophilic α-carbon of metal vinylidene, resulting in zwitterionic intermediate II. Afterwards, the metal carbenoid intermediate III generated via resonance of intermediate II undergoes 6π-electrocyclization to give intermediate IV and a final reductive elimination gives rise to the cyclized product 27.
A methylene group adjacent to a protected secondary amine, e.g., carbamate, could also be exploited as hydride donor. Remarkably, as the lone pair of nitrogen was partially transferred to carbonyl group via p–π conjugation, the electron-donating ability of nitrogen was significantly decreased and the negative charge density of the hydrogen atom could only be partially increased by nitrogen via hyper-conjugative interaction compared with tert-amine. Therefore, high valent metal salts owning stronger activation ability should be employed to facilitate the cascade process. Additionally, because of the electron-withdrawing nature of carbamates, electron density on the nitrogen atom was decreased, resulting in comparative difficulty in forming alkoxycarbonyl-iminium intermediate, which is less stable than that generated from tert-amine. Sames et al. reported a PtI4-catalyzed α-alkenylation of protected cyclic secondary amines 28, which afforded annulation products 29 (Scheme 12) [39]. Spirocyclization products 29 could also be furnished in good yield via the cascade protocol if the terminal alkyne was substituted at C(2) of cyclic amine 30. Theoretically, the platinum vinylidene I is formed via π-activation of the alkyne moiety, followed by [1,6]-hydride transfer through space to afford intermediate II, in which the nucleophilic vinyl-platinum attacks the electrophilic alkoxycarbonyl-iminium fragment to give the intermediate III. A final platinum salt elimination gives rise to the fused products 29 or 31.
Liang et al. described a palladium-catalyzed cascade [1,5]-HT/cyclization of propargylic esters 32 to construct substituted naphthylamines 33 (Scheme 13) [53]. Notably, propargylic esters substituted with electron-rich aryl groups, which led to electron-rich allenyl-palladium complex at the propargylic position, always gave better yields than the ones with electron-withdrawing substituents, and the electron-withdrawing acyl or sulfonyl group on the nitrogen was crucial to the reaction. These clues indicated the hydrogen was abstracted by nucleophilic allenyl-palladium in the form of proton. Mechanistically, the nucleophilic allenyl-palladium intermediate II is generated from the propargylic compound 32 under the catalysis of Pd(0), then [1,5]-proton transfer follows to afford the intermediate III, the direct 6π-electrocyclic ring closure (ERC) of which leads to the intermediate IV. Afterwards, IV undergoes [1,3]-hydrogen shift and elimination to afford the final product 33 (path A). Alternatively, the intermediate VI may also be formed via a [1,3]-palladium shift of the intermediate III. The following insertion of the C-Pd bond and hydrogen elimination afford the product 33 (path B).
The same group also reported a PtCl2-catalyzed hydro-functionalization reaction of allenes formed in situ from propargylic esters 34, which furnished multi-functionalized tetrahydroquinolines 35 (Scheme 14) [42]. If R3 is an electron-withdrawing group, the formation of products 35 is favored. Mechanistically, hydride is delivered initially to C(1)-carbon of platinum activated allene intermediate I formed via platinum-catalyzed [1,3]-OAc migration. The resulting vinyl-platinum species II then attacks the iminium to furnish the fused tetrahydroquinoline 35. A completely different transformation occurred in the case of propargylic ester 36 bearing a strong electron-donating 4–MeOC6H5 group in R3, furnishing an α, β-unsaturated ketone 37, which suggested that electron-donating 4–OMeC6H5 group in R3 decreased the electrophilicity of C(1), and the hydration of allene would be more favored than [1,5]-hydride shift.
Zhang et al. reported an efficient synthesis of piperidin-4-ones based on gold-catalyzed cascade process (Scheme 15) [61, 68]. One-pot sequential m-CPBA oxidation and gold-catalysis with Ph3PAuNTf2 led to an excellent yield of piperidin-4-one 39. This chemistry allowed facile preparation of 5-, 6-, and 7-membered ring-fused or spiro-piperidin-4-ones. Initially, Zhang et al. speculated that tertiary aliphatic amine N-oxide I generated via m-CPBA oxidation of tertiary amine 38 might undergo gold-catalyzed intramolecular alkyne oxidation [94] to furnish α-oxo gold carbene II, in which α-hydride would migrate to the electrophilic gold carbene, leading to the formation of zwitterion III containing an electrophilic iminium and a nucleophilic gold-enolate. A subsequent intramolecular cyclization furnished piperidin-4-one 39. Notably, the less-substituted methyl in amine 40 was preferentially involved in the ring formation with serviceable regioselectivities (5:1). In all the substrates whose tert-amine moieties are unsymmetrical, the chemistry behavior of hydrogen donors was rather unusual, i.e., the poor hydride donors were more active than good hydride donors, e.g., methyl > methylene and benzylic methylene, methylene > methine, electron-rich benzylic methylene ≈ electron-deficient benzylic methylene. Although the proposed mechanism could account for the formation of product 41 and 41′, it failed to explain the regioselectivity. Thus, the initially proposed mechanism of [1,5]-hydride transfer/cyclization is quite questionable.
Based on DFT calculation and a variety of control experiments, Zhang and Houk et al. argued that the mechanism involving the sequential ring opening and [1,5]-proton shift was energetically more favorable (Scheme 16) [62]. Presumably, the first step is a syn addition of gold-coordinated N-oxide 42 to alkyne, resulting in the formation of intermediate 43, which undergoes a hetero-retro-ene ([1,5]-proton shift) to furnish intermediate 44, thus the formation of gold-carbenoid intermediate III is avoided. The final step is a cyclization of 44 to yield piperidin-4-one derivatives 45 and regenerate the catalyst, which was calculated to be the rate-determining step. In 43, phosphine ligand makes the adjacent carbon more nucleophilic, thus the proton is abstracted from the least sterically hindered amine-substituent.
3.3 Electrophilic α,β-Unsaturated Aldehyde and Acyl Oxazolidinone as the Hydride Acceptors
Sames et al. reported a PtCl4-catalyzed α-alkylation of protected pyrrolidine 46, via which the fused cycles 47 was furnished in good yield and high diastereoselectivity (dr > 15:1) (Scheme 17) [95]. Notably, the conformational rigidity of the substrate 46 could be increased significantly by the malonate moiety, which led to a more reactive hydride acceptor [96]. Because the iminium subunit in intermediate II could be significantly destabilized by carbamate, which rendered secondary C–H bond in intermediate I serving as hydride donor less reactive, high catalyst loading (30 mol%) of highly active PtCl4 was indispensable to get decent yield (77 %).
Seidel et al. exploited the complex of Mg(OTf)2 and chiral bisoxazoline 50 to catalyze the cascade reaction of substrates 48 carrying α, β-unsaturated acyl oxazolidinone, which produced chiral tetrahydroquinolines 49 in good yields and high enantioselectivities (Scheme 18) [97]. The employment of nickel perchlorate in combination with ligand 50 also furnished 49 in good diastereo- and enantioselectivity.
Chiral secondary amine could be employed to catalyze this cascade process. Kim et al. utilized Jørgensen catalyst 53 successfully to catalyze the cascade [1,5]-HT/ring closure sequences of o-N-pyrrolidinyl-substituted cinnamaldehydes 51 via sequential iminium and enamine activation, affording chiral tetrahydroquinolines 52 in high enantioselectivities (Scheme 19, a) [98]. Products incorporated with 7- to 9-membered azacycles could also be formed with excellent enantioselectivities. The enal could also be furnished in situ via aerobic oxidation of allylic alcohol. The same group described a one-pot transformation of 3-arylprop-2-en-1-ol derivatives 54 into tetrahydroquinolines 55 via a sequence of Ru(VII)-catalyzed aerobic oxidation/1,5-hydride transfer/cyclization (Scheme 19, b) [99], in which an in situ generated enal served as hydride acceptor. TMS substituted prolinol ether 56 was exploited as chiral catalyst, achieving high levels of enantioselectivity. Additionally, the saturated aldehyde 57 could be employed as the precursor of hydride acceptor, which was transformed in situ to α,β-unsaturated iminium II via Pd(II)-catalyzed aerobic oxidation (Scheme 19, c). Via a cascade Saegusa-type oxidative enamine catalysis/1,5-HT/cyclization sequences, which was catalyzed by Pd(II) and prolinol ether 56 in a relay style [100], tetrahydroquinolines 55 was prepared in moderate yields and high levels of enantioselectivity. Furthermore, the electrophilic α,β-unsaturated iminium II could also be generated under metal-free oxidation condition (Scheme 19, d) [101]. The same group reported a Jørgensen catalyst 56-catalyzed enantioselective three-step cascade reaction of substrate 57 involving oxidation/[1,5]-hydride transfer/ring closure, [102] in which IBX was exploited as oxidative agent to transform enamine to hydride acceptor II, furnishing tetrahydroquinolines 58 in moderate yields, moderate-to-high diastereoselectivities and excellent enantioselectivities.
A similar reaction could also be implemented with enone 58 as hydride acceptor. This time, Kim et al. employed chiral primary amine 59 as chiral catalyst to prompt the cascade process [103], affording tetrahydroquinine derivatives 60 in moderate yields and with high enantioselectivities (up to 97 % ee) (Scheme 20).
Yuan et al. reported a FeCl3-catalyzed stereoselective cascade [1,5]-HT/ring closure of 61, furnishing structurally diverse spirocyclic oxindolyl tetrahydroquinolines 62 bearing contiguous quaternary or tertiary stereogenic carbon centers in high yields and with good diastereoselectivities (Scheme 21) [104]. The asymmetric reaction could be catalyzed by chiral phosphoric acid 63 (20 mol%), affording the enantio-enriched tetrahydroquinoline in 95 % yield, 94:6 dr and 54 % ee (R1 = CO2Et, R2, R3 = butylene, R4 = H). The same reaction was further investigated by Feng et al. with his well-known chiral N, N′-dioxide-scandium complex 14 [105], resulting in the optically active spirooxindolyl tetrahydroquinolines 62 in good yields (up to 97 %) with excellent diastereoselectivities (>20:1) and enantioselectivities (up to 94 % ee). Strong chiral memory effect was found using a chiral substrate.
