Abstract
The effectiveness of chemical drugs has been reduced by the resistance of cancer cells to chemical drugs, such as breast cancer as one of the most common cancers in women. Hence, it is important to study the development of more effective drugs with fewer side effects, such as herbs. Thus, the present study aimed to assess the effects of Moringa oleifera (MO) grown in Iran with anti-cancer properties in the inhibition of apoptosis and proliferation in breast cancer cells. MO extract was prepared in this study while confirming phenolic compounds, namely quercetin, gallic acid, and folic acid, through HPLC methods. Afterward, the apoptotic and anti-proliferative impacts of phenolic compounds were evaluated on 4T1 breast cancer cells via MTT, BrdU, Annexin V-FITC/PI staining, and caspases-9 and -3 activity assays. Furthermore, ELISA was applied to evaluate BAX/Bcl2 ratio. MO extract (0.02, 0.04, and 0.08 g daily for four weeks) was used to treat the BALB/c mice. The size of tumors was measured. MO reduced the proliferation significantly and induced apoptosis (P < 0.01). Furthermore, tumor volume in MO-treated mice was decreased. The reduction in tumor volume at 0.02 g dose was higher than the other two doses (P < 0.001). According to in vitro results, the apoptotic pathway was possibly induced by activating caspases-9 and -3 and an increase in the Bax/Bcl-2 ratio. Through the in vivo results, and significant reduction in tumor size, new evidence was added to the possible treatment of breast tumor provoking intrinsic apoptotic paths.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Moringa oleifera and Moringa peregrina belong to the family of Moringaceae. Moringa oleifera L. (MO) is a fast-growing tree with a natural coagulant (Balogun et al. 2020; Korsor et al. 2019). It has been historically used to clarify polluted effluents. According to studies, it has several medicinal and health values (Kou et al. 2018). Today, there are various studies on different therapeutic features of this plant. It is more amazing that all the parts of Moringa, including the roots, bark, leaves, seeds, flowers, stems, and pods, are a source of various nutrients, antioxidants, and medicines. The plant possesses different bioactive components, such as vitamins, flavonoids, isothiocyanates, phenolic acids, saponins, and tannins (Abdull Razis et al. 2014; Anwar et al. 2007; Kou et al. 2018; Zalpoor et al. 2022a, b). Previous research has indicated the following: the vitamin C content in Moringa leaves is seven times more than that in oranges; the protein content is nine times more than that in yogurt; vitamin A content is 10 times more than that in carrots; calcium content is 17 times more than that in milk; iron content is 25 times more than spinach, and potassium content is 15 times more than that bananas. Antioxidant compounds in Moringa play their role through hydrogen donation (Gopalakrishnan et al. 2016). One of the components of phenolic compounds is flavonoids, mostly employed on account of their antioxidant properties. The antioxidant mechanism of these materials is to prevent the oxygen species ROS formation by inhibition of the enzymes making up the radicals, removing essential elements included in producing free radicals, and removing and reducing radicals (Cai et al. 2004; Chumark et al. 2008; Gupta et al. 2012; Santos et al. 2012; Sreelatha and Padma 2009). Isothiocyanate compounds are the other compounds of Moringa (Lopez-Rodriguez et al. 2020). Studies have revealed that Moringa powder has protective features against gastrointestinal ulcers. It also has an even stronger effect than omeprazole as a result of isothiocyanate compounds that are both immune-regulating and anti-inflammatory. Moringa extract contains immunosuppressive features as a result of its macrophages and neutrophils effects and inhibition of phagocytosis (Baldisserotto et al. 2018). Owing to the nutritional value, MO is utilized in various illnesses, such as respiratory distress, hypercholesterolemia, hypertension, diabetes, anemia, insulin resistance, cancer, and inflammation (Asgari-Kafrani et al. 2020; Atsukwei et al. 2014; Chen et al. 2012; Gupta et al. 2012; Onah et al. 2017; Peter et al. 2011; Poussel et al. 2015; Randriamboavonjy et al. 2016). Robust anti-proliferative features of MO are also established on different kinds of cancer, which is the second cause of death and a global challenge in the world (Al-Asmari et al. 2015). All parts of the M. oleifera tree have anti-cancer properties, specifically, leaf extracts the main features of malignancy cells are the uncontrolled proliferation, invasion, and migration through the extracellular matrix to other body parts (metastasis and secondary tumors). Cancer mortality is higher in low- and middle-income countries rather than in other countries due to growing pollution, a lack of healthcare facilities, and expensive anti-cancer drugs (Khor et al. 2018a; b). Therefore, developing natural anti-cancer drugs is necessary as a strategy to overcome these challenges.
