Abstract
In recent years, thermal insulation and thermal comfort have gained great importance in buildings. Natural porous lightweight aggregates have a potential of greatly improvement on thermal insulation properties of cementitious products. However, its thermal insulation properties should not be evaluated solely with the thermal conductivity coefficient. This experimental study is devoted to determining the thermal comfort properties, namely thermal conductivity, thermal diffusivity, specific heat value, heat storage capability and heat storage efficiency of tested mortars for building applications. Within this scope, four different porous natural aggregate types were tested as lightweight aggregates for thermal insulation properties. These lightweight aggregates are pumice, volcanic slag, tuff and diatomite. The results show that the apparent porosities of the test specimens were quite high. As a result of this property, thermal conductivity values decreased considerably compared to the control specimen (up to 79.39%). Also, it is concluded that density and porosity ratio of the aggregate used in the preparation of the mortar specimens is an effective parameter on the specific heat of the material. The mortars produced by the volcanic slag, pumice, tuff and diatomite aggregates appear to be effective in heat storage. Additionally, it has been determined that the thermal characteristics of the materials change depending on the aggregate chemical structure. For this reason, the major chemical components of all mortar specimens were examined chemically, and the findings were tried to be associated with the thermal characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, one of the most important problems faced by society is climate change. One of the biggest causes of climate change is greenhouse gases released into the environment. Minimizing energy consumption is an important way to minimize greenhouse gas emissions (Wu et al. 2016). Recently, energy efficient buildings have started to be of great importance in terms of both economic and environmental factors in the world. Countries are increasingly focusing on energy efficient buildings, with over a third of the energy consumption expended by buildings (Koksal et al. 2015).
In recent years, cementitious mortars have been transformed as an important component that contributes to the energy efficiency feature in buildings (Chung et al. 2015; Kilincarslan et al. 2018; Kalkan et al. 2021). Recently, cement mortars can be produced with many different aggregate derivatives. These include materials of natural origin, semi-synthetic or artificial aggregate derivatives that provide wall compatibility, reversibility and comfort (Bayraktar et al. 2018; Zhang et al. 2019). Providing thermal comfort in optimum conditions in today's buildings has been put into practice as an inevitable rule in regulations and standards (Bilgin and Arıcı 2017; Kilincarslan et al. 2018). In addition, overheating and overcooling of the areas where people live deteriorate the thermal comfort properties and create negative effects on human psychology and physiology (Topay 2013; Cetin 2015; Topay and Parladir 2015; Cetin et al. 2018). For this reason, reducing the heat transfer of building materials in areas where people continue to live will reduce the overheating or overcooling of the environments and reduce the negative impact on people.
In order to provide the desired thermal comfort environment in buildings, especially in the outer partition wall sections, many different origin materials have been used as thermal insulation materials in buildings until today. However, it is often experienced that the technical thermal comfort performances expected from these thermal insulation materials cannot be achieved. In order to provide thermal comfort, material derivatives with high porosity, low density, low moisture content and also containing chemical components with insulating properties should be used for this purpose.
However, it can be seen that the wall structure section and/or elements do not give the desired results when only materials with low thermal conductivity value are applied as thermal insulation material in buildings for thermal comfort. In addition, providing sufficient thermal comfort in building elements and wall building components may not give sufficient results to examine only by looking at properties such as thermal conductivity values, density, and porosity values. Together with these parameters, several variable parameters and factors related to materials can be used in technical investigations in examining the thermal comfort of a building section: Moisture movement, condensation characteristic, vapor diffusion resistance factor, heat transfer characteristic, heat storage capability, cooling phenomenon and so on. The amount of energy that is required for the cooling and heating of buildings is dependent on the specific heat capacity (Cp), thermal conductivity (λ) and thermal diffusivity (α) of the building envelope (Shafigh et al. 2018). Conduction heat transfer in solids occurs through molecular vibrations and energy transport by free electrons (Bhattacharjee and Krishnamoorthy 2004). The capacity of a cement-based material to transfer heat through conduction is evaluated based on its thermal conductivity (Tong 2011; Zhang et al. 2015). The specific heat capacity of a cement bonded material is the amount of energy which is required to raise the temperature of a unit of mass by one degree. Thermal diffusivity indicates the speed of heat transfer through a cement based material in transient heat transfer conditions (Shafigh et al. 2020).
Providing comfort for thermal insulation purposes in the houses we live in and establishing temperature balance under optimum conditions is mostly related with the selection of the material that applied in the buildings. Nowadays, it has become a necessity to reduce the energy cost of buildings. For this reason, it is substantial to examine the thermal inertia behavior and thermal features of building materials that make up wall components and used for coating purposes (Mounir and Maaloufa 2015). Cement mortar is a widely used and a common construction material in buildings. Regardless of what kind of material the selected building materials are, their thermal insulation effects and thermal conductivity characteristics must be analyzed and examined.
High-performance features are the prerequisite and foundation of energy saving in buildings. Two thermophysical properties, which strongly influence the energy performance of a wall, are the volumetric heat capacity and thermal conductivity. The heat storage plays an important role, if the thermal conductivity of the material is relatively high. However, when the conductivity is relatively low, the effect of the thermal insulation is dominant. When evaluating the energy savings in buildings, the effect of interior walls is less than the effect of external walls. Nevertheless, interior walls need especially the heat storage materials with a high thermal conductivity. These requirements for materials are consistent under various climate conditions (Long and Ye 2016).
Jitka Poděbradská et al. (2003) examined the specific heat capacity of several cement mortar, three types of glass fiber reinforced composites and two types of carbon fiber reinforced composites in the temperatures between 25 and 800 °C. They determined in their study that the specific heat capacity value for silica aggregate materials creates a function characteristic that increases up to approximately 600 °C and then starts to decrease. While specific heat is fundamental agreement for various research, literature data on the thermal diffusivity of hardening process of concrete do not seem to be coherent. Constant values during hardening process are reported as well as increasing and decreasing tendencies (De Schutter and Taerwe 1995). Jasim et al. (2019) investigated the thermal properties of concrete by replacement of sand with porcelain waste. They found thermal conductivity, specific heat capacity and thermal diffusivity as 2.31 W/mK, 974 J/kgK and 0.98 mm2/s, respectively, at 50% replacement and 60 days. Talebi et al. (2020) concluded that thermal conductivity, specific heat capacity and thermal diffusivity of normal weight concrete with a compressive strength in the range of 15–62 MPa could be in the range of 1.6–3.2 W/mK, 0.92–1.16 kJ/kgK and 0.69–1.34 (× 10–6 m2/s), respectively. Jaskulski et al. (2021) found that thermal conductivity, volumetric heat capacity, specific heat, and thermal diffusivity of lightweight concrete with waste copper slag could be in the range of 0.446–0.784 W/mK, 1.50–1.59 MJ/m3K, 923–997 mm2/s and 0.291–0.492 J/kgK, respectively, for dried specimens.