If two or more hydride donators and acceptors are available in the substrate, multiple cascade processes will occur to furnish complex cyclic products. Akiyama et al. reported a fascinating Yb(OTf)3-catalyzed double C(sp3)–H bond functionalizations of benzylamine derivative 64 through a sequential dual hydride shift/cyclization process, which carries two potential hydrogen donors, i.e., methylene a and methylene b (Scheme 22) [106]. The key feature was the initial hydride shift ([1,4]- or [1,6]-hydride shift), which was completely controlled by the bulkiness of α substituent of trifluoromethyl ketone. Through variation of the R group of ketone, the sequence of functionalization of two potential hydrogen donors could be totally reversed. A bicyclo[3.2.2]nonane skeleton 65 afforded from the substrate 64a with trans-α,β-unsaturated trifluoroacetyl group (R1=H) by a sequential [1,6]-and [1,5]-hydride shift process (equation A). On the other hand, a [1,4]- and [1,5]-hydride shift occurred successively in the substrate 64b with a benzyl group α to a trifluoroacetyl group, resulting in the formation of bicyclic product 66 (equation B). The regioselectivity of initial C(sp3)–H activation was elegantly rationalized by Akiyama via DFT calculation and theoretical studies revealed that the resonance stabilization in the benzylidene carbonyl moiety and the steric repulsion of the α-substituent (R) were the key to change the reaction course.
The enone could be prepared in situ via Knoevenagel condensation. Wang et al. reported a ZnCl2-catalyzed tandem Knoevenagel condensation/1,5-hydride shift/cyclization of o-aminiobenzaldehyde 67 with substrate 68 carrying reactive methylene (Scheme 23) [107], which produced spiropyrazolo-terahydroquinoline derivatives 69 in good to high yields with good to excellent diastereoselectivities (up to 95 % yield, >95:5 dr). The strong Lewis acid, i.e., ZnCl2, could not only facilitate the formation of enone I and enolate II but also increase the electrophilicity of enone by chelation of the carbonyl of enone to promote 1,5-hydride shift.
3.4 Saturated Aldehydes and Imines as the Hydride Acceptors
The C–H bond can be functionalized not only into C–C bond but also into C–O bond and C–N bond via similar cascade processes. Mátyus et al. reported a microwave-assisted synthesis of tricyclic angularly annulated aminal 71 from ortho-dialkylaminobenzaldehdyde 70 with H2O as solvent (Scheme 24) [108, 109]. Even though the reaction was conducted under harsh conditions, i.e., at 210 °C for 50 min and employment of stoichiometric K2CO3, aminal 71 could only be obtained in low yield. According to the report by Maulide et al., Lewis acidic condition should be beneficial to the transformation [110], which was not investigated in Mátyus’ report
The annulated aminal can be employed as unstable intermediate for further C–H functionalization of tertiary amines. Maulide et al. described a Sc(OTf)3-catalyzed one-pot C–H functionalization of cyclic tertiary amines 72, in which the sacrificial reduction of a neighboring carboxaldehyde group directed addition of Grignard reagents and lithium alkynyl trifluoroborates to the α-position of amine moiety, resulting in the corresponding α-functionalized products 74 and 74′ bearing a wide range of appendages in good to excellent yields. (Scheme 25) [110]. Basically, the formation of 73/73′ from 72 is under thermodynamic control and 73 is the thermodynamically favored product. The aminals 73 and 73′ can be reversed to intermediate I and II, thus an equilibrium for interconversion exists between 73 and 73′. The more favorable iminium intermediate I (stabilized by more substituents) and less favorable iminium intermediate II can be trapped by more nucleophilic reagents, leading to the final stable products 74 and 74′, respectively.
Sames et al. reported a highly active TiF4-catalyzed intramolecular hydro-O-alkylation of aldehyde substrate 75 (Scheme 26), via which the N-protected pyrrolidine substrate 75 was transformed into a cis-fused bicyclic aminal 76 as a single diastereomer [37]. Because of the electron-withdrawing nature of carbamate and less activity of secondary C–H bond, the employment of highly active and oxophilic TiF4 with high catalyst loading (1.3 eq) was crucial for successful transformation, which resulted in the fused aminal in 68 % yield and good diastereoselectivity (≥50:1).
The in situ-generated iminium could also be exploited as hydride acceptor, via which inert C(sp3)–H bond could be functionalized to C–N bond efficiently. Seidel et al. reported a trifluoroacetic acid-catalyzed cascade [1,6]-hydride transfer/cyclization of 77 to synthesize 7,8,9-trisubstituted dihydropurine derivatives 78 (Scheme 27) [73]. TFA plays two roles in this process: (1) promotion of imine formation and (2) protonation of imine for acceleration of the hydride shift process. Meth-Cohn and Volochnyuk et al. reported similar reactions in 1967 [71] and 2007, respectively [72].
The same group also described a TfOH-catalyzed one-pot synthesis of aminals 81 from o-aminobenzaldehydes 79 and primary aromatic or aliphatic amines 80 (Scheme 28) [111]. The protonated imine I worked as hydride acceptor. Almost at the same time, Akiyama et al. reported a similar catalytic approach to synthesize quinazolines and TsOH·H2O was identified as the optimal catalyst [112].
Ketoesters could be exploited to generate iminium intermediate employed as hydride acceptor. Gong et al. reported a chiral Brønsted acid 85-catalyzed asymmetric cascade [1,5]-hydride transfer/cyclization of 2-pyrrolidinyl phenyl ketoesters 82 with anilines 83, which produced the enantio-enriched cyclic aminals 84 (Scheme 29) [113]. The iminium subunit in intermediate I served as hydride acceptor, which was generated in situ through the condensation of o-aminobenzoketone 82 with aniline 83 in the presence of 85.
Seidel et al. also reported a metal-free one-pot α-amination of cyclic secondary amines 78 with 2-aminobenzaldehyde 77, which efficiently furnished ring-fused aminals 79 in good yields (Scheme 30) [64–66]. Both the electronic structure and the geometry of the amines have profound effects on reactivities and yields. Via an extensive exploration of possible pathways using DFT calculations based on the original experimental results, Seidel and Houk proposed an unusual mechanism involving cascade [1,5]-proton transfer/cyclization [66]. Initially amino-benzaldehyde 77 reacts with secondary amine 78 to furnish hemi-aminal I, which undergoes subsequent dehydration to give quinoidal intermediates II. The nucleophilic imine subunit then abstracts a proton from the methylene adjacent to tert-amine moiety ([1,6]-proton shift), resulting in azomethine ylide III, which is rapidly protonated by ethanol. Subsequently, IV is furnished via internal proton transfer and finally ring-fused aminal 79 is formed by intramolecular nucleophilic attack. Dang and Bai et al. also reported a similar cascade process for preparation of tetrahydro-pyrimido[4,5-d]pyrimidine [114].
The electrophilic iminium could also be prepared in situ from alkyne via gold catalysis. Gong et al. disclosed a catalytic domino hydroamination/redox reaction, which could directly assemble the tertiary amine substituted 3-en-1-yne derivatives 80 and various amines 81 into cyclic aminals 82 in excellent yields and moderate to high diastereoselectivities by using the combination of gold(I) complex and TfOH (Scheme 31) [115]. Theoretically, terminal alkyne 80 undergoes gold(I)-catalyzed intermolecular hydroamination with aniline 81 to give imine intermediate II, which is protonated by Brønsted acid to give electrophilic iminium species III; this intermediate then undergoes a subsequent [1,5]-hydride transfer to generate a transient intermediate IV, which ultimately suffers an intramolecular nucleophilic attack to afford 82. The asymmetric version could be facilitated by over-stoichiometric chiral phosphoric acid and 5 mol% Ph3PAuNTf2, giving rise to enantio-enriched 82 in high yield and excellent enantioselectivity.
3.5 Electrophilic Metal Carbenoids as the Hydride Acceptors
As an electrophilic species, metal carbenoid can also serve as an ideal hydride acceptor. Saa et al. elegantly demonstrated a [Cp*Ru(cod)Cl]-catalyzed cyclization of protected alkynyl pyrrolidine 83, which furnished 1-Azaspiro[4,4]-nonane 84 carrying versatile TMS moiety as a single diastereomer in good yield (Scheme 32) [76]. Mechanistically, the ruthenium carbenoid I is afforded from N2CHTMS and [Cp*Ru(cod)Cl], which undergoes cycloaddition with 83 to give metallacyclobutene II. A subsequent ring opening of II leads to the electrophilic Ru-vinyl carbenoid III, which suffers [1,5]-hydride shift to furnish intermediate IV. Ultimately, an intramolecular nucleophilic attack gives rise to the spiro product 84.
C3-linked piperidine 85 was also readily cyclized under the optimal condition with less reactive secondary C–H bonds α to protected secondary amine as the hydride donor, furnishing the fused bicyclic piperidine 86 in good yields (Scheme 33).
3.6 Other Electrophiles as the Hydride Acceptors
The in situ-generated carbocation can be exploited as a good hydride acceptor. Zhang et al. reported an enantioselective catalytic intramolecular redox reaction of yne-enones 87 in the presence of Au(I) catalyst and chiral ligand 89, affording 7-membered tetrahydroazepines 88 in high yield and with high to excellent enantioselectivity (Scheme 34) [41, 79]. Compared with relatively inactive C–H bond α to tert-amine, oxygen of carbonyl is more nucleophilic and ready to attack the electrophilic alkyne activated by Au(I) catalyst. Basically, alkynophilic Au(I) catalyst triggers a heterocyclization (first cyclization) by activation of the alkyne to generate the furanyl carbocationic subunit in intermediate II, which is exploited as the hydride acceptor.
Nitroalkene is a versatile electron-deficient olefin, which might be exploited as the hydride acceptor as well. The cascade process was investigated by Jordis et al. with (E)-1-(2-(2-nitrovinyl)phenyl)pyrrolidine 90 as the substrate (Scheme 35) [116]. This transformation could be accomplished under thermal conditions (118 °C) in 80 h, leading to the cyclized product 91 and 91′ in 39 % yield (syn/anti = 12: 1).