Breast cancer is a very aggressive and prevalent cancer. The mortality rates caused by breast cancer remain are still high in spite of numerous proven therapeutic drugs and approaches, such as radiotherapy, hormone therapy, surgery, and chemotherapy. Moreover, the side effects of anti-cancer drugs are severe in long term (Waks and Winer 2019). Presently, various studies have been performed on applying natural compounds to treat the tumor cells effectively. The minimal side effects are the important issue regarding herbal medicine (Aung et al. 2017; Wang and Jiang 2012). Recently, there are different plant species containing almost 74% of the known anti-cancer medicines and some others under clinical trials for the treatment of cancer like Curcumin and Lycopene (Craig 1997; Winston and Beck 1999). According to epidemiological studies, there is a negative correlation between using cruciferous vegetables and the risk of breast, lung, and colon cancer (Cohen et al. 2019). It was shown that M. oleifera leaf and bark extracts effectively prevent the growth of pancreatic, colorectal, and breast cancer cells (Khor et al. 2018). Moreover, examining the mechanism of new therapeutic compound effects is valuable to treat the tumor cells effectively besides former approaches. On the other hand, using anti-cancer drugs causes severe side effects in long term. Thus, the present work aimed to assess the effects of Moringa oleifera grown in Iran with antioxidant and anti-inflammatory features in the inhibition of apoptosis and proliferation in breast cancer cells.
2 Materials and Methods
2.1 Chemicals
Bovine serum albumin (BSA), chloroform, methanol, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), gallic acid, quercetin, caffeic acid, and several others had analytical grades (Sigma-Aldrich®, US). Merck Chemical Supplies (Damstadt, Germany). The dried stems and leaves of MO were bought from Rooyan Sabz Company (Bushehr, Iran).
2.2 Preparing the Extracts
Herein, electrical masonry was applied to grind the dried stems and leaves of Moringa oleifera. By soaking 100 g of grinded powder into 1000 ml of 99% methanol and soxhleting for 24 h (with the sample-solvent ratio of 1:10 w/v), the organic solvent was eliminated by a rotary evaporator (Buchi, Switzerland; temp: 50 °C; pressure 175 mbar). Ultimately, the obtained dried extracts were gathered and kept at 4 °C.
2.3 Estimating the Phytochemicals in Moringa Total Extract
HPLC rapid analysis (Agilent 1100, SY-8100 UV/VIS detector) was utilized to estimate three flavonoids of caffeic acid, quercetin, and gallic acid based on the former standard method (Asgari-Kafrani et al. 2020) as bioactive markers. To analyze the samples, multi-solvent HPLC was used armed with Agilent Infinity, Single Pump, and Diode Array Detector. The mobile phase comprising 50% acetonitrile and 50% HPLC grade methanol was passed at a flow rate (0.9 ml/min) via Agilent ZORBAX Eclipse Plus-C18 column (4.6 × 250 mm, 5 μm) at room temperature and the wavelength of 265 nm (Asgari-Kafrani et al. 2020). The peak area achieved from the methanolic extract of Moringa oleifera was compared with the chromatogram gained from the standard sample and three flavonoids were recognized and the quantities were obtained (Sánchez-Machado et al. 2010).
2.4 Animals
Female BALB/c mice with 20–25 g were prepared from an animal house at the medical school of Isfahan University of Medical Sciences (Isfahan, Iran). The mice were treated for four weeks in standard conditions.
2.5 Breast Tumor Formation in Mice
Tumor developed by injecting a tumor cell fraction in each mouse. In addition, a 200 µL suspension of 4T1 cells (2 × 106) was injected in the area near the breast of 5-week-old female BALB/c mice based on former studies (Nigjeh et al. 2019). To feed the control group (n = 3), PBS was used as the breast tumor control. A breast tumor was developed in the other groups (six mice in each group) utilizing 4T1 cells to prepare for injection with MO extract for 28 days. The clinical abnormalities of animals were monitored for the beginning of toxicological symptoms every morning.
2.6 Animal Study Design
The anti-tumor activity and tumor size were estimated by randomly dividing 24 adult mice with matched tumor size into four groups (six mice in each group). Group I: Normal Control (NC) without any treatment, with the injection of 1 ml of distilled water. The test groups received 100 µl MO extract with various concentrations (0.02, 0.04, and 0.08 g/kg) each day. The animals were treated for four weeks. To record the tumor size of each group every week (on days 0, 7, 14, 21, and 28.), a caliper was used. The tumor volume was determined as V = (L × W × W)/2.
2.7 Cancer Cell Lines and Cell Culture
The National Cell Bank of Pasteur Institute of Iran supplied the 4T1 breast cancer cell lines (2539-CRL). The cells were cultured in RPMI-1640 with 100 U/ml penicillin G, 10% fetal bovine serum (FBS), and 100 μg/ml streptomycin, incubated in standard conditions (5% CO2 and 95% air at 37 °C).
