Abstract
The praying mantis genus Hierodula is widespread throughout temperate and tropical Asia, and its species are well recognised due to their large size. Nevertheless, the life history of many of its species remains unknown. Therefore, we investigate the biology, life cycle, mating, cannibalism, and spermatophore feeding of the species Hierodula tenuidentata. The mean incubation period of five field-collected oothecae was 36.4 days (± 0.5 SD) and thus very similar to oothecae laid in the laboratory (35.1 days ± 3.0 SD). The mean number of nymphs hatching from the field-collected oothecae was 22.0 (± 3.1 SD), while this number was considerably higher for the oothecae laid in the laboratory (97.1 ± 11.0 SD). The time needed from hatching to imago was about 89.1 days (± 0.7) and included nine moults for males and ten moults for females. The mean life span of females (93.3 days ± 5.3 SD) was longer than of males (48.6 ± 4.7 SD). We observed spermatophore feeding by the female at the end of mating; sexual cannibalism occurred during copulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Although being charismatic and well-recognised organisms, the biology and life cycle of mantids is, in general, poorly known, but has received considerable attention from biologists in recent years (Holling and Dill 1976; Eisenberg et al. 1981; Birkhead et al. 1988; Hurd et al 1994, 1995; Harris and Moran 2000; Hurd et al. 2004; Perez 2005; Allen et al. 2012; Raut and Gaikwad 2016; Vanitha et al. 2016; Greyvenstein et al. 2020; Shcherbakov and Govorov 2020; Greyvenstein et al. 2021). Hierodula Burmeister, 1838 is among the richest genera of Mantodea with 104 described species (Otte and Spearman 2005). Nevertheless, their taxonomy is not well understood and still appears to be paraphyletic with respect to the other genera in Hierodulinae subfamily (Schwarz and Roy 2019). It can be due to the scarcity of records and data on their natural and evolutionary history. Hierodula therefore is in strong need of a careful taxonomic revision to verify the validity of many species and determine their respective diagnostic characters, but also the improvement of the knowledge on their life history and biology is crucial to understand their diversification and evolution. Moreover, some species of this genus have been recently recorded outside their native ranges as alien species in Europe, with possible impact on native ecosystems (Battiston et al. 2018; Schwarz and Ehrmann 2019; Battiston et al. 2020; Moulin 2020).
Distribution and taxonomy of Hierodula tenuidentata Saussure, 1869 is still discussed. Its presence in South-East Asia is poorly known and is overlapping with similar species with doubtful records. In general, this species is rather common and widespread, but its biology is still unknown.
The aim of this study therefore is to investigate the life cycle of this species under optimal conditions evaluating how many generations this species can have per year, how many months it takes to complete their life cycle, how many nymphs hatch from an ootheca, and the influence of its size and weight on the number of nymphs. We also ask how many moults it needs to complete the life cycle, whether cannibalism is present, and how many oothecae can be laid by a single female.
2 Material and Methods
2.1 Collection, Rearing Condition, and Life History
The life cycle of Hierodula tenuidentata was studied from March 2019 to June 2020 under laboratory conditions. Five oothecae were collected accidentally right after the females laid them on the branches in nature (Kohmare-Sorkhi, Fars, Iran), and then, they were stored separated in glass jars (15 × 15 × 10 cm) at room temperature (~ 25–27 °C). Relative air humidity (RH) was maintained on a level of 40–45% by occasional misting with water. RH was measured using a HTC2 digital terrarium hygrometer (Dongguan City, China).
In total, 110 nymphs hatched from the five field-collected oothecae. Sixty of them were separated into two groups: 30 nymphs were placed in 30 separated jars (8 × 8 × 5 cm) and another 30 in a glass terrarium (20 × 20 × 15 cm) all together to examine the cannibalism in the first and second instars. The jars had three holes in their cap (each 2 mm in diameter) to allow airflow. A thin piece of a tree branch (10 mm × 10 cm) was placed into all jars for climbing and hanging purposes, especially during moults. After reaching their third instar, the nymphs that survived in the terrarium were transferred to separated jars.