Nitroalkene could also be activated by Lewis acids, e.g., Sc(OTf)3, Yb(OTf)3, and Zn(OTf)2 to increase its electrophilicity. According to the unpublished result in Pfaltz group, the cascade process could be facilitated by Yb(OTf)3, which furnished the cyclized product 91 in 60 % yield at 80 °C within 12 h (Scheme 36). The transformation was diastereospecific and only anti diastereomer was observed. The diastereoselectivity can be explained through Zimmer–Traxler transition state II in which the orientation of substituents would be pseudoequatorial, leading to the anti-product via intramolecular nucleophilic attack.
The electrophilic vinylogous iminium could also be exploited as hydride acceptor. Seidel et al. reported a diphenyl phosphate (DPP)-catalyzed cascade [1,5]-hydride shift/cyclization with doubly nucleophilic indole 93 and o-aminobenzaldehydes 92 as substrates, which gave rise to 7-membered rings 94 in good to excellent yields (Scheme 37) [80]. Mechanistically, the acid-catalyzed reaction of aldehyde 92 with indole 93 initially furnishes the electrophilic vinylogous iminium I via sequential Friedel–Crafts reaction and dehydration. A subsequent intramolecular [1,5]-hydride transfer leads to electrophilic iminium II, which traps nucleophilic C2 of indole to afford 7-membered product 94.
Sun and Xu et al. reported a Brønsted acid-catalyzed cascade dehydration/1,5-hydride shift/cyclization of 2-arylpyrroles 95 and 2-(pyrrolidin-1-yl)-, 2-(piperidin-1-yl), or 2-morpholinobenzaldehydes 96 (Scheme 38) [117], which produced structurally diverse 7-membered 1,2-pyrrole-annulated benzazepines 97 in yields of 25–65 %. Both the initial Friedel–Crafts reaction and subsequent dehydration were promoted by PTSA, resulting in the carbocationic intermediate II, which served as hydride acceptor.
The same group also reported a PTSA-catalyzed synthesis of spiroindolenines 100 from 2-substituted (Me, Et) indoles 98 and 2-(pyrrolidin-1-yl)benzaldehydes 99 via a [1,5]-hydride shift/cyclization sequence with good to excellent yields and moderate diastereoselectivity (dr 3.5:1)(Scheme 39) [118]. The major diastereoisomer product could be readily obtained with up to >20/1 d.r. by simple washing with isopropyl ether after flash chromatography. Similar to Seidel’s report, the in situ-generated vinylogous iminium intermediate I served as the hydride acceptor, which underwent subsequent [1,5]-hydride transfer and cyclization to furnish spiroindolenines 100. As C2 of indole moiety was substituted by an alkyl group, the more nucleophilic C3 will attack the iminium moiety instead, resulting in the dearomatization of indole subunit.
Gong et al. discovered a MsOH-catalyzed cascade oxidation/C(sp3)–H functionalization of unactivated terminal alkynes 101 with 102 as the oxidant, which yielded 2,3-dihydroquinolin-4(1H)-ones 103 (Scheme 40) [119]. Mechanistically, the nucleophilic nitrogen atom initially captures a proton that is delivered to alkynes subsequently. Intermediate I is formed by dearomatization of styrene cation. Afterwards, two possible pathways might operate to afford the final products. In path A, the nucleophilic attack of pyridine-N-oxide onto intermediate I generates enolate II, which undergoes sequential delocalization and [1,5]-hydride transfer/ring-closure to furnish a final intermediate III. The interaction between methane-sulfonic anion and pyridine cation in III facilitates C–H and N–O bonds cleavage to afford 103. In path B, the hydride of intermediate I migrates preferentially, which is followed by cyclization and nucleophilic attack of pyridine-N-oxide onto benzylic cation IV, resulting in the intermediate III.
Harmata et al. developed an intramolecular redox C–H activation process of alkenyl sulfoximines to synthesize 4- and 6-membered heterocycles 106 and 105 (Scheme 41) [120]. Terminal alkene activated by sulfoximines worked as hydride acceptor and allylic C–H bond served as hydride donor. Notably, the reaction time strongly influenced the formation of final products. When 104 was refluxed in toluene for 3.5 h, the 4-membered cyclic species 106 could be obtained in 41 % yield as the major product; whereas if the reaction was refluxed for around 24 h, 6-membered thiazines 105 were isolated as a mixture of diastereomers in 40 % yield. Mechanistically, an intramolecular [1,5]-hydride migration operates initially, leading to zwitterionic intermediate I. Subsequent ring closure can be formulated as the intramolecular collapse of the zwitterionic intermediate I or II. The formation of 4-membered product 106 might be kinetically favorable and reversible. Although intermediate II might be less stable than intermediate I for the reason that the allylic positive charge in intermediate I can be dispersed by more substituents, the conversion of 4-membered 106 to 105 is thermodynamically favorable and the driving force might be the release of cyclic strain of 106.
Nitrones have been widely exploited in various cycloaddition reactions. Intriguingly, these electrophilic species can also serve as the hydride acceptors. Sun and Xu et al. reported an expeditious access to structurally diverse oxadiazepines 108 via 1,5-hydride shift/cyclization of pyrrolidine- or tetrahydroisoquinoline-containing nitrones 107 with nitrones as hydride acceptors and AlCl3 was exploited as Lewis to promote the cascade process (Scheme 42) [121]. Furthermore, the nitrone 107 could be furnished in situ, which underwent subsequent 1,5-hydride shift, and ring cyclization through a one-pot process to afford 108 in good yields.
4 C(sp3)–H Bond Adjacent to Ethereal Oxygen as The Hydride Donors
In addition to the C–H bond adjacent to tert-amino moieties, methylene (or methine) adjacent to ethereal oxygen could also be employed as hydride donor. As discussed in the section of mechanistic insight, the C–H bond adjacent to ethereal oxygen is less reactive than that adjacent to tert-amine, thus more reactive hydride acceptors are required.
4.1 Electrophilic Benzylidene Malonates and Their Derivatives as the Hydride Acceptors
Sames et al. reported a Sc(OTf)3-catalyzed intramolecular hydroalkylation of isolated electron-deficient olefins (Scheme 43) [95]. Tetrahydropyrans or tetrahydrofurans carrying C(2)-linked α, β-unsaturated malonate side chain 109 and 111 were employed as substrate to furnish the spiroether product 110 and 112 in excellent yields. Notably, germinal substitution along the olefin tether was not required for efficient annulation for the reason that benzylidene malonate activated by Sc(OTf)3 were reactive enough, thus higher conformational rigidity to increase the reactivity of hydride acceptor was not indispensable [96].
The same group also reported a Sc(OTf)3-catalyzed [1,5]-hydride transfer/cyclization of ortho-vinylaryl alkyl ethers 113, via which highly substituted dihydrobenzopyran 114 could be prepared in excellent yields (Scheme 44) [122].
SnCl4 could also be employed as Lewis acid to efficiently catalyze the cascade process for synthesis of a benzopyran skeleton 116 from benzyloxy benzylidene malonate 115 (Scheme 45) [123]. Notably, the methyl ortho to the alkoxy group or the benzylidene moiety could enhance the reactivity drastically compared with non-substituted substrate. This remarkable enhancement of the reactivity could be well rationalized by following two factors: (1) the conformational behavior of the benzyloxy group and (2) the “buttressing effect”. In the case of 115 having an o-methyl group, the conformational equilibrium largely shifted to the left conformer 115a because of the severe steric repulsion. As a consequence, the “buttressing effect” between the methyl group and the benzyloxy group made the hydrogens on the benzyl group much closer to benzylidene electrophilic carbon. As a result of the synergetic effect of these two factors, hydride can be delivered more readily to the acceptor, thus both catalyst loading and reaction time could be dramatically reduced.
4.2 Electrophilic Activated Alkyne and Allene as the Hydride Acceptors
Yamamoto et al. reported a PtBr2-catalyzed cyclization of 1-ethynyl-2-(1-alkoxybut-3-enyl)-benzenes 117, which furnished functionalized indenes 118 in good to allowable yields (Scheme 46) [124]. Notably, the allyl group substituted at benzylic position was indispensable for the success of this cyclization, without which the reaction did not work at all. This observation suggests that the coordination of olefin to platinum at a right position/geometry might be essential for the indene formation.
Chatani et al. employed other alkynophilic metals such as PtCl2, PtCl4, and [RuCl2(CO)3]2 to catalyze the cyclization of non-allyl substituted 1-ethynyl-2-(1-alkoxyalkyl) benzenes 119 and 121 under mild condition, via which the desired indenes 120 and 122 were furnished in high yields (Scheme 47) [40]. In contrast to Yamamoto’s results [124], the substrates without allyl group still worked well.
The same group also investigated catalytic cyclization of 2-alkyl-1-ethynylbenzene derivatives carrying silyl ether groups as in 123 and 125 (Scheme 48) [40]. In contrast to Liu’s result [44], when silyl ether-substituted 2-methy-1-ethynylbenzene was subjected to the reaction, only silyl ether-substituted indenes 124 and 126 were afforded in good to excellent yields.
Liu et al. also reported a TpRuPPh3(CH3CN)2·SbF6-catalyzed cyclization of 2-alkyl-1-ethynylbenzenes 127 bearing a siloxy group, which produced synthetically valuable 1-indanones 128 or 1H-1-indenols 129 in reasonable yields and in short periods (Scheme 49) [44]. Basically, terminal alkyne subunit is transformed to ruthenium-vinylidene I initially, which undergoes a [1,5]-hydride shift to give ruthenium-containing 1,3,5-hexatriene II. A subsequent 6π-electrocyclization of intermediate II furnishes ruthenium-containing cyclohexadiene III, which suffers reductive elimination to produce 1-substituted-1H-indene 128. Afterwards, the cationic ruthenium catalyst attacks C(2) carbon of indene to form benzyl cation IV, followed by [1,2]-hydride shift to afford ruthenium cyclopentylidene V. Ultimately, 1-indanone 129 is produced via a second hydride shift and hydrolysis.