The rapid increase of urbanization and industrialization over recent years confront humankind with serious environmental problems. The major factor is greenhouse gases, particularly carbon dioxide (CO2). The buildings and construction sectors together are responsible for 36% of global energy consumption and almost 40% of total direct and indirect CO2 emissions (Kalkan et al., 2021). For this reason, it is extremely important to examine the thermal performance of the materials to be used in buildings in order to reduce environmental concerns. In this paper, in order to increase energy efficiency in buildings and to improve thermal comfort properties, a series of technical parameters and evaluations of thermal comfort aspects that are not frequently used today, and technical performances of four different types of natural porous aggregates in cementitious mortars were experimentally analyzed in detail to use them as raw materials in building materials. These natural porous aggregates are pumice, volcanic slug tuff and diatomite. A comparative analyses and experimental test results will be given as numerical evaluations based on the parameters such as thermal diffusivity, specific heat value, thermal conductivity, heat storage capability and heat storage efficiency in this paper for cementitious lightweight mortars (CLM) produced with different natural porous aggregate types. Also, as a new approach, the interaction of geochemical components forming the material matrix is also discussed in characterizing the thermal properties of the material in cementitious lightweight mortar combinations. In terms of these parameter values, it is discussed which material can perform higher than others. In addition, the parameters that affect the material performance are presented comparatively.
2 Materials and Methods
2.1 Materials Used
2.1.1 Cement
Ordinary Portland cement, which is one of the most widely used cement types in worldwide, is used as binder material in the cementitious mortar combinations. Ordinary Portland cement, which is comparable to EN 197–1 CEM 1 42.5R (42.5 N/mm2), used in the preparation of the cementitious lightweight mortar specimens produced with the four different natural porous aggregates was supplied from a local supplier. Some chemical components of the cement used are given in Table 1.
2.1.2 Natural Porous Aggregates
Four different types of natural porous aggregates were investigated in this experimental work namely, pumice (PU), volcanic slag (VS), tuff (TF) and diatomite (DT) aggregates. In the preparation of the experimental test specimens, all these aggregates were first classified in 0–4 mm size and then were used as separate mixing components to represent natural porous aggregate types.
Pumice (PU) aggregate was used in this research as lightweight natural porous aggregate material. Pumice is a spongy and porous geological material. During the formation of pumice, gases in the body rapidly leaves the body and because of sudden cooling, it contains numerous pores. These pores are generally formed from disconnected hollows. Thus, pumice has very high sound and heat insulation, and low permeability. Pumice is widely used as lightweight concrete aggregate. Also, pumice is used in production of lightweight building elements such as brick, masonry block, panel, etc. In recent years, pumice has been used in cementitious lightweight mortars for the purpose of heat and sound insulation (Kalkan and Gündüz 2016). In this work, pumice aggregates were obtained from Kayseri pumice quarries in Turkey. The material was resized to 0–4 mm as fine lightweight natural aggregate in order to use in cementitious lightweight mortar combinations. Its average dry unit weight was 580 ± 40 kg/m3. The crushing resistance of the pumice aggregate made according to TS EN 13,055 (Turkish Standards Institution, 2016) is 3.38 ± 0.15 N/mm2 on average. The average water absorption ratio by weight is 34.4% for the saturated surface dry condition of PU aggregate sample.
Volcanic slag (VS) aggregate is a porous lightweight aggregate and is glassy volcanic rock type with basaltic-andesitic composition, which is formed as a result of the leaking of lava with basaltic character along the cracks due to various volcanic activities so that it is also named as basalt lava. Also, it is sometimes named as cinders or volcanic cinder (Gass et al. 1971; Rittmann and Rittmann 1976). Slag formations, which have a porous and porous structure, formed as a result of volcanic events, are found in many parts of the world where volcanic activities are present. It is rich in highly vesicles volcanic glass, which gives it high porosity and low density (Ercan et al. 1978). Its forms when blobs of gas-charged lava are thrown into the air during an eruption and cool in flight, falling as dark volcanic rock containing cavities crated by trapped gas bubbles (Ercan et al. 1983). Volcanic slag materials are typically reddish to black in color, mostly due to its high iron content. The surface of some volcanic slag may have a dark green iridescent color; oxidation may lead to a deep reddish-brown color (Demirdag and Gunduz 2008). Volcanic slag aggregate specimens were obtained from the quarries in the Aegean region of Turkey. The aggregate material was crushed, screened, and resized as to 0/4 mm as fine lightweight natural aggregate in order to use in cementitious lightweight mortar combinations. Its average dry unit weight was 730 ± 35 kg/m3. The crushing resistance of the volcanic slug aggregate made according to EN 13,055–1 is 6.43 ± 0.18 N/mm2 on average. The average water absorption ratio by weight is 30.7% for the saturated surface dry condition of VS aggregate sample.
Tuff (TF) stone is of volcanic origin and can be in white, off-white, and creamy colors. It has a porous structure with andesite and/or basalt compounds in patches. Tuff stone specimens were obtained from the quarries in the Aksaray region of Turkey. Due to the peculiarities of the region, it has been widely used in the region for a long time, and it has been used in stone house construction in the form of cut stone. Since it has a soft character, it is easy to process and shape, and can become a more compact natural material by hardening as a result of contact with air, wind and sun. The tuffs, which show distinct stratification in places, show volcano-sedimentary features. The tuff stone material was crushed, screened and resized as to 0–4 mm as fine lightweight natural aggregate in order to use in cementitious lightweight mortar combinations. Its average dry unit weight was 660 ± 55 kg/m3. The crushing resistance of the tuff stone made according to EN 13,055–1 is 4.71 ± 0.11 N/mm2 on average. The average water absorption ratio by weight is 31.8% for the saturated surface dry condition of TF aggregate sample.
Diatomite (DT) is sediment composed of fossilized siliceous shells of unicellular microscopic diatom algae from the algae classes. These creatures, which multiply very quickly and exist in proportions that cannot be determined in the body of water, when they lose their vitality, they sink to the bottom of the water and pile up. They are generally found in the form of rock masses and vein structures in nature (Özbey and Atamer 1987; Aruntaş 1996; Mineral Commodity Summaries 2009). Diatomite is a white, chalky, and sedimentary rock type. It can be found in nature and easily turns into a very fine and white-beige powder when broken. This diatomite powder has an abrasive feature, and it is very light because of its highly porous structure. Diatomite is a hard-shelled type of algae composed of silica and diatom fossils (Mineral Commodity Summaries 2009). Although diatomite is predominantly composed of silica, the rock hardness (on Mohs scale) is around 1.5–2 and the grain hardness is between 4 and 6.5. Dry unit weight varies between 150 and 750 kg/m3 depending on its chemical composition. Diatomite aggregate specimens used in the research program were obtained from Kazan region of Ankara/Turkey. 0–4 mm diatomite aggregates with an average dry unit weight of 340 ± 30 kg/m3 were used throughout the experimental studies. The crushing resistance of the diatomite aggregate made according to EN 13,055–1 is 1.14 ± 0.07 N/mm2 on average. The average water absorption ratio by weight is 47.8% for the saturated surface dry condition of DT aggregate sample.
The sieve analysis of the lightweight aggregates was represented in Fig. 1.
The chemical composition of the lightweight aggregates was determined by the X-ray fluorescence (XRF) elemental analysis spectrometer. The chemical composition of the aggregates is given in Table 1 in oxide form.
2.1.3 Slaked Lime
Calcium hydroxide or slaked lime is an inorganic material. Its chemical formula is Ca(OH)2. It is obtained by mixing calcium oxide (called lime or quicklime) with water. Slaked lime used in this experimental work was supplied from a local supplier as CL80 form. In accordance with the TS EN 459–1 (Turkish Standards Institution, 2010) standard, the Ca(OH)2 ratio of the slaked lime used in the study is minimum 80%, the total CaO+MgO ratio is minimum 88%, the MgO ratio is maximum 3%, the LOI is maximum 7%. The maximum grain size of the lime is 200 μm and the unit volume mass is 500 kg/m3.