2.8 Apoptosis Assay and Cell Viability
2.8.1 Cytotoxicity Assay
To determine the cytotoxicity effect of MO, applying 3‐(4,5‐dimethylthiazole‐2‐yl) ‐2,5‐diphenyltetrazolium bromide (MTT) assay was considered in 4T1 cells. The cells (5 × 103 cells/well) were treated with MO within the range of concentrations (0, 16, 32, 64, 96, 128, 256, 512, and 1024 µg/ml) for 24 h. Adding MTT to each well (0.5 mg/ml) was performed at room temperature (37 °C) for 4 h followed by incubations. MTT solution was then discarded and substituted with dimethyl sulfoxide (100 μl per well). The plate was shortly shaken and absorbance was read at 570 nm through Microplate Spectrophotometer (Synergy H1 Hybrid Multi‐Mode; Bio‐Tek, Tokyo, Japan).
2.8.2 Bromodeoxyuridine Cell Proliferation Assay
To mature cell proliferation, bromodeoxyuridine (BrdU) assay kit was used (Cat.6813; Cell Signaling Technology, Danvers, MA) as previously described (Barez et al. 2020). Culturing 4T1 cell line in 96‐well plates in the 1.5% PBS medium for 24 h, they were then treated with MO at various concentrations (0, 16, 32, 64, 96, 128, 256, 512, and 1024 µg/ml) in 24 h. BrdU solution was then added and incubated for 12 h (10 μL/well). Ultimately, the cells were incubated with the Fixing/Denaturing Solution. Adding the secondary antibody and its substrate, an ultimate reaction was started to produce a yellow color. Finally, the stop solution (1 M) was added to read the absorbance at 370 nm with Microplate Spectrophotometer (Synergy H1 Hybrid Multi‐Mode; Bio‐Tek).
2.8.3 Detecting Cell Death Through Annexin V/PI Staining
To measure the effects of MO on programmed cell death, Annexin V-FITC/PI kit (Biovision) was utilized as described previously (Barez et al. 2021). After 24 h, the cells were plated in 6-well plates and were exposed to MO extract for 24 h (76.5, 153, and 306 µg/ml). The subsequent steps were based on the protocol of the manufacture. FACS Caliber flow cytometer (BD Bioscience) quantified with the FlowJo software yielded the results.
2.8.4 Measuring the Bax and Bcl-2 Levels with ELISA Kits
To determine Bax and Bcl-2 protein levels, ELISA kits (Zellbio GmbH, Germany) were employed following the manufacturer’s protocol as a duplicate in untreated and treated cells. The cells were plated (5 × 105 cells/well) in 6-well plates and were subjected to MO extract (76.5, 153, and 306 µg/ml) for 24 h and lysed based on the ELISA kit instruction for extracting total proteins. Afterward, the primary antibodies from each kit were added to the extraction for binding to Bax and Bcl-2 protein, and these interactions were detected by Horseradish peroxidase (HRP)-conjugated secondary antibody. Ultimately, the absorbance was measured at 450 nm via a microplate reader. To calculate Bax and Bcl-2 protein levels, the standard curve was used.
2.9 Statistical Analysis
To process and analyze all the data, GraphPad Prism was applied. The results stated as the mean ± SD were the average of independent triplicate experiments (three replications). By use of a statistical nonparametric one-way analysis of variance (ANOVA) test after the Tukey Post-hoc test, data were compared. The statistical significance was set at P value < 0.05.
3 Results
3.1 HPLC Quality Control of Moringa oleifera Extract Through Bioactive Markers
The existence of Flavonoids in the Moringa oleifera methanolic extract was confirmed through the preliminary phytochemical study. To perform flavonoid analysis, the HPLC method was utilized. Phenolic compounds, such as gallic acid, quercetin, and caffeic acid, were identified in MO extract through the HPLC technique (Table 1).
Figure 1 depicts the chromatogram of the methanolic extract. The retention times of the standards were compared with methanolic extract and three phenolic compounds were recognized (Fig. 1).
According to the analysis of chromatograms, methanolic extract possessed the highest caffeic acid level (2.2 ± 0.71 mg/100 g) with a retention time of 3.61 min and the highest amount of quercetin was obtained (3.08 ± 0.36 mg/100 g) with a retention time of 3.78 min. Ultimately, the retention time of 3.59 min was obtained for Gallic acid (2.1 ± 0.88 mg/100 g).