Liang et al. reported a PtCl2-catalyzed transformation of 3-(2-alkyl)phenyl-propynyl acetate 130 to prepare naphthalenyl acetate 131 (Scheme 50) [45]. The electrophilic Pt–allene complexes formed in situ worked as hydride acceptors. Mechanistically, the Pt(II)-promoted [1,3]-OAc shift leads to the formation of platinum-activated allenyl ester I, which undergoes a [1,5]-hydride shift to form 1,3,5-hexatriene II. A subsequent 6π-electrocyclic ring closure affords intermediate III, which further eliminates the methoxy group, resulting in rearomatization to afford 131.
Liu et al. reported a PPh3AuCl/AgSbF6-catalyzed cycloisomerization of allenene acetal functionality 132, via which bicyclo[3.2.1]oct-6-en-2-ones 133 were prepared in high yields, high chemoselectivities, and high stereoselectivities (Scheme 51) [125]. In most cases, only one single stereoisomer of the resulting cyclic products was formed despite their molecular complexities. Mechanistically, substrate 132 initially undergoes Au(I)-catalyzed allene cyclization to give electrophilic Au(I)-alkenyl carbenoid I, which abstracts acetalic hydride through [1,5]-hydride transfer, leading to the formation of Au(I)-η1-allyl species II containing a dimethoxymethyl cation. A subsequent SE2′ addition of Au(I)-η1-allyl functionality at this oxocarbeniums opposite the neighboring methyl group affords tricyclic species III with its methyl group on the same side as the adjacent hydrogen and ethyl group. A final acid-catalyzed deprotection leads to 133.
Urabe et al. described a Rh2(TFA)4-catalyzed cyclization of alkynyl ethers 134, which afforded dihydropyrans 135 in good yield (Scheme 52) [126]. Ring closure proceeded in a highly regioselective manner, and no isomeric five-membered product 136 was detected. Notably, the sulfonyl moiety was critical for the success of this reaction. Mechanistically, initial coordination of Rh(II) to alkyne subunit generates a cationic carbon β to sulfonyl group, which abstracts a hydride from methylene α to ethereal oxygen, generating a zwitterionic intermediate III. A final intramolecular nucleophilic attack furnishes the product 135.
Sames et al. developed an α-alkenylation of cyclic ethers to synthesize both annulation and spirocyclization products (Scheme 53) [39]. Four types of electronically diverse hydride donors were investigated and remarkably the selection of suitable catalysts was crucial to the success of cascade processes. As to substrate 137, in which relatively unreactive secondary C–H bond was exploited as hydride donor, no aromatic group was available to stabilize the oxocarbenium generated via [1,5]-HT. Hypervalent platinum catalyst PtI4 was the optimal catalyst, which affected complete conversion of 137 to furnish the product 138 in 86 % yield, whereas PtI4 only led to complete decomposition of 141 despite higher reactivity of its tertiary C–H bond. Less active platinum catalyst K2PtCl4 was the optimal catalyst to produce spirocycles 142 in 70 % isolated yield. With K2PtCl4 as catalyst, substrate 143 could also be transformed into fused product 144 in 62 % yield. Although hydride donor in 143 was a less active secondary C–H bond, the oxocarbenium generated upon [1,5]-HT could be stabilized by adjacent phenyl group, thus less active K2PtCl4 could facilitate the conversion. The brominated derivative 139 gave a lower yield of compound 140 (33 %) even though highly active PtI4 was employed, showing a considerable sensitivity of this reaction to electron-withdrawing substituents, particularly in para- and ortho-position due to their destabilization of oxocarbenium intermediates. Theoretically, the platinum vinylidene I is formed initially, followed by through-space [1,6]-hydride transfer to produce zwitterionic intermediate II, which affords the final product via a sequential C–C bond formation and platinum salt elimination.
Gagosz et al. reported Au(I) catalyst 147-catalyzed alkylation of alkynyl ethers which produced cyclohexane 146 as major product (Scheme 54) [127]. Theoretically, the electrophilic activation of the alkyne 145 by Au(I) initiates a [1,5]-hydride shift to furnish oxocarbenium ion I, interaction of which with the pendant nucleophilic vinyl-gold moiety affords cyclopropenium intermediate II. Carbocation IV, which would finally collapse into cyclohexene 146 after elimination of the gold(I) catalyst might be generated via a [1,2]-alkyl shift on Au-carbene intermediate III.
In contrast to C(2)-linked terminal alkynes 145, gold-catalyzed alkylation of C(3)-linked tetrahydrofurans bearing terminal alkyne functions 148 mainly led to the formation of major product exo-methylene cyclopentanes 149 and minor products 150 (Scheme 55). This reversed selectivity might be explained by the relative stability of intermediates III and V. Steric constrains should be weaker for the fused bicyclic intermediate V (in Scheme 55) than intermediate III (in Scheme 54), thus allowing a rapid [1,2]-hydride shift, which leads to VI rather than a [1,2]-alkyl shift, which leads to III.
Gold-activated allene can also be employed as hydride acceptor. Gagosz et al. demonstrated that a phosphite gold complex 154-catalyzed intramolecular hydroalkylation of allenes 151, which afforded the spiro compound 152 and undesired fused bicyclic compound 153 in 30 and 61 % yields, respectively (Scheme 56) [128]. The selectivity could be reversed if HNTf2 was exploited. Under Brønsted acidic condition, the transformation was slower but furnished exclusively the desired spiro compound 152 in an excellent yield. The two reactions under Au(I) or Brønsted acid catalysis proceeded under very mild conditions in a stereoselective manner and two new contiguous asymmetric centers were formed.
A similar complete divergence in product selectivity was observed when the substrates 155 possessing a benzyl ether moiety were treated with either gold complex 154 or HNTf2 (Scheme 57) [128]. Under gold catalysis, tetrahydropyran 157 was obtained in 94 % yield, while tetrahydropyran 156 was produced in 84 % yield with HNTf2 as the catalyst. The stereoselective formation of compound 156 can be explained by the highly ordered chair-like transition state IV, which leads to carbocation V from oxocarbenium I. The relative trans relationship between the phenyl and isopropenyl substituents in product 156 results from the pseudoequatorial orientation of the phenyl and isopropylidene group in transition state IV. An analogous disposition explained the cis relationship between the phenyl group and the alkyl substituent at C(6).
4.3 Electrophilic α, β-Unsaturated Aldehydes and Ketones as the Hydride Acceptors
The electrophilicity of alkene subunit of α, β-unsaturated aldehydes can be dramatically increased if activated by BF3·Et2O, which serves as an ideal hydride acceptor. Sames et al. described a BF3·Et2O-catalyzed intramolecular hydroalkylation reaction of α, β- unsaturated aldehydes 158, via which spirocycles 159 could be furnished in good yields at ambient temperature as a mixture of diastereomers (Scheme 58) [95].
In addition to tertiary C–H bonds, secondary C–H bonds could also be directly functionalized (Scheme 59) [95]. Compared with tertiary C–H bond, secondary C–H bond was less reactive, therefore more active Lewis acid, i.e., PtCl4 (10 mol%) was employed to facilitate hydroalkylation of enal 160, giving rising to the fused annulation product 161 in 40 % yield and good diastereoselectivity (dr >15: 1).
In addition to the more active α, β-enal, hydroalkylation of less active enones 162 also proceeded smoothly (Scheme 60) [95]. Under the catalysis of 30 mol% of BF3·Et2O and with comparatively active tertiary C–H as hydride donor, the substrate 162a or 162b carrying α, β-unsaturated methyl and phenyl ketone units were efficiently transformed into spirocycles 163a and 163b, respectively, in excellent yields and moderate diastereoselectivities.
The 2,3-disubstituted tetrahydropyran 165 could be obtained in high diastereoselectivity via treatment of acyclic ether 164 with a substoichiometric amount of BF3·Et2O at elevated temperature (Scheme 61) [95]. The less active secondary C–H bond acted as hydride donor and less active methyl ketone was employed as hydride acceptor. Because the oxocarbenium could be stabilized by adjacent phenyl group, relatively mild Lewis acid BF3·Et2O (75 mol%) was active enough to promote the cascade process efficiently, resulting in the formation of desired product 165 in high yield (90 %) and good diastereoselectivity (dr >15: 1).
A simple and economical intramolecular hydroalkylation of olefins was elegantly demonstrated by Sames et al. based on the generation of highly reactive alkenyl-oxocarbenium intermediates I in situ from acetals (Scheme 62) [38]. Under the catalysis of BF3·Et2O, the cyclic product 167 could be obtained in good yield and diastereoselectivity from acetal 166 within 1 h. Direct comparison of 166 to the corresponding unprotected aldehyde showed a drastic increase in both reactivity and chemical yield, as well as an improvement in diastereoselectivity. Theoretically, BF3·Et2O opens cyclic acetal 166 to generate oxocarbenium intermediate I, which activates conjugated alkene moiety for subsequent hydride abstraction. After hydride transfer, the resulting oxocarbenium-enol ether intermediate II undergoes rapid C–C bond formation and the acetal can be reformed from the new oxocarbenium species III, producing 167. The observed high stereoselectivity can be explained by the favorable transition state II in which all the substituents are in equatorial positions.
The intramolecular hydroalkylation of enones could be readily implemented as well via this strategy (Scheme 63) [38]. The addition of ethylene glycol had a dramatic effect on the reaction rate as demonstrated in the cyclization of enones 168; the reaction could be completed within 12 h, furnishing the cyclized products 169 in good yields but with low diastereoselectivities. This strategy could not only drastically increase the reaction rate but also improve the isolated yields and stereoselectivities.