2.1.4 Polymer and Filling Materials
Cellulose ethers are commonly used as additives to improve the quality of cement-based materials. As admixtures, they improve the properties of mortars such as water retention, workability, and open time. Polysaccharides such as starch derivatives are also used to improve the consistency of the fresh material (Patural et al. 2011). Cellulose derivatives are obtained through a chemical modification consisting in the etherification in hydroxyl groups present in cellulose with of organic groups. Brachaczek (2019) examined the effects of three different derivatives of cellulose ether (hydroxypropyl)methyl cellulose (HPMC), (hydroxyethyl)methyl cellulose (HEMC), methyl ethyl hydroxyethyl cellulose (MEHEC) on the technical performance of cement-based renovating mortars, such as consistency, water retention and adhesion properties, and reported that cellulose ether plays a very active role in regulating the consistency of the mortar (Brachaczek 2019). These three different types of cellulose ethers are known as currently used admixtures in the production of factory-made mortars. In the literature, it has been experienced that cellulose additives with a comparable viscosity in the use of cellulose ether additives in production of cement-based mortars have a different effect on the consistency and a different effect on the water holding capacity in the matrix structure (Patural et al. 2011; Brachaczek 2019; Souza et al. 2021; Spychał and Czapik 2021). As in most composite mortar mixes, < 0.20% by weight of (hydroxyethyl)methyl cellulose (HEMC) additive was added to all the mixes in order to increase the consistency of the mortar mixes, improve the bond strength and ensure workability. This cellulose additive is a nonionic cellulose ether produced from natural polymer material by processing the etherification reaction. It is in the form of a kind of odorless, tasteless, and non-toxic white powder. It dissolves in hot and cold water, forms a transparent viscous solution and is insoluble in common organic solvents. It is a commercial product used to provide thickening, gelling, emulsifying, stabilizing, water retention and good workability properties in cement-based mortars.
It is important to use water repellents to minimize water uptake in cementitious mortars. The access of water to the interior of the hardened mortars and mortars is highly detrimental to the structural integrity of the wall, and the presence of water in the mortar creates a disadvantage that adversely affects its thermal properties. However, water dissolves soluble salts, causing efflorescence. It is also involved in freeze-thaw cycles and can cause serious mechanical damage to the wall (González-Sánchez et al. 2020). For this reason, these disadvantages can be minimized by adding water-repellent additives to the mortar mixes. Sodium oleate is one of the additives used to provide water repellent properties in cementitious mortars and/or lime-based mortars and grouts. Sánchez et al. (2020) has been used sodium oleate as a water-repellent agent to reduce the water absorption of air lime-based grouts for historic masonry repair. Stolz et al. (2019) reported the comparison of the oleochemical hydrophobic effects and water resistance properties of sodium oleate additive on cement-based plaster mortars with the properties of different water-repellent additives in a long-term field study. Tang (2021) investigated the use and effects of calcium stearate, zinc stearate, sodium oleate, silane emulsion and siloxane emulsion, which are additives, used as water-repellent agents in cement-based mortar applications and building materials. He emphasized that the sodium oleate additive can be a more effective water repellent in cementitious products since it is a reactive hydrophobic additive. In research study, in order to minimize the water absorption capacity of the mortar due to the high porosity of naturally porous aggregates, sodium oleate was added as a water reducing agent, too. Sodium oleate used in the study is an additive used for water repellent in powder form, which is commercially obtained as a result of the reaction of sodium hydroxide and oleic acid. It is water soluble with partial decomposition. With the effect of the mixed water of the mortar, it creates a basic environment with pH > 8. Its density is 0.9 g/cm3 on average.
The use of quartz sand as a filling material in fine cementitious composite mortar mixtures is an application preferred by most researchers. They can be used both in mortar mixtures and in high-strength concrete applications, especially due to the fact that the mortar provides high strength and durability properties and exhibits pozzolanic activating properties in very fine dimensions (Lin et al. 2018; Liew et al. 2020; Khan and Abbas 2021). Within the scope of this study, natural quartz sand (QS) with a fine grain size of 125 µm, obtained from the commercial market, was used as filling material in the preparation of the experimental test samples. The bulk density of quartz sand is in the range of 1450 + 100 kg/m3 on average, and the grain shapes are mostly in the form of oval-shaped and round grains.
2.2 Methods
2.2.1 Mix Design
In order to analyze the influence of natural porous aggregates on thermal properties in cementitious lightweight mortar (CLM) specimens, a control mixture without using any natural porous aggregate was produced and coded as M-R. This standard control mixture used through this research was obtained only mixing cement, slaked lime, natural quartz sand and polymer additives. Then, in order to examine the influence of natural porous aggregates on thermal properties in cementitious lightweight mortar specimens, four different mortar series were produced as alternative and comparative CLM specimens with the use of constant amount of natural porous aggregates. The materials used in the production of mortars and the proportions as kg per cubic meter of these materials are given in Table 2.
In the mortar composition prepared for control purposes, only fine quartz powder was used as the main aggregate material and a normal density mortar design was planned. In order to convert this mortar design into a lightweight mortar format, lightweight aggregate derivatives with lower bulk density than quartz sand powder were applied in all the mixtures in progress by replacing them in equivalent weight ratios. In addition, due to the importance of aggregate porosity in obtaining lightweight mortar, natural lightweight aggregates with larger size and relatively higher porosity than quartz powder can be used in the size range of 0–4 mm. Therefore, mixtures were designed based on weight percent values and all natural porous aggregate ratios were replaced with quartz sand. As it can be seen from Table 2, when the total kg/m3 unit value formed by the materials used in each mixture is considered as the weight % values of the usage amounts of the materials in the recipe composition, it is seen that the cement ratios are 28% by weight. Also, slaked lime and polymer admixture ratios are as 5 and 1.2% by weight, respectively, for a more accurate comparison. Particle sizes of all natural porous aggregates used in mortar mixtures were sized as 0/4 mm in order to facilitate comparison between the specimens. However, water absorption capacity of the aggregates is different due to the different porosity ratios of the aggregates used in this study. Mixing water amounts should be adjusted in order to create the equivalent consistency value in each mortar mixture. Therefore, using different W/C ratios for each mixture, it was controlled by the flow table method, ensuring the equivalent consistency. All fresh CLM mixtures are symbolically presented in Fig. 2.
To ensure the consistency of the experiments, 50 samples of 50 × 50 × 50 mm3 cubes were produced in each series of mixture combinations. Thus, a total of 250 cube samples were prepared for five different batch mixes. Fifteen of these prepared samples were used in the reputational analysis of compressive strength, apparent density, and apparent porosity values for each series. 15 test cube samples were used for water absorption tests. Another 15 cube samples were used for the test samples to be cut into pieces to be used in the specific heat value measurements. In addition, leftover 5 cube samples in each series were kept for further observational studies. For each mixture, six pieces of 200 × 400 × 30 mm3 rectangular specimens were also cast to determine the other thermal properties of the specimens. After casting, all testing specimens were removed from the molds after 24 h. All samples removed from the molds were cured in water for the first 14 days, and then curing was continued in the laboratory under normal ambient conditions until the test day.
2.2.2 Consistency and Apparent Density
Consistency of the fresh CLM specimens was examined by flow table test according to the TS EN 1015–3 (Turkish Standards Institution, 2000) standard. Apparent dry density measurements were carried out according to the TS EN 1015–10 (Turkish Standards Institution, 2001) standard.