4 Anti-proliferative Effects of MO Extract (Stems and Leaves)
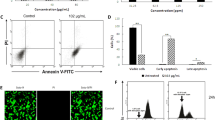
The effect of MO extract on reducing cell viability and proliferation was determined by treating 4T1 cells with MO extract at various concentrations (0, 16, 32, 64, 96, 128, 256, 512, and 1024 µg/ml) for 24 h. Proliferation and viability were then measured with MTT and BrdU assays. According to Fig. 2a, cell viability was decreased significantly in MO-treated cells compared with the control cell lines in a dose-dependent manner. IC50 of MO extract was achieved at 153 µg/ml. According to the BrdU method, the proliferation rate was reduced significantly, initiating at 64 µg/ml (P < 0.05) compared with untreated cells (Fig. 2b). Afterward, the apoptotic effects of MO extract were assessed utilizing flow cytometry. Based on the flow cytometry results, MO extracts incremented Annexin-FITC/PI-positive cells at various MO extract concentrations (76.5, 153, and 306 µg/ml) compared with the control group (Fig. 2c). The cells were treated with MO extract in IC50 dosage or 153 µg/ml and also in additional lower (76.5 µg/ml) and higher dosage (306 µg/ml).
MO extract had cytotoxic outcomes in breast cancer cells. 4T1 cells were treated with MO extract and then their viability was assessed with a MTT assay, b the anti-proliferation effect measured by BrdU kit and c apoptotic cell percentage was assessed using Annexin–FITC/PI analysis. These analyses were triplicated in three separate experiments (n = 9). Results were presented as mean ± SD and P < 0.05 (*), P < 0.01 (**) considered as statistically significant
Apoptotic cells were increased significantly at 306 μg/ml (P < 0.01) and 153 (P < 0.05) concentrations of MO extract in treated-4T1. According to our results, MO extract has a great potent apoptotic and anti-proliferative effect in 4T1 cells in breast tumor cells.
4.1 MO Extract Apoptosis Induced by Activation of Caspases-3 and -9 and Upregulated Bax/Bcl-2 Ratio
We clarified the molecular mechanism of the apoptotic effect of MO extract by evaluating caspase-3 and caspase-9 activity. The cells were treated with MO extract in IC50 dosage or 153 µg/ml and also in additional lower (76.5 µg/ml) and higher dosage (306 µg/ml) (Fig. 3a, b).
MO extract enhanced activation of caspases-3, 9 and Bax, Bcl-2 in breast cancer cells. FT1 cells were treated with MO, and then, apoptotic effect was assessed with evaluating (a, b) the caspase 3 and 9 activities. Activities were assessed in the extracted total protein of each sample by the specific fluorometric assay kit and (c, d) also measurement of Bax and Bcl‐2 protein levels were done using fluorometric assay kit and absorbance read by ELIZA reader. These analyses were triplicated in three separate experiments (n = 9). Results were presented as mean ± SD and P < 0.05 (*) and P < 0.01 (**) considered as statistically significant
The levels of caspase-3 were incremented (4.4 ± 0.37-fold; P < 0.05, 7.1 ± 0.34 -fold; and 8.7 ± 0.6 -fold P < 0.01, respectively) in the existence of MO extract compared to the untreated cells. In presence of the three dosages of MO extract, the levels of caspase-9, respectively, were 2.1 ± 0.30-fold; P > 0.05, 5.1 ± 0.4-fold; P < 0.05, and 6.91 ± 0.3; P < 0.01.
We further proved the MO-induced apoptosis in 4T1 cells by measuring Bax (pro-apoptotic) and Bcl2 (anti-apoptotic) protein levels in several concentrations of MO (76.5, 153, and 306 µg/ml) through the ELIZA technique. According to our data, MO extract increments Bax and decrements Bcl-2 protein expression in a dose-dependent manner (Fig. 3c, d).
We found a considerable increment Bax (P < 0.05 at 153 μg/ml and P < 0.01 at 306 μg/ml) and a decrease in Bcl-2 (P < 0.05 at 153 and 306 μg/ml) protein in cells treated with MO compared with the control group. Thus, an apoptotic state was indicated in the breast cancer cell line. In total, the data revealed that MO extract improved intrinsic apoptosis path through activating caspase-3 and caspase-9 and increasing Bax/Bcl-2 protein ratio.
4.2 Effects of MO Extract on Breast Tumor Size
The induction of apoptotic pathway with MO extract in breast tumor cells in vivo was determined through a xenograft model utilizing 4T1 cells in BALB/c mice. In this research, normal mice were treated with 0.02, 0.04, and 0.08 g of MO extract. No mortality or toxicity symptoms were found in any groups (results not presented). At 0.02 g MO-treated mice, the highest anti-tumor activity was found to be 90% smaller than that of the untreated control group following the 4-week treatment (P < 0.001; Fig. 4).