During the first and the second instars, the nymphs were fed with fruit flies (Drosophila melanogaster Meigen 1830), i.e. three individuals per nymph every second day. Latter instars were fed with living mealworm larvae (Tenebrio molitor Linnaeus 1758).
All jars were checked daily, and all parameters (i.e. hatching, feeding, mating, oviposition, fecundity, and longevity) were recorded. All dead or uneaten preys were removed to avoid contamination or disturbance. After the final moult, the sex of each individual was recorded.
To observe mating behaviour and sexual cannibalism, seven males and seven females, two weeks after their final molting, were picked randomly and each couple were placed in a glass terrarium (40 × 40 × 30 cm) filled with some natural elements such as rocks, wood branches for hiding and moving. Before their encounter, males and females were fed with a sufficient amount of food (four adult mealworms for each female and two adult mealworms for each male). Mated females were fed with two to four mealworms daily and checked for the egg cases they laid which were recorded.
Fifteen oothecae were used for examining the correlation between oothecae size, weight, and hatching number of nymphs. Ten oothecae were watered for one day to soften, and then, they were dissected in order to observe the oothecae inner structure and egg arrangement under the microscope.
Low temperature was used as killing agent, and then specimens were pinned, spread and dried. Length, width, and height of each ootheca were recorded, based on Brannoch et al. (2017). The measurements of each nymphal instar, adults, and oothecae were recorded using a digital calliper with 0.01 mm sensitivity. The bodyweight of females and the oothecae was measured by a Mettler digital laboratory-scale balance analytical PC440 with 0.001 g sensitivity. Photographs were made with a Canon EOS 700D digital camera. The descriptive statistics (means and standard error) were calculated using Microsoft Excel 2019. Collection codes are assigned to all specimens and oothecae. The identifications were carried out by the first author (Z.M.) using Battiston et al. (2010), and the wasps were identified by H. Lotfalizadeh, chalcidoid specialist. The materials are preserved in the following collections (Iran): ZMPC—personal collection of the first author, Kangan; ZM-CBSU—Zoological Museum of Shiraz University.
3 Results
Family Mantidae Latreille, 1802.
Genus Hierodula Burmeister, 1838.
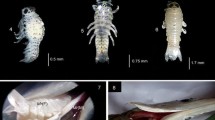
Hierodula tenuidentata Saussure, 1869 (Fig. 1).
3.1 Biology
The field-collected oothecae (Fig. 2) were deposited by the females at the end of branches of pomegranate trees. They were collected immediately after the females finished their laying ootheca which they were found accidentally, so the incubation period was determined from the day we placed them in the laboratory. They were up to 24 mm in length, and their shapes were globular with a pointed apex. Nymphs hatched from the upper rim of the ootheca. Information on oothecae weight, length, and hatching number of nymphs is given in Table 1. All nymphs of a single ootheca hatched within a single day. All of the oothecae were parasited by Podagrion pachymerum (Walker 1833) (Fig. 2c) in their wild habitat. Information on the mean number of wasps that emerged from the oothecae is given in Table 1.
3.2 Development of Stages
Sixty nymphs were reared in total, but only 25 of them (i.e. 41.7%; 7 males, 18 females) completed their life cycle. In the first nymphal stage, the average length of the body increased by 11.3% in each stage (Table 2). Nymphs were appropriately supplied with their prey (mealworms, and fruit flies) which was reared by the first author in the laboratory. Before each moulting, nymphs will tend to be motionless and stop feeding. As the size of the nymph increases, the consumption of the prey also increases. The period between first to third instars has a high mortality rate, but mortality decreases in the last instars. The high mortality rate may have been affected by the diet or other laboratory conditions. Adult females had a longer life span (93 days ± 6 SD) than males, (48 ± 4 SD) (Table 3). Females were more voracious and consumed more prey than males, and their abdomens are much bigger and broader than those of males. Males have much longer antenna than the females. They are so similar in apparencies and the difference is by their size and their thickness, as the males are smaller and slender than the females.