2.2.3 Water Absorption and Apparent Porosity
Water absorption and apparent porosity tests were determined at 28 days only. All testing samples were firstly were dried in an oven at 50 ± 5 °C for 24 h to minimize damage to the microstructure from excessive drying (Zhang and Zong 2014) for determining the water absorption and apparent porosity properties. Within the scope of the study for the water absorption test, first all the samples were dried in a ventilated oven environment for 48 h at 110 °C until they reached the dry constant mass. The unit weights of the dry samples were measured and recorded. Afterwards, these samples were immersed in water under normal atmospheric pressure in a closed vessel with a temperature of 22 ± 2 °C and kept for 24 h. Then, the unit weights of the samples were re-measured and put back into the water, and this process was continued for at least 96 h, and the unit weights of the samples were recorded every 12 h. In this duration, the samples whose weights reached the constant weight were recorded as the saturated sample weight. The percent difference between the initial dry weights of the samples and the weight values in the saturated state was calculated and the water absorption rates of the samples were defined in this way.
Porosity is the percentage relationship between the volume of the pore space and the total volume of the specimen. The method to adopted was that postulated by Ribeiro et al. (2010) in which the apparent porosity of the test samples was verified using the technique based on the Archimedes principle for Portland cement mortars. The samples were first weighed in the dry state (Ms). It was then immersed in water for 24 h until fully saturated, after which the immersed mass (Mi) and wet mass (Mu) were determined. Thus, apparent porosity (PA) was calculated according to Eq. (1) (Ribeiro et al. 2010).
where ρL is the liquid density (in this in case, water, ρL = 1.0 g/cm3 at 25 °C).
2.2.4 Compressive Strength
Compressive strength of all specimens was tested at 7, 14 and 28 days according to ASTM C109 standard. 5 × 5 × 5 cm cubic specimens were produced for compressive strength test.
2.2.5 Thermal Characteristic Feature
2.2.5.1 Thermal conductivity
Thermal diffusivity and thermal conductivity are two terms used in building physics approaches that examine thermal and numerical analysis. Although thermal conductivity is a frequently used term in building physics, thermal diffusivity is a term that is rarely used in building physics. The thermal conductivity of a material is a measure of the ability of that material to transmit heat through it. Thermal conductivity values of test specimens were carried out by hot box apparatus. Hot box test was performed on 3 × 20 × 40 cm3 test specimens. Thermal conductivity measurements were made with the setup in which the measurement methodology of hot box standards can be applied under laboratory conditions. This laboratory scale hot box device is a device that measures in steady state via conduction. For each mixture series 3 pieces of thermal conductivity specimens were produced.
2.2.5.2 Specific heat
Specific heat value can be defined as the measure of a hardened mortar's ability to absorb heat from the environment where it is used. It is an important factor in terms of specifying the fact of heating and cooling under an ambient condition where the mortar is applied. The specific heat value of a hardened mortar is the amount of heat necessary to increase the temperature of the unit mass by one degree in a given temperature environment. This value differs from the point value, as it is the average value in a certain temperature zone. The energy that the molecules of a substance have in relation to rotation, translation and vibrational movements is called the internal energy of the body. For any material, specific heat value can basically be defined as two different technical parameters. The first of these is called as specific heat value at constant pressure and generally symbolized by the "Cp" parameter, and the second is specific heat value at constant volume and symbolized by the "Cv" parameter. The unit of both specific heat values is specified as "kcal/kgoC" or "J/kgK".
In order to determine the specific heat value of the hardened CLM specimens, a calorimeter device with defined technical characteristics in the literature was used throughout the experimental studies (Pan et al. 2016). This experimental method is based on the principle of determining the temperature at the resulting test point by adding a certain amount of test specimen with a certain mass and high temperature to the water at low temperature. In order to determine the specific heat value of the hardened test mortar samples, 6 rectangular prism-shaped pieces of each sample with an average unit weight of 130 ± 20 g were cut from the samples. At the beginning of the experiment, the sample samples were pre-drying in a ventilated oven to remove the moisture inside. Afterwards, the unit weight was determined with a scale that can be measured with 3% sensitivity, and the necessary conditioning for the test was done. The symbolic view of a specific heat calorimeter used in the measurement technique is given in Fig. 3. The heat received by the water and the container can be calculated. The specific heat value of the material was calculated by equating this value with the relation of the heat given by the hot material. In the application of this investigation, the use of water as the calorimetric liquid requires approximately 60 °C (within the temperature limit from 100 to 20 °C) to the environment. By applying this measuring principle, specific heat values of all hardened CLM specimens were experimentally tested after 28 days of curing period.
2.2.5.3 Thermal diffusivity coefficient
The thermal diffusivity of a material is the thermal inertia of that material. Thermal diffusivity measures the ability of a material to conduct thermal energy relative to its ability to store thermal energy. For example, metals transmit thermal energy rapidly (cold to touch) whereas wood is a slow transmitter. In general sense insulators have low thermal diffusivity. The main difference between thermal conductivity and thermal diffusivity can be predicted from here. Thermal conductivity is closely related to thermal spread. The relationship between two sizes can be expressed as an equation. Mathematically, the thermal diffusivity of a material can be defined depending on its specific heat value, density, and thermal conductivity value.
Thermal diffusivity coefficient shows how quickly heat spreads over a material's surface and body. Thermal diffusivity is not a commonly used term, unlike thermal conductivity. It is an important physical feature of a material which helps to understand the ability of a material to conduct heat relative to stored heat per unit volume. The thermal diffusivity of a material is symbolized by "α" and its unit is "m2/s". The thermal diffusivity can be algorithmically described as the ratio of the amount of heat transmitted in a material to the amount of heat stored in that material. This means that the higher the thermal diffusivity, the higher the thermal conductivity. Therefore, materials with higher thermal dissipation ability quickly pass heat through them. Also, thermal spreading of a solid material is extremely sensitive to the geochemical components of the materials that make up the matrix structure as well as its porosity. This approach can be formulated as follows (Eq. 2):
where;
α: thermal diffusivity (m2/s),
λ: thermal conductivity (W/mK),
ρt: oven dry apparent density (kg/m3),
Cp: Specific heat (J/kgK).
If a material has high thermal conductivity coefficient or low heat capacity, it could be said that this material has high thermal diffusivity. The high thermal diffusivity indicates that the heat dissipation from the material’s surface to the interior is high, while the low thermal diffusivity represents that the heat is absorbed in a large amount by converting it into heat energy. In such materials, the heat conduction amount is also at low levels. From this point of view, it is desirable that the thermal diffusivity, in other words, the heat dissipation values of the hardened mortars in terms of insulation performance (value should be as small as possible). When formulating a mortar for insulation purposes, it should be considered that it consists of components that will provide low thermal diffusivity to the product in terms of mixture components.
2.2.5.4 Heat Storage Property
When considered in the context of usage or application thickness of building materials, "heat storage property" within the material can also be examined as a performance parameter. If this approach will be dealt with in terms of amount of heat required for 1 °C temperature increase in 1 cm thickness application, similar phenomenon can clearly be seen. The amount of heat stored depends on the specific heat of the medium, the temperature change, and the amount of storage material and it is expressed by Eq. 3 (Kumar & Shukla, 2015).
where;
ΔQ: the heat stored (J),
m: the mass of material (kg),
Cp: Specific heat (J/kgK).
dT: the temperature difference.