Tu et al. reported a Macmillan’s catalyst 172-catalyzed asymmetric α-alkylation of tetrahydrofuran 170 containing an α,β-unsaturated aldehyde, via which chiral spiroether 171 could be prepared (Scheme 64) [129]. The sequential [1,5]-hydride transfer/cyclization was facilitated via cascade iminium/enamine activation. The presence of strong acid was indispensable to ensure sufficient electrophilicity of the iminium intermediate. Theoretically, substrate 170 reacts with 172 to give iminium intermediate I. Owing to the steric interaction of the bulky tert-butyl group, the E enamine II is formed preferentially upon [1,5]-HT, which exists in two possible conformers III and IV. Because of dipole repulsion between the cyclic-oxocarbenium and enamine moieties in conformer III, IV is the more favored conformer, which undergoes intramolecular C–C bond formation to afford the final product 171.
4.4 Saturated Aldehyde and Ketone as the Hydride Acceptors
Sames et al. described a BF3·Et2O-catalyzed intramolecular hydro-O-alkylation of aldehyde substrates 173, which led to spiroketal products 174 (Scheme 65) [37]. Because oxocarbenium generated upon hydride migration is also a highly electron-deficient species, which would compete for hydride with aldehyde, thus how to deliver hydride to acceptor is challenging. Sames’ protocol to address this problem was to employ BF3·Et2O (30 mol%) for activation of carbonyl of 161, rendering hydride acceptor more electrophilic to “snatch” hydride. Tetrahydropyran substrate 173 could be converted to spiroketal 174 in high isolated yield at ambient temperature. Remarkably, the final cyclization step was a reversible process, which was under thermodynamic control, thus the cascade process was highly diastereoselective. Both 6, 6-scaffolds and 5, 6 spiroketals could be obtained in excellent yields.
The cis-fused bicyclic acetal 176 could also be afforded as a single diastereomer via this strategy with 175 as the substrate (Scheme 66) [37]. Mild Lewis acid, e.g., BF3·Et2O, was inactive and only the stronger oxophilic Lewis acid TiF4 (20 mol%) could facilitate the transformation for the reason that secondary C–H was less reactive than the tertiary C–H bond in 173.
In addition to aldehyde substrates, less electrophilic ketone 177 could also be converted into spiroketal 178 in 30 % yield and excellent diastereoselectivity under the mediation of stoichiometric TiF4 (Scheme 67) [37]. BF3·Et2O was not active enough to promote this hydro-O-alkylation, which might be ascribed to the poor reactivity of methyl ketone.
4.5 Electrophilic Metal Carbenoid as the Hydride Acceptors
Saa et al. discovered a ruthenium-catalyzed diastereoselective cyclization of linear alkynyl ether 179 involving cyclic and acyclic ethers, which produced spirocycles 180 in fairly good yields (Scheme 68) [76]. The ring size of the cyclic ether had a dramatic effect on the reaction time and yields.
Similarly, acetalic C–H bond could be exploited as hydride donor as well, and via ruthenium-catalyzed cyclization of linear alkynyl acetals 181, spirobicycles 182 could be furnished in fairly good yield (Scheme 69) [76], along with the formation of linear hydroxyester 183, which was generated by hydrolysis of intermediate I. Notably, rigid cyclic acetal afforded a higher yield of spiro compound in comparison to the linear acetals [96].
[1,6]-hydride shift/cyclization process could occur when specially designed dioxolane substrate 184 was subjected to the reaction, affording 5,6-spirocyclic product 185 in excellent yield (Scheme 70) [76]. The examples showed again the hydride could be delivered through space and the distance between hydride donor and acceptor was not an issue. A comparison of the cyclizations of dioxolanes 181 and 184 shows the more rapid formation of the 1,4-dioxaspiro[4,5]decane 185 versus 1,4-dioxaspiro[4,4] nonane 182, which clearly indicated that the conformation of metallic intermediate played a definitive role during the course of the reaction.
Fukuyama et al. exploited highly reactive rhodium carbenoids as hydride acceptors to construct tetrahydrofuran moieties in his total syntheses for many times. The mechanism should be the cascade [1,5]-Hydride migration/cyclization, whereas Fukuyama et al. argued that their reactions proceed via metal carbene C–H insertion reactions [130–135].
4.6 Ketenimines and Carbodiimides as Hydride Acceptors
Alajarin and Vidal et al. discovered dihydroquinolines and spirocyclic dioxolano-quinazolines 187 could be readily accessed via cascade [1,5]-hydride transfer/6π-ERC under thermal conditions (Scheme 71, a) [58]. Basically, with active ketenimine or carbodiimide as hydride acceptor whose central carbon atom is highly electrophilic, the cascade process is facilitated by the hydricity of the acetalic C–H bonds in 186. After hydride migration, the 1,3,5-conjugated hexatriene I can be generated, which had a long conjugate system and can be stabilized to a large extent. The ketenimines 188 bearing ether moiety could also be transformed exclusively into dihydroquinolines 189 (Scheme 71, b) [56].
The same group described a Sc(OTf)3-catalyzed three-step cascade reaction of 190 involving hydride shift/cyclization/hydrolysis as well, which produced indanones 191 (Scheme 72) [70]. Hydride was delivered to acceptor in an uncommon [1,4]-manner and Lewis acid activation was indispensable. Sc(OTf)3 was the preferable Lewis acid that could catalyze the cascade process. Because of the oxophilicity of Sc(OTf)3, it not only catalyzed the cascade process but also promoted the hydrolysis of acetalic function.
4.7 Electron-Withdrawing Group Activated Allene as Hydride Acceptor
Allene could be activated not only by carbophilic transition metals but also by electron-withdrawing groups at the terminal carbon atom. Alajarin, Sanchez-Andrada, and Vidal et al. disclosed that 2-(1,3-dioxolan-2-yl)phenylallenes 192 containing a range of electron-withdrawing substituents such as phosphinyl, alkoxycarbonyl, sulfonyl at the cumulenic C3 position could be converted into 1-(2-hydroxy)-ethoxy-2-substituted naphthalenes 193 under thermal conditions (Scheme 73) [52]. Mechanistically, an initial [1,5]-hydride shift of the acetalic H atom onto the central cumulene carbon atom affords the conjugated 1,3,5-hexatriene I, which undergoes a subsequent 6π-electrocyclic ring-closure of I to furnish spirocycle intermediate II. A final aromatization step with concomitant ring opening of 1,3-dioxolane fragment produces the substituted naphthalenes 193.
5 C(sp3)–H Bond Adjacent to Sulfur as the Hydride Donor
The methylene (or methine) adjacent to sulfur atom can also work as hydride donor. With reactive electrophilic moieties as hydride acceptors, the cascade [1,5]-hydride transfer/cyclization can occur to give thio-heterocycles. Alajarin and Vidal et al. contributed much to this chemistry.
5.1 1,3-Dithiolane as the Hydride Donor and Benzylidene Malonate as the Hydride Acceptors
Alajarin and Vidal et al. described a Sc(OTf)3-catalyzed cascade [1,4]-hydride shift/cyclization of substrate 194 carrying 1,3-dithiolane, which produced spirocyclic products 195 in moderate to good yields (Scheme 74) [70]. Tertiary C–H bond in 1,3-dithiolane worked as hydride donor. Because Sc(OTf)3 is not thiophilic but oxophilic, the dithiolane moiety could remain intact after the cascade [1,4]-hydride shift/cyclization. Compared with substrate 190 carrying 1,3-dioxolane, the disturbing hydrolysis of the acetalic function was thoroughly suppressed.
5.2 Ketenimine and Carbodiimide as the Hydride Acceptors
Alajarin and Vidal et al. discovered that under thermal conditions (refluxed in toluene), the single thioether 196 carrying ketenimine moiety could be transformed into 4-ethylthio-3,4-dihydroquinoline 197 in good yield via cascade [1,5]-hydride transfer/6π-ERC (Scheme 75) [56].
6 Benzylic C(sp3)–H Bond as the Hydride Donors
Although a range of cascade reactions of heteroatom-containing substrates (X = NR, O, or S) have been described, their corresponding carbon analogues (X = CH2 or CHR) have been rarely investigated, which might be ascribed to difficulties posed by the rate-determining step of [1,5]-hydride transfer without the assistance of adjacent heteroatom. Sames et al. disclosed that the secondary and tertiary benzylic C–H bond without an adjacent heteroatom could also be exploited as hydride donor. After [1,5]-hydride transfer, the carbocation that develops on benzylic carbon can be stabilized by adjacent electron-rich aromatic groups and alkyl groups via π–p conjugation and hyperconjugation, respectively.
6.1 Electrophilic Benzylidene Malonates and Their Derivatives as the Hydride Acceptors
Sames et al. elegantly demonstrated a PtCl4-catalyzed cascade process involving benzylic methines that lacked the stabilization of α-heteroatom (Scheme 76) [95]. The aryl substrate 198 and thiophene substrate 200 were consumed up within 24 h at 50 °C. Although the electrophilic alkene was activated by two electron-withdrawing carboxylate groups and the cation generated upon [1,5]-hydride transfer could be stabilized by adjacent aromatic group, high-valent PtCl4 was still indispensable for successful transformation, under the catalysis of which hexa-substituted cyclohexanes 199 and 201 could be obtained in moderate to good yield.
Fillion et al. reported a one-pot construction of tetrahydrobenzo-[b]fluoren-11-ones 204 under the catalysis of Sc(OTf)3 (Scheme 77) [136]. The substrates 202 carrying highly electrophilic benzylidene Meldrum’s acids and benzylic methylene or methine functions could undergo a cascade [1,5]-hydride shift/cyclization to afford spirocycles 203, which suffered subsequent intramolecular Friedel–Crafts acylation to generate tetracycles 204.
Akiyama et al. reported a Sc(OTf)3-catalyzed construction of 3-aryltetralin skeleton 206 from simple phenethyl derivatives 205 (Scheme 78) [59]. The electronic and steric properties of the aromatic ring adjacent to C–H bond serving as hydride donor significantly influenced the reactivity of this transformation.
Yu and Luo et al. reported a catalytic enantioselective benzylic C(sp3)–H functionalization of 207 via a [1,5]-hydride transfer/cyclization sequence with the chiral complex of copper(II) and side-armed bisoxazoline 209 as catalyst, which provided tetrahydronaphthalene derivatives 208 in moderate to high yield with up to 69 % ee (Scheme 79).