MO extract reduced tumor breast cancer size in animal model at lower dosage. Normal Control (NC) without any treatment, with the injection of 1 ml of distilled water. The test groups received 100 µl MO extract with various concentrations (0.02, 0.04, and 0.08 g) each day. Tumor measurement was done in 4 weeks. The highest anti-tumor activity was observed at 0.02 g MO-treated mice in compare to non-treated control group. Results were presented as mean ± SD and P < 0.05 (*) and P < 0.001 (***) considered as statistically significant
5 Discussion
The highest mortality in the world is related to cancer (Ribeiro and Nardocci 2013). Recently, there has been significant progress in cancer therapies, namely monoclonal antibodies or CAR-T cell therapy (Mohan et al. 2021). Meanwhile, chemical drugs cause numerous problems, such as non-economic way, side effects recurrence of tumor, and clinical disadvantage for other non-cancerous tissue (Chari 2008; van Rijt and Sadler 2009). Therefore, traditional drug treatment is still regarded and necessary in the cancer therapy field. Natural plants have low toxicity and are normally utilized as complementary drugs along with chemical drugs (Gordaliza 2007; Song et al. 2014; Sun et al. 2020). Among the most important traditional plants is Moringa oleifera (MO), for which former studies have indicated a great potential therapeutic feature against different diseases, particularly anti-cancer activities (Bose 2007; Jung 2014; Pangastuti et al. 2016; Tiloke et al. 2018). Moringa oleifera contains several anti-inflammatories, antioxidants, and anti-cancer compounds (Purwal et al. 2010). Mainly, such features are related to the phytochemicals of Moringa. According to literature, natural anti-cancer compounds normally interfere with the cancer cell apoptosis and proliferation pathways (Al-Asmari et al. 2015; Sreelatha et al. 2011). Researchers have shown an interest in investigating the anti-cancer activity of the Moringa extract through in vitro studies (Bhattacharya et al. 2018; Gopalakrishnan et al. 2016). Our study presents the first model for determining the anti-cancer features of the extracts of Moringa stems and leaves gathered from different regions of Iran. We determined the anti-cancer characteristics of MO extracts in vitro and in vivo on the 4T1 breast cancer cell line and BALB/c mice. First, we assessed the cell death effects of a mix of MO extract from stems and leaves on 4T1 breast cancer cell line by MTT assay. Based on our results, cell death was promoted by treating 4T1 cells with MO extract (Fig. 2a). Also, in this study, the proliferative effect of stems and leaves MO extract was determined by BrdU assay for the first time. The study presented a significantly reduced cell proliferation and viability in 4T1 cells. Furthermore, according to the flow cytometry results, apoptosis was induced greatly through MO extract (at 153 µg/ml) in the 4T1 breast cancer cell line (Fig. 2c). These data revealed great anti-proliferative and apoptotic impacts of whole MO extract (combined stems and leaves) on the cells. Based on our findings, combined stems and leaf MO extract could reduce cell viability and induce apoptosis at 153 μg/ml in 4T1 cells in compare with non-treated 4T1 cells (Fig. 2a) in other similar studies such as Alsamari et al. also represented the apoptotic effects and toxicity of leaves and bark MO extracts separately. They recognized various compounds in MO extract with anti-cancer features, such as D-allose, isopropyl isothiocyanate, eugenol, and hexadecanoic acid ethyl ester. All phytochemicals possess a definite structure with long-chain hydrocarbons, an aromatic ring, and sugar moiety. Alsamari et al. found a considerable reduction in cell survival, a significant increment in the apoptotic cells, and a cell cycle arrest in MDA-MB-231 cell line through Dead Cell and Cell Cycle Kits in 250 μg/ml concentration (Al-Asmari et al. 2015). According to our results, it seems that a combined stems and leaves MO extract can induce apoptosis in lower dosage on 4T1 cells. However, our study was limited and it needs more research. In another study, Charoensin established the anti-cancer activity of the MO extract among HepG2 (hepatic malignancy), Caco-2 (colorectal malignancy), and MCF-7 (breast cancer cell line) via MTT assay. They demonstrated that the methanol MO extract (250 µg/ml) kills cancer cells (Charoensin 2014). In addition, MO extract induces cell death in other tumor cells. Parvathy et al. have shed light on the cytotoxic effects of Moringa leaf extracts (IC50: 0.32 µg/ml) on U266B1 cell line (lymphocyte B malignancy) (Parvathy and Umamaheshwari 2007; Milugo et al.). Nair et al. have investigated the HeLa cell line using MTT assay, thereby showing that MO extract caused dose-dependent cytotoxicity in cervical cells (IC50: 70 µg/ml) (Nair and Varalakshmi 2011). These studies have not indicated the exact molecular cytotoxic pathways. Killing of tumor cells by most anti-cancer herbal components has been linked to activation of apoptosis pathways in cancer cells. Extrinsic and intrinsic are the two main pathways of apoptosis. In both pathway of apoptosis, a family of Cys (Cysteine) proteases, named caspases are activated. caspases performance a role in proteolytic cascade and remove the dying cell. Caspase-9 and -8 are related to intrinsic and extrinsic, respectively. Understanding the molecular events that induce apoptosis in cancer cells by anti-cancer components provides exact information for developing more valuable and novel therapies beside of chemotherapy (Fulda and Debatin 2006). Therefore, we assessed intrinsic apoptosis pathways by evaluating caspase-3 and -9 activities. We found that combined stems and leaf MO extract activates the caspase-3 and caspase-9 (Fig. 3a, b). The induction of intrinsic mitochondrial apoptosis was indicated. Mohd Fisall et al. found that Dichloromethane fraction of Moringa oleifera leaf methanolic extract selectively inhibits breast cancer cells (MCF7) by induction of apoptosis via upregulation of Bax, p53 and caspase 8 expressions (Mohd Fisall et al. 2021). Their study showed that leaf methanolic MO induces apoptosis via extrinsic pathway. In addition, we evaluated and Bcl-2 and Bax expression to confirm mitochondrial apoptosis. Activation of caspases is regulated by Bcl-2 family proteins mitochondrial apoptosis. We observed the up-regulating pro-apoptotic Bax with the down-regulation of anti-apoptotic Bcl-2 showing an active mitochondrial apoptosis (Fig. 3c, d). For various cancers, previous studies stablished that induction of intrinsic mitochondrial apoptosis is a desirable target for new therapeutic strategies (Fulda and Debatin 2006).