3 Results and Discussion
3.1 Consistency and Apparent Density
Consistency of the fresh CLM specimens was examined by flow table test according to the TS EN 1015–3 (Turkish Standards Institution, 2000) standard, and the test results are shown in Table 3. Examining Table 3, it can be easily seen that the flow diameter values of all the mixture specimens is at an equivalent consistency as 145 ± 5 mm in order to make a healthy comparison of the technical evaluations of CLM specimens.
Density of the hardened CLM specimens was determined as the value of apparent dry density. Apparent dry densities of CLM specimens are also given in Table 3. The specimens with natural porous aggregates (M-PU, M-VS, M-TF, M-DT) had lower apparent dry density than that of the control specimens (M-R). Although the particle sizes and weight usage ratio are the same in the CLM mixtures, it is seen that the aggregate type that provides the lowest density value among natural porous aggregates is diatomite aggregate. However, it is seen that aggregate types that can be used to obtain mortar with low apparent dry density are listed as pumice, tuff, and volcanic slag.
3.2 Water Absorption and Apparent Porosity
The water absorption tests on CLM specimens were carried out at the same ages as 28 days. Water absorption and parent porosity values of CLM specimens are given in Table 3. Also, graphical representation of water absorption and parent porosity values of test specimens are given in Fig. 4. It is seen that the water absorption value of the control specimens is quite low compared to the values of natural porous aggregate mortar specimens as expected. Among the specimens, the highest water absorption value was obtained in specimens with diatomite aggregated specimens, and its water absorption capacity is 5.8 times higher than the control mixture due to the high porosity of the aggregate component. This phenomenon is followed by specimens of mixtures with pumice aggregates, tuff aggregates and volcanic slag aggregates as 3 times, 2.5 times and 2.4 times, respectively, provides that it is at lower values.
Test results of apparent porosity are presented in Table 3. Specimens with high water absorption ability and low unit volume mass value had high porosity due to the higher amount of open pores. Among the specimens, the highest apparent porosity value was obtained in specimens produced with diatomite aggregates, and its apparent porosity is 2.6 times higher than the control mixture due to the high open and closed pores of the aggregate component. This phenomenon is followed by specimens of mixtures with pumice aggregates, tuff aggregates and volcanic slag aggregates as 1.9 times, 1.72 times and 1.67 times, respectively, provided that it is at lower values. Since the natural structural form of diatomite aggregates has a high ratio of open and closed pores, the apparent porosity of the test specimens made with this aggregate type was obtained as the highest value. The porosity ratio of other aggregates with pumice, tuff and volcanic slag was found to be almost close to each other. Higher the porosity, lower will be the strength of the hardened CLM specimens. The high rate of porosity can also represent the high ratio of open pores in the specimen matrix structure as well as the high ratio of closed pores. This shows that there is more stable air in the matrix structure, especially in units with closed pores, and allows it to gain a more advantageous and more insulating form, especially in terms of thermal performance.
3.3 Compressive Strength
The 28 days compressive strength values of all specimens are shown in Table 3. Also, graphical representation of compressive strength values of test specimens was given in Fig. 5. In 28 days compressive strength test stages, compressive strength values of all CLM produced with natural porous aggregates remained under control mixture combinations. Increasing of porosity of the matrix structure and decreasing the unit volume weight in the specimens can explain this phenomenon. Natural porous aggregate materials have high open and closed pores; it affects the strength of CLMs in a negatively.
It is well known that the dry density and the compressive strength are directly proportional. It is seen that the compressive strength value of the control test specimens is 2.17–4.04 times higher than the strength values of natural porous aggregate test specimens and the unit volume weight is also higher. According to the experimental test results, as the porosity ratio of the aggregate components increased, the unit weights of the specimens decreased and accordingly the compressive strength values decreased. This decrease in compressive strength can be explained by the porosity and soft structure of natural porous aggregates. However, the compressive strength values of all specimens produced in this study remain within the limit values specified in the TS EN 998–1 (2017) standard. According to this standard, mortars to be used for thermal insulation purposes are required to take part in the CSI or CSII compressive strength classes. According to 28 days compressive strength test results, all hardened CLM specimens are located in the range CSI or CSII classes. Compressive strength of hardened CLM specimens varies in a range between 0.92 and 1.72 MPa. These values are suitable for interior and exterior plaster applications that are capable to satisfy mechanical requirements in TS EN 998–1 (2017) standard.
3.4 Thermal Characteristic Feature
3.4.1 Thermal Conductivity and Thermal Diffusivity
Thermal conductivity and thermal diffusivity of all hardened CLM specimens were experimentally determined for oven dry condition after 28 days of curing period and the test results are given in Table 4 as well as chemical components of hardened mortars specimens, which are determined by XRF analysis. According to the test results, thermal conductivity of control mixture was 0.698 W/mK, and thermal conductivity of hardened CLM specimens with using different natural porous aggregate were changed between 0.144 and 0.261 W/mK. As apparent porosity ratio increases, the thermal conductivity coefficient of the hardened CLM specimens decreases. A similar phenomenon is observed in the change in the density value of the material, too. In other words, the hardened mortar gets a more insulating form. It can be said that materials with relatively lower thermal conductivity coefficient are more heat insulating. The higher the porosity of the natural porous aggregate used in the mixture components, the lower the thermal conductivity value in parallel with the formation of low density. For example, diatomite aggregate specimens have a thermal conductivity value of 47% at a high porosity rate and 1.5 times lower than the thermal conductivity value of specimens with pumice aggregate with 34% porosity. A similar phenomenon was also observed for other mixtures, too.
Within the scope of the study, the thermal conductivity values of all hardened mortar test samples in the oven-dry condition were determined first and this value was taken into consideration in the thermal performance calculations. However, in practical applications, it is inevitable that the insulation properties of lightweight mortars may vary depending on the possible moisture content under service conditions. In this context, the thermal conductivity values were tested secondary to this humidity condition, assuming the test specimens of mortar hardened under service conditions could contain an average of 6% moisture content. According to the findings based on 6% moisture content, the thermal conductivity values of the M-R, M-PU, M-VS, M-TF and M-DT samples were obtained as 2.637, 0.397, 0.616, 0.501 and 0.376 W/mK, respectively. In the context of these data, it can be thought that the change in the thermal performances of the test samples of M-R, M-PU, M-VS, M-TF and M-DT may change with an increasing trend of 3.78, 1.88, 2.36, 1.97, 1.69 times, respectively, when the average 6% moisture acceptance is taken into account.
3.4.2 Specific Heat Value
The experimental analysis findings of the specific heat value (Cp) of the CLM specimens under constant pressure are given in Table 5.
When examining Table 5, it can be easily seen that the specific heat values of the CLM specimens produce different results at different temperature values of the material. The value where the calorimetric temperature difference (ΔTavr) as a result of interpolation formed between the data obtained for each material in statistical analysis is 60 °C on average is defined as the specific heat value (Cp) of hardened CLM specimens under constant pressure.
The specific heat of a material can optionally be considered as a function of the density of the material. The higher the density of a material, the lower the specific heat of that material can be expected. From this point of view, all test results were statistically evaluated according to the material density and the specific heat in a linear regression. This evaluation is given in Fig. 6.
The test findings pointed that specific heat of control mixture without any natural porous aggregate was 731 J/kgK. Besides, specific heat of hardened CLM specimens with utilizing the different natural porous aggregates were changed between 840 and 1144 J/kgK. When apparent porosity ratio increases in the matrix, the specific heat value of the hardened CLM specimens increases. This phenomenon also indicates that the hardened CLM specimens absorb heat from the environment and become a more reliable insulating form.