Nymphs were light green (Fig. 2d) when they emerged and active immediately after hatching and searching for prey. The nymphs moulted ten times for the females and nine times for the males in order to reach the adult.
Group number two which included 30 nymphs that were placed together in a terrarium showed cannibalism in the first two instars even when plenty of food was available. Seven out of 30 (23%) were eaten by the others.
3.3 Mating (Fig. 3a & b)
After mating, we observed spermatophore feeding in all of seven female cases that were mated. The females fold themselves to reach their genitalia and remove the spermatophore with their mouthparts and consume it (Fig. 3c & d).
3.4 Oviposition
The females in their natural habitat laid their oothecae mostly on pomegranate trees (Punica granatum), especially delicate branches. The reared females laid oothecae on the sticks placed in their jars. Four out of seven laid their first ootheca after seven days, two after eight days, and one after five days. Most oothecae were laid at night. The oothecae were very soft and light greenly coloured in the first hour after being laid; afterwards, they dried and hardened and turned into light brown within two hours; after one day, their colour turns to dark brown. According to the total calculated adult lifespan, two of the mated females laid five oothecae, four of them three, and one only one. Laying of oothecae was done from a downward direction. Observation of sagittal section of dorsally dissected oothecae showed that egg chambers were arranged in a circular pattern, with rows containing from two to five eggs each, arranged next to each other (Fig. 2b).
4 Discussion
According to our study, it seems that this species is univoltine and has one generation per year. The nymphs emerged in spring (i.e. May and early June) and reach adulthood in summer (late August). At the end of summer and early autumn (mostly September), the females lay their oothecae. In autumn and winter, the eggs remain unhatched. According to Tables 1 and 4, the number of emerged nymphs varies from oothecae collected and developed in nature with oothecae that were reared in laboratory conditions. This may be due to the more challenging access to nutritional resources and therefore to the number of eggs produced by the parents or to the level of parasitisation that in the oothecae left in the wild is considerable. It is likely that a concurrence of these two factors in nature leads to reduced hatching on average of 32% per ootheca. According to Table 5, the number of laid oothecae depends on the female diet, size, weight, and consumption of males. Females that consumed the male during copulation laid larger ootheca and had more eggs. Increasing fecundity was reported by Birkhead et al. 1988, and their study showed that sexual cannibalism in Hierodula membranacea can increase fecundity in female mantids. Nevertheless, this assumption needs further verification in future studies. In our study, females had one more moulting than males, which suggests that this “extra” moult is directed towards resource acquisition for egg production (Birkhead et al. 1988; Barry et al. 2008). If larger body mass allows for greater egg production and enhances the ability to produce pheromones (Allen et al. 2012), then larger females are predicted to have a fitness advantage. Also, the male and females in our study had a higher number of moults (ten times for females, and nine times for males) than the other mantids in the Mantidae family. Members of Mantidae usually have 7–8 moults in order to reach the adulthood, therefore having 9–10 moults in our study can be possibly caused by feeding the later instars (first and second instars were fed by fruit flies) only by mealworms, which were the only food we could provide for them. In fact, the food limitation can affect the mantid survivorship, the times to reach adulthood, and also egg production (Hurd et al. 1994). In our study, we observe that the prey type and the using just one type of prey like mealworms can affect these three components in the mantids life cycle and we observe that the females with sexual cannibalism that consumes the males laid more oothecae which contains more eggs and had more offspring than the females did not consume the male during mating.