6.2 Activated Alkynes as the Hydride Acceptors
Liu et al. reported a TpRuPPh3 (CH3CN)2·PF6-catalyzed cycloisomerization of cis-3-en-1-ynes I or their precursor alcohols 210, which afforded cyclopentadiene 211 (Scheme 80) [43]. Mechanistically, 210 undergoes ruthenium-catalyzed dehydration to afford the real substrates cis-3-en-1-ynes I, which is converted into ruthenium-vinylidene intermediate II via [1,2]-shift of alkynyl hydrogen. A [1,5]-Hydride shift ensues to generate ruthenium haxa-1,3,5-triene III, which undergoes 6π-electrocyclic ring closure and reductive elimination to furnish cyclopentadiene IV. Ultimately, the most stable regioisomer 211 is yielded via a [1,5]-hydrogen shift.
Liu et al. reported a TpRuPPh3(CH3CN) .2 SbF6 (10 mol%)-catalyzed cyclization of 2-alkyl-1-ethynylbenzene derivatives 212, which yielded 1-substituted-1H-indene products 213 in moderate to good yields (Scheme 81) [44]. The counterions were critical to the success of the reaction.
He et al. described a PtCl2-catalyzed intramolecular cyclization of o-isopropyl or o-benzyl arylalkynes 214, which yielded functionalized indenes 215 (Scheme 82) [69]. In contrast to a previous report [44], the terminal carbon of alkyne in this reaction was substituted with aryl substituent. Notably, CuBr (2.0 equiv.) was indispensable to achieve high yield. Theoretically, platinum(II)-activated electrophilic alkyne initially abstracts a hydride from benzylic C–H in [1,4]-manner to generate a benzylic carbocation I, which subsequently intercepts nucleophilic alkenyl-platinum(II) to afford the 5-membered ring.
Chatani et al. reported a cycloisomerization of 1-alkyl-2-ethynylbenzenes catalyzed by PtCl2, PtCl4, and [RuCl2(CO)3]2 for preparing substituted indenes [40]. Remarkably, the benzylic primary C–H bond could participate in this cascade process to afford indene in 44 % yield. In contrast to Zhang’s report [61], it is the hydride that transfers in [1,5]-manner this time.
Liu et al. described a [IPrAuCl]/AgNTf2-catalyzed oxidative cyclizations of cis-3-en-1-ynes 216 with 8-methylisoquinoline oxide 218 as oxidant, which gave rise to cyclopentenone skeletons 217 (Scheme 83) [67]. Basically, the initially formed gold-containing enol ether I has a high energy barrier to overcome to form hypothetical carbenoid III. Instead, I undergoes a rapid [1,5]-hydrogen shift to generate intermediate II. Remarkably, hydrogen is transferred in the form of proton because hydrogen is captured by electron-rich gold-alkenyl subunit and the electron-withdrawing substituent in the benzylic position is beneficial to the cascade process. Afterwards, a subsequent cyclization of II leads to 217. This proposed mechanism explains the observation that an acidic C–H bond can accelerate this oxidative cyclization. Similar mechanisms of transferring proton in [1,5]-manner have also been described by Zhang et al. [62, 64–66].
6.3 Electrophilic Imine, Hydrazone, Oxime Ester as the Hydride Acceptors
In the total synthesis of d-Homosteroid, Tietze et al. reported a BF3·OEt2-catalyzed cascade [1,5]-hydride transfer/cyclization of 219, which produced an unusually bridged steroid alkaloid 220 in 85 % yield at room temperature (Scheme 84) [137–139]. Although benzylic methine and imine in 219 are comparatively inactive hydride donor and acceptor, with the assistance of electron-donating methoxy group at para-position and activation by BF3·OEt2, hydride could migrate to imine moiety readily. A subsequent nucleophilic attack of amino group on carbocation on II led to the formation of bridged steroidal azacycles 220. In addition to phenyl substituted imine, hydrazones and oxime ethers could also work as hydride acceptor in the presence of Lewis acid. Frank et al. described a similar BF3·Et2O-catalyzed intramolecular hydro-N-alkylation of hydrazones and oxime ethers [140].
Akiyama et al. described a Sc(OTf)3-catalyzed reaction to access isoquinoline skeleton 222 (Scheme 85) [141]. The tosyl imine formed in situ and benzylic methylene worked as hydride acceptor and donor, respectively. Sc(OTf)3 played dual roles, one of which was to promote the condensation of benzaldehyde 221 and tosylamide. Because the hydride donor in the reaction was inactive benzylic methylene, electron-withdrawing tosyl group was indispensable to increase the electrophilicity of C=N bond, additionally Sc(OTf)3 could further activate the imine. The methodology was elegantly elaborated in the formal synthesis of (±)-tetrahydropalmatine 225.
Sames et al. reported a highly stereoselective intramolecular amination of benzylic C(sp3)–H bonds via cascade [1,5]-HT/cyclization of N-tosylimine 227 generated in situ from aliphatic aldehyde 226, which constructed 2-arylpiperidines 228 and 3-aryl-1,2,3,4-tetrahydroisoquinolines (Scheme 86) [96]. Remarkably, the conformational freedom of substrates had a profound influence on the chemical behaviors of hydride acceptors: the substrates with high conformational rigidity had higher reactivities than those with high conformational freedom. The cascade [1,5]-HT/cyclization was highly stereoselective, which could be rationalized by the reversible cyclization step. The high stereoselectivity resulted from thermodynamic control and the aryl ring preferred to adopt an axial orientation in diastereomer II to avoid the steric interaction (pseudo-allylic strain) with the sulfonamide group in diastereomer I.
Akiyama et al. reported a Brønsted acid-catalyzed synthesis of 3-aryl-1-trifluoromethyltetrahydroisoquinolines 230 and 230′ by a benzylic [1,5]-hydride shift-mediated C–H bond functionalization (Scheme 87) [142], which features the diastereo-divergent synthesis of 3-aryl-1-trifluoromethyltetrahydroisoquinolines 230 and 230′ by tuning the substituents on nitrogen atom. The trifluoromethylketimine derived from para-anisidine and activated by Tf2NH served as hydride acceptor and the substituents on ketimines had dramatic impacts on the diastereoselectivities: cis-product 230 could be furnished as major product when R was PMP group, whereas the diastereoselectivity was reversed with R as hydrogen.
6.4 Ketenimine and Carbodiimide as Hydride Acceptors
Alajarin et al. described a concise protocol for synthesis of 3,4-dihydroquinolines 232 and 3,4-dihydroquinazolines 234 (Scheme 88) [55]. Triphenyl substituted methines and electrophilic ketenimine/carbodiimide worked as hydride donors and acceptors, respectively. Because of the high electrophilicity of ketenimine and carbodiimide, and highly stabilizing effect of adjacent three aromatic groups, thermal conditions alone could efficiently facilitate the cascade process, under which 231 and 233 could be transformed into 232 and 234, respectively, in moderate to good yield. Mechanistically, the C–H bond of the triarylmethane fragment is cleaved via a [1,5]-hydride shift to give conjugated 1,3,5-hexatriene I or II, which suffers subsequent 6π-electrocyclic ring closure to produce the sterically congested 232 and 234.
Thibaudeau and Evano et al. reported a TfOH or Tf2NH-catalyzed keteniminium-initiated cationic polycyclization of ynamides 235 (Scheme 89) [143], which provided a straightforward access to polycyclic nitrogen heterocycles 236 possessing up to three contiguous stereocenters and seven fused cycles. Basically, the reaction is initiated by protonation of electron-rich alkyne of ynamide XX, yielding a highly reactive N-tosyl- or N-acyl-keteniminium ion I, which served as hydrogen acceptor. A [1,5]-sigmatropic hydrogen shift would then ensue to generate conjugated iminium II (in resonance with the bis-allylic carbocationic form III). The first cycle would be formed by a 4π conrotatory electrocyclization, producing IV in the manner of Nazarov reaction. Finally, a second cyclization between the benzylic carbocation IV and the arene/alkene subunit leads to the formation of polycycle 236.
7 Non-benzylic C(sp3)–H Bonds as the Hydride Donors
The cascade [1,5]-hydride transfer/cyclization summarized above have entailed the electronic assistance of adjacent heteroatoms or aromatic groups for stabilizing the carbocation formed upon hydride shift. However, hydride abstraction from an aliphatic, non-benzylic position is still a challenging task, and its realization would improve the usefulness of the cascade strategy in synthetic organic chemistry. Because of the lack of electronic assistance from adjacent heteroatom or aromatic group, the dissociation energy of C–H bond in aliphatic non-benzylic position is quite high. It was not until 2011 that Akiyama et al. managed to employ non-benzylic methylene as the hydride donor.
7.1 Electrophilic Benzylidene Malonates and Their Derivatives as the Hydride Acceptors
Akiyama et al. first reported an unprecedented cascade [1,5]-hydride transfer/cyclization with non-benzylic methine as hydride donor (Scheme 90) [74]. Treatment of benzylidene barbituric acid 237 with 3 mol% Sc(OTf)3 in refluxing ClCH2CH2Cl for 24 h could furnish the desired tetraline 238 in excellent yield. Notably, the substrate with a linear side chain 239 did not give the desired product 240, even with a catalyst loading of 30 mol%. This result suggested that the substitution degree at the hydride-releasing carbon atom was crucial for the success of the cascade process.
7.2 Gold-Activated Alkynes as the Hydride Acceptors
Barluenga et al. elegantly demonstrated an intriguing manifold reactivity of alkynylcyclopropanes 241 bearing a spirane core (Scheme 91) [144]. Remarkably, comparatively inactive non-benzylic secondary C–H bond could work as hydride donor. The cyclopropane moiety was crucial for the success of the cascade process, which rendered inactive methylene group closer to electrophilic alkyne moiety. Thus, the proximate hydride could be readily abstracted by electrophilic alkyne activated by gold catalyst. The diverse fate of the resulting cationic gold species could be efficiently controlled by simple option of appropriate catalyst (either [(JohnPhos)Au(MeCN)]-[SbF6] or [(IPr)Au-(NTf2)]) and reaction temperature. A range of cyclic structures, e.g., pentalene derivatives 242 and 244, and bicycles 243 could be accessed with complete selectivity. Mechanistically, the gold-alkyne coordination triggers a [1,5]-hydride transfer with concomitant ring opening of cyclopropane. The generated 1,4-enallenyl gold intermediate I might then undergo a cyclization via intermediate II to afford tricycle 242. The subjection of 242 to harsher reaction conditions leads to the formation of 243 and 244, and III might be their common intermediate, which is formed by regioselective gold-catalyzed C–C bond cleavage. The formation of 243 can be rationalized by a [1,2]-alkyl migration of III and metal elimination of IV. Alternatively, deprotonation of III and a subsequent protodemetallation of V would provide pentalene 244.