The experimental study findings showed that both the material origin, density and porosity ratio of the aggregate used in the preparation of the mortar specimens is an effective parameter on the specific heat of the material. Compared to the control specimen, the porosity of volcanic slag aggregated specimens increased by 66.7%, while the rate of increase in specific heat value was determined to be 14.9%. Similarly, the porosity of tuff aggregated specimens increased by 77.2%, while the rate of increase in specific heat value was determined to be 28.6%. Although it is seen that the pumice aggregate type plays a more effective role in terms of specific heat value compared to volcanic slag and tuff aggregate, the porosity of pumice aggregated specimens increased by 88.9%, while the rate of increase in specific heat value was determined to be 34.9% compared to the control specimen. In this context, the porosity of the diatomite aggregate mixtures used as the highest porous aggregate, compared to the control specimen, increased by 161.1%, while the rate of increase in specific heat value was 56.5%. Thus, diatomite aggregate is the most active aggregate type among these four different aggregate types with natural porosity. In this way, when the rate of apparent porosity in the matrix of hardened structure increases, the specific heat increases because of the increased porosity in the material, and also the material gains more ability to absorb heat from the environment where a hardened mortar produced with natural porous aggregates is applied in a structure.
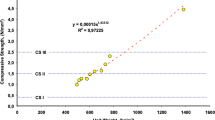
It has been observed that the chemical composition of the mass forming the matrix of the aggregate and hardened mortar plays a considerable role in the change of the specific heat value of the natural porous aggregate mortar compositions as well as the apparent porosity. In this context, the major chemical components of all hardened CLM specimens were examined and the findings were tried to be associated with the specific heat value of the material. The major chemical components obtained from the hardened CLM specimens are given in Table 4. In this study, although it is seen that the presence of silicon dioxide in the material matrix structure is more effective than the other major components, SiO2 is an important value because it represents the acidity and alkalinity criterion of the materials geochemically. While the presence of high SiO2 increases the acidity degree of the material, the presence of low SiO2 is a known fact that it gives basic character. When this is examined, it is seen that the change in specific heat value can be examined in more detail by making an empirical ratio definition depending on the SiO2 and apparent porosity form of the material. This empirical ratio is defined as the square root of the apparent porosity value (P1) of the material multiplied by the SiO2 ratio, as an exponential expression. With this empirical ratio, it has become more meaningful to examine the specific heat values of all hardened CLM specimens, and the matrix structure exhibits a feature that can be evaluated as a function of its physical property and geochemical content. All test results were statistically analyzed with linear regression according to this empirical ratio and the specific heat. This analysis is shown in Fig. 7.
In this statistical analysis, it can be concluded that the specific heat value of the material increases as the empirical ratio obtained by multiplying the SiO2 and porosity rates of the hardened CLM specimens increases. When examined in terms of material components, a high SiO2 ratio of 70.4% and a low porosity ratio of 18% in the control specimen constituted a value of 35.6% according to this approach, and it has the lowest Cp value among the specimens. Compared to the values of the control specimen, although the SiO2 ratio is lower, the empirical ratio of the volcanic slag aggregated specimens is 38.2%, and the Cp value is higher at 14.9%. This phenomenon is since the apparent porosity of the aggregate material is higher than the control specimen.
Similarly, the empirical ratio of specimens with tuff aggregates is 42.6% and the Cp value is 28.6% higher than the control specimen. Subsequently, the empirical ratio values of the specimens with pumice aggregates and diatomite aggregates are 44.6 and 55.8%, respectively, and they are 34.9% and 56.5% higher than the Cp value of the control specimen. This examination shows that high specific heat value of the material and high porosity ratio, as well as high SiO2 ratio, is important. In cases where it is necessary to keep the specific heat values of the materials within certain limit values in the production and/or applications of building materials, it is important to arrange the porosity and the SiO2 composition in a balanced ratio during the material production process.
3.4.3 Thermal Diffusivity
The findings of the thermal diffusivity (α) of the CLM specimens are given in Table 4. The test results indicate that thermal diffusivity of control mixture without using any natural porous aggregate was 0.65 m2/s. On the other hand, thermal diffusivity of hardened CLM specimens with using the different natural porous aggregates were changed between 0.19 and 0.30 m2/s. Being a general sense, the ratio of apparent porosity increases in the matrix, the thermal diffusivity value of the hardened CLM specimens decreases.
The study findings showed in similar to specific heat analyses that both the material origin, density and porosity ratio of the aggregate used in the preparation of the mortar specimens is an effective parameter on the thermal diffusivity of the material. Compared to the control specimen, the porosity of volcanic slag aggregated specimens increased by 66.7%, while the rate of increase in thermal diffusivity value was determined to be 53.6%. Similarly, the porosity of tuff aggregated specimens increased by 77.2%, while the rate of increase in thermal diffusivity value was determined to be 58%. Although it is seen that the pumice aggregate type plays a more effective role in terms of thermal diffusivity value compared to volcanic slag and tuff aggregate, the porosity of pumice aggregated specimens increased by 88.9%, while the rate of increase in thermal diffusivity value was determined to be 64.1% compared to the control specimen. When the analysis results are evaluated, it can be observed that increasing the porosity reduces the heat diffusion through the mortar structure. This phenomenon can be evaluated as heat is transferred much more slowly through the material in the application case and the material will exhibit a more resistant structure against heat passages. Thus, the less heat diffused, the higher the insulation property for heating or cooling purposes in a material structure. If it is needed to quantify this situation, in the hardened CLM specimens, the CLM mixture produced with diatomite aggregates was found to provide 71% less heat dissipation than the control mixture. Thus, it can be seen that diatomite aggregate is the most active aggregate type among these four different aggregate types with natural porosity. The materials with high thermal conductivity coefficient or low heat capacity have high heat diffusion coefficients.
It has been observed as like specific heat analyses that the chemical composition of the mass forming the matrix of the aggregate and hardened mortar also plays a significant role in the change of the thermal diffusivity of the natural porous aggregate mortar compositions as well as the apparent porosity. Just as in the specific heat evaluation, the thermal diffusivity value of all hardened CLM specimens was also examined in terms of the previously declared empirical ratio. In this examination, it was seen that there is a relationship between the value of the empirical ratio and the thermal diffusivity value of the material, which can be considered linear with a statistical approach. With this empirical ratio, it has become more meaningful to examine the thermal diffusivity values of all hardened CLM specimens, and the matrix structure exhibits a feature that can be evaluated as a function of its physical property and geochemical content, too. All research findings statistically evaluated is given in Fig. 8.
In contrast to specific heat evaluation, it is seen that the thermal diffusivity of the material decreases as the empirical ratio obtained by multiplying the SiO2 and porosity rates of the hardened CLM specimens increases. When examined in terms of material components, a high SiO2 ratio of 70.4% and a low porosity ratio of 18% in the control specimen constituted a value of 35.6% according to this approach, and it has the highest α value among the specimens. Compared to the values of the control specimen, although the SiO2 ratio is lower, the empirical ratio of the volcanic slag aggregated specimens is 38.2%, and the α value is lower at 53.6%. This phenomenon is because the apparent porosity of the aggregate material is higher than the control specimen. Similarly, the empirical ratio of specimens with tuff aggregates is 42.6% and the α value is 58% lower than the control specimen. Subsequently, the empirical ratio values of the specimens with pumice aggregates and diatomite aggregates are 44.6 and 55.8%, respectively, and they are 64.1 and 71% lower than the α value of the control specimen. As can be seen when the thermal diffusivity of the CLM mortar specimens is examined, the hardened mortar material developed with natural porous aggregates with high porosity and SiO2 ratio is lower than the average heat dissipation property, porosity and silica ratio properties of other types of mortars that are relatively lower.