Furthermore, we evaluated the anti-tumor activity of MO in vivo on mice challenging with 4T1 cells mimicking human mammary cancer. The tumor size was significantly reduced by the extract within a 4-week test in a dose-dependent manner (Fig. 4). The treated mice challenging with 4T1 cells represented a greatly reduced size of tumor compared to the control group (non-treated mice). By the end of the fourth week, the tumors amazingly disappeared approximately in the treated mice, consistent with former in vivo studies. Similarly, Jung et al. studied the in vivo anti-cancer activity of MO extract soluble in cold water on HepG2 cells. The results of flow cytometry determined the DNA damage effects and cell cycle arrest on the HepG2 cells (Charoensin 2014). Krishnamurthy et al. accomplished the in vitro and in vivo studies to determine the potent anti-cancer properties of the Moringa extract on Hep2 human epidermoid cancer cell line. They treated the cells and Swiss albino mice with Soxhlet extractions in n-hexane, chloroform, ethyl acetate, and 50% methanol. Their results implied a potent toxicity effect of MO on cancer cells and reduced the tumor size within 14 days (Krishnamurthy et al. 2015). Also, Zunica et al. have evaluated the anti-cancer effects of moringa seed extract alone and in combination with chemotherapy in obese female mice by MDA-MB-231-derived xenograft tumors. Moringa supplementation improved insulin sensitivity but did not attenuate tumor growth (alone) in compare to chemotherapy agents. Seed extract of MO in combination with chemotherapy agents got worse in tumor progression. According to their animal study, Seed extract of MO just reduced angiogenesis, not tumor growth (Zunica et al. 2021). However, in the present study, we observed a reduction in tumor growth after treating mice with combined stems and leaf MO extract, but its anti-tumor effectiveness was decreased on breast tumor by increasing the dose of whole extract (As mentioned in Fig. 4). This may be due to the aggregation of the effective compounds of the whole stems and leaf MO extract on the tissue or a side effect in higher concentrations, which requires more detailed cellular and molecular studies.
6 Conclusion
According to our data, the whole stems and leaf MO extract reduced the viability and proliferation of breast malignancy cells and greatly induced intrinsic apoptotic pathways within 4T1 cells. In addition, this whole MO extract magnified caspases-9 and -3 activities and enhanced BAX/Bcl-2 ratio. The in vivo results showed a significant reduction in tumor size after 28 days. Importantly, we suggested that attentiveness should be taken about dosage and side effects of whole moringa extracts for breast cancer treatment.