7.3 Electrophilic Imine as the Hydride Acceptor
One more example of using aliphatic non-benzylic methine as hydride donor was also reported by Akiyama et al. in which the tosyl imine I generated in situ from 245 was employed as hydride acceptor (Scheme 92) [141]. Three-step cascade transformations involving imine formation/[1,5]-hydride shift/cyclization occurred to afford isoquinoline with Sc(OTf)3 as catalyst. In order to get decent yield, 30 mol% catalyst loading was employed, whereas the tetraline 246 was obtained only in 32 % yield even with a prolonged reaction time (72 h).
8 Conclusions and Perspectives
In this review, we have introduced the recent advances of cascade [1,n]-hydrogen transfer/cyclization as a versatile protocol to directly functionalize inactive C(sp3)–H bond into C–C, C–N, and C–O bonds in an atom-economic manner. A variety of hydrogen donors and acceptors as well as different activation modes of hydrogen acceptors have been well organized and discussed. This methodology has proven to be powerful in delivering molecular complexity, especially in the synthesis of a variety of useful and pharmaceutically important heterocycles, e.g., 5-, 6-, 7-membered fused or spiro-hetero, or full carbon cycles. The facile construction of C–C, C–N, and C–O bonds and ring skeletons renders this strategy attractive to access value-added molecules from readily available starting materials. In addition to the benefit of atom-economy, the reaction conditions are mild, and expensive transition metal catalysts are not required in many cases.
Despite the significant developments in recent years, there is still much left to do in this chemistry, for instance, the types of hydride donors and acceptors are still rather limited. More C(sp3)–H α to heteroatoms, which have lone pairs, e.g., phosphorus, bromine, chlorine, etc., are likely to be exploited as hydrogen donors and more diverse electron-deficient species can be employed as hydrogen acceptors.
Another hot research topic in this area is the asymmetric catalytic version of this reaction, and more efficient and selective catalytic systems needs to be developed. Although this methodology has shown an appealing application to construct complex moieties, it has been rarely exploited in total syntheses and the synthetic potential of this methodology in natural product synthesis has to be further exploited.
Finally, the mechanistic pathway behind this reaction should be studied in depth and some problems need to be addressed such as the influencing factors of the hydride transfer and the essence of the hydrogen transferred. On the basis of the above clarifications and deeper understanding of these transformations, discovery of new highly active and selective catalysts for this methodology and development of novel reactions are expected in the future.
Abbreviations
- Cbz:
-
Benzyloxycarbonyl
- Cod:
-
1,5-Cyclooctadiene
- CSA:
-
Camphorsulfonic acid
- DCE:
-
1,1-Dichloroethane
- DFT:
-
Density functional theory
- DMF:
-
N,N-dimethylformamide
- DNBS:
-
2,4-Dinitrobenzensulfonic acid
- DPP:
-
Diphenyl phosphate
- ERC:
-
Electrocyclic ring closure
- Fmoc:
-
9-Fluorenylmethoxycarbonyl
- HT:
-
Hydrogen transfer
- IBX:
-
O-iodoxybenzoic acid
- m-CPBA:
-
meta-Chloroperbenzoic acid
- MW:
-
Microwave
- MS:
-
Molecular sieves
- Pg:
-
Protecting group
- PTSA:
-
p-Toluenesulfonic acid
- RT:
-
Room temperature
- TCE:
-
1,1,2-Trichloroethane
- TFA:
-
Trifluoroacetic acid
- TMS:
-
Trimethylsilyl
References
Deno NC, Peterson HJ, Saines GS (1960) Chem Rev 60:7
Russell AE, Miller SP, Morken JP (2000) J Org Chem 65:8381
Yoshizawa K, Toyota S, Toda F (2001) Tetrahedron Lett 42:7983
Evans DA, Hoveyda AH (1990) J Am Chem Soc 112:6447
Evans DA, Nelson SG, Gagne MR, Muci AR (1993) J Am Chem Soc 115:9800
Node M, Nishide K, Shigeta Y, Shiraki H, Obata K (2000) J Am Chem Soc 122:1927
Ooi T, Ichikawa H, Maruoka K (2001) Angew Chem Int Ed 40:3610
Ishihara K, Kurihara H, Yamamoto H (1997) J Org Chem 62:5664
Ooi T, Otsuka H, Miura T, Ichikawa H, Maruoka K (2002) Org Lett 4:2669
Suzuki T, Morita K, Tsuchida M, Hiroi K (2003) J Org Chem 68:1601
Godula K, Sames D (2006) Science 312:67
Chen X, Engle KM, Wang DH, Yu JQ (2009) Angew Chem Int Ed 48(28):5094
Giri R, Shi BF, Engle KM, Maugel N, Yu JQ (2009) Chem Soc Rev 38(11):3242
Gunay A, Theopold KH (2010) Chem Rev 110:1060
Mátyus P, Éliás O, Tapolcsányi P, Polonka-Bálint Á, Halász-Dajka B (2006) Synthesis (16):2625–2639. doi:10.1055/s-2006-942490
Peng B, Maulide N (2013) Chem Eur J 19:13274
Haibach MC, Seidel D (2014) Angew Chem Int Ed 53:5010
Wang L, Xiao J (2014) Adv Synth Catal 356:1137
Pan SC (2012) Beil J Org Chem 8:1374
Wang M (2013) ChemCatChem 5:1291
Pinnow J (1895) Ber Dtsch Chem Ges 28:3039
Katritzky AR, Rachwal S, Rachwal B (1996) Tetrahedron 52:15031
Koufaki M, Theodorou E, Galaris D, Nousis L, Katsanou ES, Alexis MN (2006) J Med Chem 49:300
Lee SB, Lin CY, Gill PMW, Webster RD (2005) J Org Chem 70:10466
Toyota M, Omatsu I, Asakawa Y (2001) Chem Pharm Bull 924
Nozoe S, Morisaki M, Iitaka Y, Takahashi N, Tamura S, Ki K, Shirasaka M (1965) J Am Chem Soc 87:4968
Entzeroth M, Blackman AJ, Mynderse JS, Moore RE (1985) J Org Chem 50:1255
Aoki S, Watanabe Y, Sanagawa M, Setiawan A, Kotoku N, Kobayashi M (2006) J Am Chem Soc 128:3148
Macias FA, Galindo JLG, Varela RM, Torres A, Molinillo JMG, Fronczek FR (2006) Org Lett 8:4513
Katsoulis IA, Kythreoti G, Papakyriakou A, Koltsida K, Anastasopoulou P, Stathakis CI, Mavridis I, Cottin T, Saridakis E, Vourloumis D (2011) ChemBioChem 12(8):1188
Boivin TLB (1987) Tetrahedron 43:3309
Elliott MC, Williams E (2001) J Chem Soc Perkin Trans 1(19):2303
Marmsater FP, West FG (2002) Chem Eur J 8:4347
Hale KJ, Hummersone MG, Manaviazar S, Frigerio M (2002) Nat Prod Rep 19(4):413
Haustedt LO, Hartung IV, Hoffmann HM (2003) Angew Chem Int Ed 42(24):2711
Wang L, Li P, Menche D (2010) Angew Chem Int Ed 49(48):9270
Pastine SJ, Sames D (2005) Org Lett 7:5429
McQuaid KM, Sames D (2009) J Am Chem Soc 131:402
Vadola PA, Sames D (2009) J Am Chem Soc 131:16525
Tobisu M, Nakai H, Chatani N (2009) J Org Chem 74:5471
Zhou G, Liu F, Zhang J (2011) Chem Eur J 17:3101
Xia X, Song X, Wang N, Wei H, Liua X, Liang Y (2012) RSC Adv 2:560
Datta S, Odedra A, Liu R (2005) J Am Chem Soc 127:11606
Odedra A, Datta S, Liu R (2007) J Org Chem 72:3289
Shu X, Ji K, Zhao S, Zheng Z, Chen J, Lu L, Liu X, Liang Y (2008) Chem Eur J 14:10556
Meth-Cohn O (1996) Adv Heterocycl Chem 65:1
Nijhuis WHN, Verboom W, Reinhoudt DN (1987) J Am Chem Soc 109:3136
Nijhuis WHN, Verboom W, El-Fadl AA, Hummel GJV, Reinhoudt DN (1987) J Org Chem 54:209
Chen L, Zhang L, Lv J, Cheng J, Luo S (2012) Chem Eur J 18:8891
Zhang L, Chen L, Lv J, Cheng J-P, Luo S (2012) Chem Asian J 7:2569
Barluenga J, Fananas-Mastral M, Aznar F, Valdes C (2008) Angew Chem Int Ed 47:6594
Alajarin M, Bonillo B, Marin-Luna M, Sanchez-Andrada P, Vidal A (2013) Chem Eur J 47:16093
Zhao S, Shu X, Ji K, Zhou A, He T, Liu X, Liang Y (2011) J Org Chem 76:1941