This, as mentioned above, shows that in mortars with low heat emission values, depending on the product components, the heat in their bodies is absorbed and absorbed in large proportions by converting to heat energy and represents that they have low heat convection values. This is an extremely important parameter in terms of mortar materials to be applied in the construction industry for insulation purposes.
3.4.4 Heat Capacity
If Table 5 is to be examined as the arithmetic multiplying the material density and Cp values in terms of "material heat capacity", a striking issue could be interpreted as while the heat capacity value (heat storage capability) of the material is low, the thermal diffusivity of the same material can be higher. This situation can represent that such materials will not absorb heat on the surface or their bodies and will make a relatively faster heat dissipation to the indoor environment. However, the high thermal diffusivity means high heat dissipation from the material surface to the interior, and the low thermal diffusivity represents that the heat is absorbed in a large amount as heat energy. In such materials, the amount of heat conduction is also low. In this context, it is desirable that the thermal diffusivity, which is the heat dissipation value, should be low in the hardened CLM specimens in insulation performance point of view.
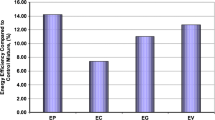
Control mortar specimens need a heat of approximately 2577 cal for 1 cm thickness and 1 m2 surface application area for a 1 °C temperature increase in the surface temperature. Similarly, pumice, volcanic slag, tuff, and diatomite aggregated CLM specimens need a heat of approximately 2171, 2080, 2236 and 1830 cal, respectively. Due to the high apparent porosity value of the hardened mortar and its geochemical components, the surface temperature of the matrix structures with low heat dissipation, as mentioned in the above sections, can increase the surface temperature more rapidly as they store less heat. This can be considered as an important parameter in terms of thermal comfort. In other words, it is a desirable general opinion that the mortars to be developed for insulation should have low heat storage capabilities. Figure 9 shows the heat storage efficiency of CLM specimens with natural porous aggregates as % value, which was analyzed by considering the heat storage capabilities of the control mortar.
If Table 4 and Fig. 9 are examined together, it can be observed that CLM specimens with diatomite aggregate are much heat storage efficient (%29), when compared with the control specimen. This phenomenon can be explained as the pores forming the inner structure of the diatomite make the heat difficult to transmit. Similarly, the CLMs produced with the volcanic slag, pumice, and tuff aggregates, known to be very resistant to heat, appear to be more effective in heat storage, respectively. This phenomenon means that less heat is stored in the material structure. Also, the material will exhibit a more resistant structure against heat storage.
4 Conclusions
In this research, a comparative analysis was carried out on thermal characteristics of lightweight mortars using four different lightweight porous aggregates. According to the results of the experimental studies, it could be concluded that:
-
1.
The lowest density mortar was produced with diatomite aggregate. Also, specimens produced with the other natural porous aggregates are also quite lightweight products.
-
2.
The highest apparent porosity value was obtained with diatomite aggregates (2.6 times higher than control) due to high open and closed pores. Apparent porosity of M-PU, M-TF, and M-VS as 1.9, 1.72 and 1.67 times higher, respectively. High porosity shows more stable air in the matrix structure and allows it to gain a more advantageous and insulating form, especially in thermal performance.
-
3.
High porosity has negative effect on strength of the hardened CLMs. Compressive strength of all test CLMs remained under compressive strength of control mixture.
-
4.
Thermal conductivity of control mixture was 0.698 W/mK. Thermal conductivity of hardened CLMs with using natural porous aggregates were changed between 0.261 and 0.144 W/mK (the lowest is M-DT). As the ratio of apparent porosity increases, the thermal conductivity of CLM specimens decreases.
-
5.
The higher the density, the lower the specific heat of the material might. Specific heat of M-R was 731 J/kgK. Besides, specific heat of hardened CLM specimens with different natural porous aggregates were changed between 840 and 1144 J/kgK.
-
6.
Chemical composition of aggregate and hardened mortar plays an important role in the change of specific heat value of the CLM. Presence of SiO2 in the material matrix structure is more effective than the other major components. Specific heat value of the materials increases as (SiO2*P1)0.5 of the hardened CLM specimens increases. When it is necessary to keep the specific heat values of the materials within certain limit values, it is important to arrange the porosity and the SiO2 composition in a balanced ratio in material production.
-
7.
Thermal diffusivity of control mixture was 0.65 m2/s. Thermal diffusivity of hardened CLM specimens with using the different natural porous aggregates were changed between 0.19 and 0.30 m2/s. As the ratio of apparent porosity increases in the matrix, the thermal diffusivity value of the hardened CLM specimens decreases. That means that heat is transferred much more slowly through the material and the material will exhibit a more resistant structure against heat passages. Therefore, the less heat diffused, the higher the insulation property for heating or cooling purposes. The M-DT was found to provide 71% less heat dissipation than M-R. The thermal diffusivity of the material decreases as the empirical ratio increases. When evaluated in terms of thermal insulation, thermal diffusivity should be as small as possible.
-
8.
Control mortar specimens need a heat of approximately 2577 cal for 1 cm thickness and 1 m2 surface application area for a 1 °C temperature increase in the surface temperature. Similarly, pumice, volcanic slag, tuff, and diatomite aggregate CLMs need a heat of approximately 2171, 2080, 2236 and 1830 cal, respectively. Mortars to be developed for insulation should have low heat storage capabilities.