There are various studies regarding Mantodean’s life history and biology (Holling and Dill 1979; Eisenberg et al. 1981; Birkhead and Young 1987; Hurd et al. 1994, 1995; Iwasaki 1996; Harris and Moran 2000; Hurd et al. 2004; Perez 2005; Allen et al. 2014; Raut and Gaikwad 2016; Greyvenstein et al. 2020; Shcherbakov and Govorov 2020; Greyvenstein et al. 2021), but no study was done concerning Hierodula tenuidentata life history and biology. Therefore, the results obtained from this study are compared to studies that were done on Hierodula ventralis Giglio-Tos, 1912, and Hierodula patellifera (Serville 1839).
We have noticed that the morphology and the unique characteristics of oothecae of this species are similar to the oothecae of Hierodula ventralis and Hierodula patellifera (Leong 2009; Raut et al. 2014).
The parameters like size, shape, and also the colour of a mantid oothecae can be influenced by various biotic and abiotic factors such as temperature, food availability, humidity, genetics, the presence of males (Robert 1937; Breland and Dobson 1947; Hurd et al. 1995), natural enemies that parasite or prey on their eggs such as Chalcidoids (Eupelmidae, Torymidae, Ichneumonoidea, etc.) (Mirzaee et al. 2021a), Dermestid beetles such as Dermestes sp., Orphinus sp., and Thaumaglossa sp. (Coleoptera: Dermestidae) (Kershaw 1910; Hawkeswood 2003); other arthropods such as Chernes sp. (Pseudoscorpiones: Chernetidae), Synageles persianus (Araneae: Salticidae) (Mirzaee et al. 2021b). These factors (biotic and abiotic) can influence not only the structure of oothecae but also the population dynamics of mantids in the wild.
The incubation period for field-collected oothecae was 36.4 days (± 0.5 SD) (Table 1) which is slightly different for the oothecae that were laid in captive conditions (35.1 ± 3.0 SD) (Table 4). The incubation period recorded for other species like Hierodula ventralis and Hierodula patellifera was 25 days, Leong (2009 did not mention the laboratory conditions he used, but Raut et al. 2014 used laboratory conditions at 25 to 30 °C and 75–80% RH which is different with our study (Leong 2009; Raut et al. 2014), and it shows that the nymphal development in those two species is shorter than in H. tenuidentata. We assume that this difference can be due to the effect of photoperiod and temperature on the biology of nymphs.
The maximum number of oothecae produced by Hierodula tenuidentata in this study was five; however, in other studies, such as Leong 2009, the female of Hierodula patellifera laid only one ootheca, and in Raut et al. 2014, the female of Hierodula ventralis laid two oothecae.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Allen LA, Barry KL, Holwell GI (2012) Mate locationand antennal morphology in the praying mantid, Hierodulamajuscula. Aust J Entomol 51:133–140
Barry KL, Holwell GI, Herberstein ME (2008) Femalepraying mantids use sexual cannibalism as a foraging strategyto increase fecundity. Behav Ecol 19:710–715
Battiston R, Picciau L, Fontana P, Marshall J (2010) Mantids of theEuro-Mediterranean area. World Biodiversity Association Hand -books, Verona, Italy, p 240
Battiston R, Leandri F, di Pietro W, Andria S (2018) The giant asian mantis Hierodula tenuidentata saussure 1869 spreads in Italy: a new invasive alien species for the European fauna? (Insecta Mantodea). Biodivers J 9(4):399–404. https://doi.org/10.31396/biodiv.jour.2018.9.4.399.404
Battiston R, Amerini R, Di Pietro W, Guariento LA, Bolognin L, Moretto E (2020) A new alien mantis in Italy: is the indochina mantis Hierodula patellifera chasing the train for Europe? Biodivers Data J 8:e50779