References:
Abdull Razis AF, Ibrahim MD, Kntayya SB (2014) Health benefits of Moringa oleifera. Asian Pac J Cancer Prev 15(20):8571–8576
Al-Asmari AK, Albalawi SM, Athar MT, Khan AQ, Al-Shahrani H, Islam M (2015) Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PLoS ONE 10(8):e0135814
Anwar F, Latif S, Ashraf M, Gilani AH (2007) Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res Int J Dev Pharmacol Toxicol Eval Nat Prod Deriv 21(1):17–25
Asgari-Kafrani A, Fazilati M, Nazem H (2020) Hepatoprotective and antioxidant activity of aerial parts of Moringa oleifera in prevention of non-alcoholic fatty liver disease in Wistar rats. S Afr J Bot 129:82–90
Atsukwei D, Eze ED, Adams MD, Adinoyi SS, Ukpabi CN (2014) Hypolipidaemic effect of ethanol leaf extract of Moringa oleifera Lam. in experimentally induced hypercholesterolemic wistar rats. Int J Nutr Food Sci 3(4):355–360
Aung TN, Qu Z, Kortschak RD, Adelson DL (2017) Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int J Mol Sci 18(3):656
Baldisserotto A, Buso P, Radice M, Dissette V, Lampronti I, Gambari R et al (2018) Moringa oleifera leaf extracts as multifunctional ingredients for “natural and organic” sunscreens and photoprotective preparations. Molecules 23(3):664
Balogun TA, Buliaminu KD, Chukwudozie OS, Tiamiyu ZA (2020) Anti-cancer potential of Moringa oleifera on BRCA1 gene: systems biology. bioRxiv. https://doi.org/10.1101/2020.12.19.392423
Barez SR, Atar AM, Aghaei M (2020) Mechanism of inositol-requiring enzyme 1-alpha inhibition in endoplasmic reticulum stress and apoptosis in ovarian cancer cells. J Cell Commun Signal 14(4):403
Barez SR, Attar AM, Aghaei M (2021) MicroRNA-30c-2-3p regulates ER stress and induces apoptosis in ovarian cancer cells underlying ER stress. EXCLI J 20:922
Bhattacharya A, Tiwari P, Sahu PK, Kumar S (2018) A review of the phytochemical and pharmacological characteristics of Moringa oleifera. J Pharm Bioallied Sci 10(4):181
Bose CK (2007) Possible role of Moringa oleifera Lam. root in epithelial ovarian cancer. Medscape Gen Med 9(1):26
Cai Y, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74(17):2157–2184
Chari RV (2008) Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc Chem Res 41(1):98–107
Charoensin S (2014) Antioxidant and anticancer activities of Moringa oleifera leaves. J Med Plants Res 8(7):318–325
Chen K-H, Chen Y-J, Yang C-H, Liu K-W, Chang J-L, Pan S-F et al (2012) Attenuation of the extract from Moringa oleifera on monocrotaline-induced pulmonary hypertension in rats. Chin J Physiol 55(1):22–30
Chumark P, Khunawat P, Sanvarinda Y, Phornchirasilp S, Morales NP, Phivthong-Ngam L et al (2008) The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J Ethnopharmacol 116(3):439–446
Cohen A, Burgos-Aceves MA, Bar-Ziv N, Smith Y (2019) Cruciferous vegetables consumption and lung cancer prevention: Epidemiological studies and molecular mechanisms. J Xiangya Med 4:21
Craig WJ (1997) Phytochemicals: guardians of our health. J Am Diet Assoc 97(10):S199–S204
Fulda S, Debatin K-M (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25(34):4798–4811
Gopalakrishnan L, Doriya K, Kumar DS (2016) Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci Hum Wellness 5(2):49–56
Gordaliza M (2007) Natural products as leads to anticancer drugs. Clin Transl Oncol 9(12):767–776
Gupta R, Mathur M, Bajaj VK, Katariya P, Yadav S, Kamal R, Gupta RS (2012) Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J Diabetes 4(2):164–171
Jung IL (2014) Soluble extract from Moringa oleifera leaves with a new anticancer activity. PLoS ONE 9(4):e95492
Khor KZ, Lim V, Moses EJ, Abdul Samad N (2018) The in vitro and in vivo anticancer properties of Moringa oleifera. Evid Based Complement Altern Med
Korsor M, Ntahonshikira C, Bello HM, Kwaambwa HM (2019) Growth performance of Moringa oleifera and Moringa ovalifolia in central namibia semi-arid rangeland environment. Agric Sci 10(02):131
Kou X, Li B, Olayanju JB, Drake JM, Chen N (2018) Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients 10(3):343
Krishnamurthy PT, Vardarajalu A, Wadhwani A, Patel V (2015) Identification and characterization of a potent anticancer fraction from the leaf extracts of Moringa oleifera L.