References
Aruntaş HY (1996) Diatomit, özellikleri, kullanım alanları inşaat sektöründeki yeri. Cimento Ve Beton Dünyası 1(4):27–32
Bayraktar OY, Saglam-Citoglu G, Caglar H, Caglar A, Arslan M, Cetin M (2018) The mechanical properties of the different cooling requirements of high-temperature plaster. Fresenius Environ Bull 27(8):5399–5409
Bhattacharjee B, Krishnamoorthy S (2004) Permeable porosity and thermal conductivity of construction materials. J Mater Civ Eng 16(4):322–330
Bilgin F, Arıcı M (2017) Effect of phase change materials on time lag, decrement factor and heat-saving. Acta Phys Pol A 132(3):1102–1105
Brachaczek W (2019) Influence of cellulose ethers on the consistency, water retention and adhesion of renovating plasters. IOP Conf Ser Mater Sci Eng 471(3):32020
Cetin M (2015) Determining the bioclimatic comfort in Kastamonu City. Environ Monit Assess 187(10):1–10
Cetin M, Adiguzel F, Kaya O, Sahap A (2018) Mapping of bioclimatic comfort for potential planning using GIS in Aydin. Environ Dev Sustain 20(1):361–375
Chung O, Jeong S-G, Kim S (2015) Preparation of energy efficient paraffinic PCMs/expanded vermiculite and perlite composites for energy saving in buildings. Sol Energy Mater Sol Cells 137:107–112
De Schutter G, Taerwe L (1995) Specific heat and thermal diffusivity of hardening concrete. Mag Concr Res 47(172):203–208
Demirdag S, Gunduz L (2008) Strength properties of volcanic slag aggregate lightweight concrete for high performance masonry units. Constr Build Mater 22(3):135–142
Ercan T, Dinçel A, Metin S, Türkecan A, Günay E (1978) Uşak Yöresinin Neojen Havzaları Jeolojisi. Türkiye Jeoloji Kurumu Bülteni 21(2):104
Ercan T, Türkecan A, Dinçel A, Günay E (1983) Kula-Selendi (Manisa) dolaylarının jeolojisi. Jeoloji Mühendisliği 17:3–29
Gass IG, Smith PT, Wilson RCL (1971) Understanding the Earth. The MIT Press
González-Sánchez JF, Taşcı B, Fernández JM, Navarro-Blasco Í, Alvarez JI (2020) Combination of polymeric superplasticizers, water repellents and pozzolanic agents to improve air lime-based grouts for historic masonry repair. Polymers 12(4):887
Jasim MJ, Noh MZ, Zaidan SA, Ibrahim MHW, Durumin-Iya SG, Abdullah MM (2019) Thermal properties of concrete by replacement sand with porcelain waste. J Adv Res Fluid Mech Therm Sci 59(2):291–298
Jaskulski R, Dolny P, and Yakymechko Y (2021) Thermal and mechanical properties of lightweight concrete with waste copper slag as fine aggregate. Arch Civil Eng 67(3)
Kalkan SO, and Gündüz L (2016) A study on the usage of denim waste as reinforcement element in composite mortars on exterior building application. In: 12 International Congress on Advances in Civil Engineering, Istanbul, (pp 1–7)
Kalkan ŞO, Gündüz L, İsker AM (2021) A comparative analysis on the effects of pumice, tuff and conventional aggregates on energy efficiency performance in new generation composite mortars. Arab J Geosci 14(11):1–8
Khan ZAMAA, Abbas ZK (2021) Some properties of RCC containing silica sand powder exposed to MgSo4 solution. IOP Conf Ser Mater Sci Eng 1094(1):12051
Kilincarslan Ş, Davraz M, Akça M (2018) The effect of pumice as aggregate on the mechanical and thermal properties of foam concrete. Arab J Geosci 11(11):1–6
Koksal F, Gencel O, Kaya M (2015) Combined effect of silica fume and expanded vermiculite on properties of lightweight mortars at ambient and elevated temperatures. Constr Build Mater 88:175–187
Kumar A, Shukla SK (2015) A review on thermal energy storage unit for solar thermal power plant application. Energy Proc 74:462–469
Liew MS, Aswin M, Danyaro KU, Mohammed BS, Al-Yacouby AM (2020) Investigation of fibers reinforced engineered cementitious composites properties using quartz powder. Materials 13(11):2428
Lin R-S, Wang X-Y, Zhang G-Y (2018) Effects of quartz powder on the microstructure and key properties of cement paste. Sustainability 10(10):3369
Long L, Ye H (2016) The roles of thermal insulation and heat storage in the energy performance of the wall materials: a simulation study. Sci Rep 6(1):1–9
Mineral Commodity Summaries 2009. (2009). In Mineral Commodity Summaries. https://doi.org/10.3133/mineral2009
Mounir S, Maaloufa Y (2015) Thermal inertia for composite materials white cement-cork, cement mortar-cork, and plaster-cork. Energy Proc 74:991–999
Özbey G, Atamer N (1987) Kizelgur (Diatomit) Hakkında Bazı Bilgiler, 10. Türkiye Madencilik Bilimsel Teknik Kongresi, Ankara, pp 493–502. https://www.maden.org.tr/resimler/ekler/aeae10ea1c6433c_ek.pdf [in Turkish]
Pan J, Zou R, Jin F (2016) Experimental study on specific heat of concrete at high temperatures and its influence on thermal energy storage. Energies 10(1):33
Patural L, Marchal P, Govin A, Grosseau P, Ruot B, Deves O (2011) Cellulose ethers influence on water retention and consistency in cement-based mortars. Cem Concr Res 41(1):46–55
Poděbradská, J., Pavlík, J., Toman, J., & Černý, R. (2003). Specific heat capacity of cementitious composites in high-temperature range. In: Proceedings of the Thermophysics 2003 Meeting of the Thermophysical Society Working Group of the Slovak Physical Society, (pp 18–23)
Ribeiro DV, Labrincha JA, Morelli MR (2010) Use of red mud as addition for portland cement mortars. J Mater Sci Eng 4(8):1–8
Rittmann, A, and Rittmann L (1976) Volcanoes. Putnam. https://books.google.com.tr/books?id=Xl7wAAAAMAAJ
Shafigh P, Asadi I, Akhiani AR, Mahyuddin NB, Hashemi M (2020) Thermal properties of cement mortar with different mix proportions. Mater Constr 70(339):e224–e224
Shafigh P, Asadi I, Mahyuddin NB (2018) Concrete as a thermal mass material for building applications-a review. J Build Eng 19:14–25
Souza, A. T., Carvalhais, C. de A., & Santos, W. J. dos. (2021). Analysis of mortar coating with different types and proportions of chemical admixtures that have water retentivity properties. Revista IBRACON de Estruturas e Materiais 14
Spychał E, Czapik P (2021) The influence of cement type on the properties of plastering mortars modified with cellulose ether admixture. Materials 14(24):7634
Stolz HJ, Kehren G, Fluegel N (2019) Effects of oleochemical hydrophobing agents on plasters-results of a long-term field study. ZKG Int 72(9):61–67
Talebi HR, Kayan BA, Asadi I, Hassan Z (2020) Investigation of thermal properties of normal weight concrete for different strength classes. J Environ Treat Tech 8:908–914
Tang HJ (2021) Impact of Various Types Of Water Repellent Agent Towards Concrete Engineering Performance. UTAR, Malaysia
Tong, X. C. (2011). Characterization methodologies of thermal management materials. In Advanced materials for thermal management of electronic packaging Springer, New York, pp 59–129
Topay M, Parladir MO (2015) Suitability analysis for alternative tourism activities with the help of GIS: a case study of Isparta province. J Agric Sci 21(2):300–309
Topay M (2013) Mapping of thermal comfort for outdoor recreation planning using GIS: the case of Isparta Province (Turkey). Turk J Agric for 37(1):110–120
TS EN 998–1, (2017) Specification for mortar for masonry-Part 1: Rendering and plastering mortar
Turkish Standards Institution (2000) TS EN 1015–3-Methods of test for mortar for masonry- Part 3: Determination of consistence of fresh mortar (by flow table)
Turkish Standards Institution (2001) TS EN 1015–10, Methods of test for mortar for masonry-Part 10: Determination of dry bulk density of hardened mortar
Turkish Standards Institution (2010) TS EN 459–1-Building lime–Part 1: Definitions, specifications and conformity criteria
Turkish Standards Institution (2016). TS EN 13055-Lightweight aggregates
Wu MH, Ng TS, Skitmore MR (2016) Sustainable building envelope design by considering energy cost and occupant satisfaction. Energy Sustain Dev 31:118–129
Zhang J, Chen B, Yu F (2019) Preparation of EPS-based thermal insulation mortar with improved thermal and mechanical properties. J Mater Civ Eng 31(9):4019183
Zhang SP, Zong L (2014) Evaluation of relationship between water absorption and durability of concrete materials. Adv Mater Sci Eng 2014:1–8
Zhang W, Min H, Gu X, Xi Y, Xing Y (2015) Mesoscale model for thermal conductivity of concrete. Constr Build Mater 98:8–16
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
GÜNDÜZ, L., Kalkan, Ş.O. The Effect of Different Natural Porous Aggregates on Thermal Characteristic Feature in Cementitious Lightweight Mortars for Sustainable Buildings. Iran J Sci Technol Trans Civ Eng 47, 843–861 (2023). https://doi.org/10.1007/s40996-022-00937-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40996-022-00937-3