Alajarin M, Bonillo B, Ortin M-M, Sanchez-Andrada P, Vidal A (2011) Eur J Org Chem 1896–1913. doi:10.1002/ejoc.201001372
Alajarin M, Bonillo B, Ortin M, Sanchez-Andrada P, Vidal A, Orenes R (2010) Org Biomol Chem 8:4690
Alajarin M, Bonillo B, Orenes RA, Ortin MM, Vidal A (2012) Org Biomol Chem 10(48):9523
Brunet P, Wuest JD (1996) J Org Chem 61:2020
Alajarin M, Bonillo B, Ortin M, Sanchez-Andrada P, Vidal A (2006) Org Lett 8:5645
Mori K, Sueoka S, Akiyama T (2011) Chem Lett 40(12):1386
Apeloig Y, Karni M (1988) J Chem Soc Perkin Trans II:625
Cui L, Peng Y, Zhang L (2009) J Am Chem Soc 131:8394
Noey EL, Luo Y, Zhang L, Houk KN (2012) J Am Chem Soc 134:1078
Yu P, Lin JS, Li L, Zheng SC, Xiong YP, Zhao LJ, Tan B, Liu XY (2014) Angew Chem Int Ed 53(44):11890
Zhang C, De CK, Mal R, Seidel D (2008) J Am Chem Soc 130:416
Richers MT, Deb I, Platonova AY, Zhang C, Seidel D (2013) Synthesis 45:1730
Dieckmann A, Richers MT, Platonova AY, Zhang C, Seidel D, Houk KN (2013) J Org Chem 78:4132
Bhunia S, Ghorpade S, Huple DB, Liu R (2012) Angew Chem Int Ed 51:2939
Cui L, Ye L, Zhang L (2010) Chem Commun 46(19):3351
Yang S, Li Z, Jian X, He C (2009) Angew Chem Int Ed 48:3999
Alajarin M, Marin-Luna M, Vidal A (2011) Adv Synth Catal 353(4):557
Meth-Cohn O, Naqui MA (1967) Chem Commun 1157
Volochnyuk DM, Shivanyuk AN, Tolmachev AA, Ryabukhin SV, Plaskon AS (2007) J Org Chem 72:7417
Che X, Zheng L, Dang Q, Bai X (2008) Synlett (15):2373–2375. doi:10.1055/s-2008-1078212
Mori K, Sueoka S, Akiyama T (2011) J Am Chem Soc 133:2424
Reinhoudt DN, Visser GW, Verboom W, Benders PH, Pennings MLM (1983) J Am Chem Soc 105:4775
Cambeiro F, Lopez S, Varela JA, Saa C (2012) Angew Chem Int Ed 51(3):723
Orlemans EM, Lammerink BHM, Veggel FCJMV, Verboom W, Harkema S, Reinhoudt DN (1988) J Org Chem 53:2278
Dunkel P, Turos G, Bényeib A, Ludanyic K, Matyus P (2010) Tetrahedron 66:2331
Zhou G, Zhang J (2010) Chem Commun 46:6593
Haibach MC, Deb I, De CK, Seidel D (2011) J Am Chem Soc 133:2100
Cao T, Fan W, Zhu C (2013) Org Lett 15:2254
Lo VK-Y, Wong M-K, Che C-M (2008) Org Lett 10:517
Kuang J, Ma S (2010) J Am Chem Soc 132:1786
Sugiishi T, Nakamura H (2012) J Am Chem Soc 134:2504
Meth-Cohn O, Suschitzky H (1972) Adv Heterocycl Chem 14:211
Verboom W, Reinhoudt DN (1990) Recl Trav Chim Pays-Bas 109:311
Quintela JM (2003) Recent Res Dev Org Chem 7:259
Ruble JC, Hurd AR, Johnson TA, Sherry DA, Barbachyn MR, Toogood PL, Bundy GL, Graber DR, Kamilar GM (2009) J Am Chem Soc 131:3991
Murarka S, Zhang C, Konieczynska MD, Seidel D (2009) Org Lett 11:129
Mori K, Ehara K, Kurihara K, Akiyama T (2011) J Am Chem Soc 133:6166
Cao W, Liu X, Wang W, Lin L, Feng X (2011) Org Lett 13:600
Toth L, Fu Y, Zhang HY, Mandi A, Kover KE, Illyes TZ, Kiss-Szikszai A, Balogh B, Kurtan T, Antus S, Matyus P (2014) Beilstein J Org Chem 10:2594
Barluenga J, Fañanás-Mastral M, Fernández A, Aznar F (2011) Eur J Org Chem 1961–1967. doi:10.1002/ejoc.201001717
Xiao J, Li X (2011) Angew Chem Int Ed 50(32):7226
Pastine SJ, McQuaid KM, Sames D (2005) J Am Chem Soc 127:12180
Vadola PA, Carrera I, Sames D (2012) J Org Chem 77:6689
Murarka S, Deb I, Zhang C, Seidel D (2009) J Am Chem Soc 131:13226
Kang YK, Kim SM, Kim DY (2010) J Am Chem Soc 132:11847
Suh CW, Kim DY (2014) Org Lett 16(20):5374
Suh CW, Woo SB, Kim DY (2014) Asian J Org Chem 3(4):399
Zhang S-L, Xie H-X, Zhu J, Li H, Zhang X-S, Li J, Wang W (2011) Nat Commun 2:211
Kang YK, Kim DY (2014) Chem Commun 50(2):222
Kang YK, Kim DY (2013) Adv Synth Catal 355:3131
Han Y, Han W, Hou X, Zhang X, Yuan W (2012) Org Lett 14:4054
Cao W, Liu X, Guo J, Lin L, Feng X (2015) Chem Eur J 21(4):1632
Mori K, Kurihara K, Yabe S, Yamanaka M, Akiyama T (2014) J Am Chem Soc 136(10):3744
Zhao T, Zhang H, Cui L, Qu J, Wang B (2015) RSC Adv 5(105):86056
Kaval N, Dehaen W, Mátyus P, Eycken EV (2004) Green Chem 6:125
Kaval N, Halasz-Dajka B, Vo-Thanh G, Dehaen W, Eycken JV, Matyus P, Loupy A, Eyckena EV (2005) Tetrahedron 61:9052
Jurberg ID, Peng B, Woestefeld E, Wasserloos M, Maulide N (2012) Angew Chem Int Ed 51:1950
Zhang C, Murarka S, Seidel D (2009) J Org Chem 74:419
Mori K, Ohshima Y, Ehara K, Akiyama T (2009) Chem Lett 38:524
Du Y, Luo S, Gong L (2011) Tetrahedron Lett 52:7064
Zheng L, Yang F, Dang Q, Bai X (2008) Org Lett 10:889
He YP, Wu H, Chen DF, Yu J, Gong LZ (2013) Chem Eur J 19(17):5232
Rabong C, Hametner C, Mereiter K, Kartsev VG, Jordisa U (2008) Heterocycles 75:799
Wang P-F, Huang Y-P, Wen X, Sun H, Xu Q-L (2015) Eur J Org Chem 2015(30):6727
Wang PF, Jiang CH, Wen X, Xu QL, Sun H (2015) J Org Chem 80:1155
Chen D, Han Z, He Y, Yu J, Gong L (2012) Angew Chem Int Ed 51:12307
Gao X, Gaddam V, Altenhofer E, Tata RR, Cai Z, Yongpruksa N, Garimallaprabhakaran AK, Harmata M (2012) Angew Chem Int Ed 51(28):7016
Chang YZ, Li ML, Zhao WF, Wen X, Sun H, Xu QL (2015) J Org Chem 80(19):9620
McQuaid KM, Long JZ, Sames D (2009) Org Lett 11:2972
Mori K, Kawasaki T, Sueoka S, Akiyama T (2010) Org Lett 12:1732
Bajracharya GB, Pahadi NK, Gridnev ID, Yamamoto Y (2006) J Org Chem 71:6204
Bhunia S, Liu R (2008) J Am Chem Soc 130:16488
Shikanai D, Murase H, Hata T, Urabe H (2009) J Am Chem Soc 131:3166
Jurberg ID, Odabachian Y, Gagosz F (2010) J Am Chem Soc 132:3543
Bolte B, Gagosz F (2011) J Am Chem Soc 133:7696
Jiao Z, Zhang S, He C, Tu Y, Wang S, Zhang F, Zhang Y, Li H (2012) Angew Chem Int Ed 51:8811
Koizumi Y, Kobayashi H, Wakimoto T, Furuta T, Fukuyama T, Kan T (2008) J Am Chem Soc 130:16854
Kurosawa W, Kan T, Fukuyama T (2003) J Am Chem Soc 125:8112
Kurosawa W, Kan T, Fukuyama T (2003) Synlett 1028–1030
Kurosawa W, Kobayashi H, Kan T, Fukuyama T (2004) Tetrahedron 60:9615
Reddy RP, Lee GH, Davies HML (2006) Org Lett 8:3437
Wakimoto T, Miyata K, Ohuchi H, Asakawa T, Nukaya H, Suwa Y, Kan T (2011) Org Lett 13:2789
Mahoney SJ, Moon DT, Hollinger J, Fillion E (2009) Tetrahedron Lett 50:4706
Woelfling J, Frank É, Schneider G, Tietze L (1999) Angew Chem Int Ed 38:200
Wölfling J, Frank E, Schneider G, Tietze LF (1999) Eur J Org Chem 3013–3020
Wölfling J, Frank E, Schneider G, Tietze LF (2004) Eur J Org Chem 90–100. doi:10.1002/ejoc.200300500
Frank É, Schneider G, Kádár Z, Wölfling J (2009) Eur J Org Chem 3544–3553. doi:10.1002/ejoc.200900301
Mori K, Kawasaki T, Akiyama T (2012) Org Lett 14:1436
Mori K, Umehara N, Akiyama T (2015) Adv Synth Catal 357(5):901
Theunissen C, Metayer B, Henry N, Compain G, Marrot J, Martin-Mingot A, Thibaudeau S, Evano G (2014) J Am Chem Soc 136(36):12528
Barluenga J, Sigueiro R, Vicente R, Ballesteros A, Tomas M, Rodriguez MA (2012) Angew Chem Int Ed 51(41):10377
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection “Hydrogen Transfer Reactions”; edited by Gabriela Guillena, Diego J. Ramón.
Rights and permissions
About this article
Cite this article
Wang, L., Xiao, J. Hydrogen-Atom Transfer Reactions. Top Curr Chem (Z) 374, 17 (2016). https://doi.org/10.1007/s41061-016-0018-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-016-0018-2