Lopez-Rodriguez NA, Gaytán-Martínez M, de la Luz Reyes-Vega M, Loarca-Piña G (2020) Glucosinolates and isothiocyanates from moringa oleifera: chemical and biological approaches. Plant Foods Hum Nutr 25:1–11
Milugo T, Omosa L, Owuor B, Oyugi J, Ochanda J, Wamunyokoli F. Anti-cancer activities of crude extracts from kenyan Moringa oleifera Lam and Rauwolfia caffra against selected cancer cell lines
Mohan M, Maatman TC, Schinke C (2021) The role of monoclonal antibodies in the era of bi-specifics antibodies and CAR T cell therapy in multiple myeloma. Cancers 13(19):4909
Mohd Fisall UF, Ismail NZ, Adebayo IA, Arsad H (2021) Dichloromethane fraction of Moringa oleifera leaf methanolic extract selectively inhibits breast cancer cells (MCF7) by induction of apoptosis via upregulation of Bax, p53 and caspase 8 expressions. Mol Biol Rep 48(5):4465–4475
Nair S, Varalakshmi K (2011) Anticancer, cytotoxic potential of Moringa oleifera extracts on HeLa cell line. J Nat Pharm 2(3):138–142
Nigjeh SE, Yeap SK, Nordin N, Rahman H, Rosli R (2019) In vivo anti-tumor effects of citral on 4T1 breast cancer cells via induction of apoptosis and downregulation of aldehyde dehydrogenase activity. Molecules 24(18):3241
Onah IA, Onukwube GI, Odoh CE, Odimegwu DC (2017) Moringa oleifera, an adjuvant for respiratory snycytial virus vaccine. Aust J Basic Appl Sci 11(12):95–101
Pangastuti, A., Amin, I. F., Amin, A. Z., & Amin, M. (2016). Natural bioactive compound from Moringa oleiferaagainst cancer based on in silico screening. J Teknol 78(5)
Parvathy MVS, Umamaheshwari A (2007) Cytotoxic effect of Moringa oleifera leaf extracts on human multiple myeloma cell lines. Trends Med Res 2:44–50
Peter A, Walter A, Wagai S, Joseph O (2011) Antibacterial activity of Moringa oleifera and Moringa stenopetala methanol and n-hexane seed extracts on bacteria implicated in water borne diseases
Poussel M, Penven E, Richard C, Mercy M, Chabot F, Paris C (2015) Occupational asthma to “the miracle tree” (Moringa oleifera): first description. European Respiratory Society, New York
Purwal L, Pathak A, Jain U (2010) In vivo anticancer activity of the leaves and fruits of Moringa oleifera on mouse melanoma. Pharmacologyonline 1:655–665
Randriamboavonjy JI, Loirand G, Vaillant N, Lauzier B, Derbré S, Michalet S et al (2016) Cardiac protective effects of Moringa oleifera seeds in spontaneous hypertensive rats. Am J Hypertens 29(7):873–881
Ribeiro ADA, Nardocci AC (2013) Socioeconomic inequalities in cancer incidence and mortality: review of ecological studies, 1998–2008. Saude e Sociedade 22:878–891
Sánchez-Machado DI, Núñez-Gastélum JA, Reyes-Moreno C, Ramírez-Wong B, López-Cervantes J (2010) Nutritional quality of edible parts of Moringa oleifera. Food Anal Methods 3:175–180
Santos AF, Argolo AC, Paiva PM, Coelho LC (2012) Antioxidant activity of Moringa oleifera tissue extracts. Phytother Res 26(9):1366–1370
Song Y-H, Sun H, Zhang A-H, Yan G-L, Han Y, Wang X-J (2014) Plant-derived natural products as leads to anti-cancer drugs. J Med Plant Herb Ther Res 2:6–15
Sreelatha S, Padma P (2009) Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr 64(4):303–311
Sreelatha S, Jeyachitra A, Padma P (2011) Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem Toxicol 49(6):1270–1275
Sun W, Shahrajabian MH, Cheng Q (2020) Traditional Iranian and Arabic herbal medicines as natural anti-cancer drugs. Agrociencia 54(1):129–142
Tiloke C, Anand K, Gengan RM, Chuturgoon AA (2018) Moringa oleifera and their phytonanoparticles: potential antiproliferative agents against cancer. Biomed Pharmacother 108:457–466
van Rijt SH, Sadler PJ (2009) Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov Today 14(23–24):1089–1097
Waks AG, Winer EP (2019) Breast cancer treatment: a review. JAMA 321(3):288–300
Wang J, Jiang Y-F (2012) Natural compounds as anticancer agents: experimental evidence. World J Exp Med 2(3):45
Winston C, Beck L (1999) Phytochemicals: health protective effects. Can J Diet Pract Res 60(2):78
Zalpoor H, Nabi-Afjadi M, Forghaniesfidvajani R, Tavakol C, Farahighasreaboonasr F, Pakizeh F et al (2022b) Quercetin as a JAK–STAT inhibitor: a potential role in solid tumors and neurodegenerative diseases. Cell Mol Biol Lett 27(1):1–17
Zalpoor H, Bakhtiyari M, Liaghat M, Nabi‐Afjadi M, Ganjalikhani‐Hakemi M (2022a) Quercetin potential effects against SARS‐CoV‐2 infection and COVID‐19‐associated cancer progression by inhibiting mTOR and hypoxia‐inducible factor‐1α (HIF‐1α). Phytother Res
Zunica ER, Yang S, Coulter A, White C, Kirwan JP, Gilmore LA (2021) Moringa oleifera seed extract concomitantly supplemented with chemotherapy worsens tumor progression in mice with triple negative breast cancer and obesity. Nutrients 13(9):2923
Acknowledgements
The authors are gratefully acknowledging from research council of Falavarjan Branch, Islamic Azad University.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yousefirad, A., Rastegari, A.A., Shahanipour, K. et al. Evaluation of Proliferative Inhibition Effect of Moringa oleifera Total Extract on Breast Cancer: An In Vitro and In Vivo Study. Iran J Sci 47, 653–662 (2023). https://doi.org/10.1007/s40995-023-01434-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-023-01434-6