Birkhead TR, Lee KE, Young P (1988) Sexual cannibalism in the praying mantis Hierodula membranacea. Behaviour 106:112–118
Bolu H, Ozaslan C (2015) Mantis religiosa L. (Mantodea: Mantidae) a new host for Podagrion pachymerum walker (Hymenoptera: Torymidae) in Turkey. A&f 61:183–187
Brannoch SK, Wieland F, Rivera J, Klass K, Bethoux O, Svenson GJ (2017) Manual of praying mantis morphology, nomenclature and practices (Insect, Mantodea). ZooKeys 696:1–100
Breland OP (1941) Podagrion mantis ashmead and other parasites of praying mantid egg cases (Hym.: Chalcidoidea; Dipt.: Chloropidae). Ann Entomol Soc Am 34:99–113. https://doi.org/10.1093/aesa/34.1.99
Eisenberg RM, Hurd LE, Bartley JA (1981) Ecological consequences of food limitation for adult mantids (Tenodera aridifolia sinensis saussure). Am Midl Nat 106:209–218
Greyvenstein B, Du Plessis H, Moulin N, Van den Berg J (2020) Distribution of Galepsus spp in Southern Africa and life history of Galepsus lenticularis (Mantodea: tarachodidae). Insects 11:119. https://doi.org/10.3390/insects11020119
Greyvenstein B, du Plessis H, van den Berg J (2021) Life history of the false flower mantid (Harpagomantis tricolor linnaeus, 1758) (Mantodea: Galinthiadidae) and its distribution in southern Africa. J Orthoptera Res 30(1):17–26. https://doi.org/10.3897/jor.30.52816
Harris SJ, Moran MD (2000) Life history and population characteristics of the Mantid Stagmomantis carolina (Mantodea: Mantidae). Entomol Soc Am. 29(1):64–68
Hawkeswood TJ (2003) Notes on the biology and food items of three Australian dermestidae (Coleoptera). Calodema 1:1–4
Holling CS, Dunbrack RL, Dill LM (1976) Predator size and prey size:presumed relationship in the mantid Hierodula coarctata saussure. Can J Zool 54:1760–1764
Hurd LE, Eisenberg RM, Fagan WF, Tilmon KJ, Snyder WE, Vandersall KS, Datz SG, Welch JD (1994) Cannibalism reverses male-biased sex ratio in adult mantids: female strategy against food limitation? Oikos 69:193–198
Hurd LE, Eisenberg RM, Moran MD, Rooney TP, Gangloff WJ, Case VM (1995) Time, temperature, and food as determinants of population persistence in the temperate mantidTenodera sinensis (Mantodea: mantidae). Environ Entomol 24:348–353. https://doi.org/10.1093/ee/24.2.348
Hurd LE, Prete FR, Jones TH, Singh TB, Co JE, Portman RT (2004) First identification of a putative sex pheromone in a praying mantid. J Chem Ecol 30:155–166
Iwasaki T (1996) Comparative studies on the life histories of two praying mantises, Tenodera aridifolia (Stoll) and Tenodera angustipennis saussure (Mantodea: Mantidae) I. temporal pattern of egg hatch and nymphal development. Appl Entomol Zool 31:345–356. https://doi.org/10.1303/aez.31.345
Kershaw JC (1910) The formation of the ootheca of a Chinese mantis, Hierodula saussuri. Psyche 17:136–141. https://doi.org/10.1155/1910/78374
Leong TM (2009) Oviposition and hatching in the praying mantis, Hierodula patellifera (Serville) in Singapore (Mantodea: Mantidae: Paramantinae). NiS 2:55–61
Mirzaee Z, Ebrahimi M, Sadeghi S, Battiston R, Hajian M (2021a) Mantid ootheca (Insecta: Mantodea: Hierodula) or a home for many arthropods? Iran J Sci Technol Transection A 45:1925–1931. https://doi.org/10.1007/s40995-021-01184-3
Mirzaee Z, Lotfalizadeh H, Sadeghi S (2021b) Chalcidoid parasitoids (hymenoptera: Torymidae and Eupelmidae) of mantids (Mantodea) oothecae in Iran. Phytoparasitica. https://doi.org/10.1007/s12600-021-00965-1
Moulin N (2020) When citizen science highlights alien invasive species in France: the case of indochina mantis, Hierodula patellifera (Insecta, Mantodea, Mantidae). Biodivers Data J 8:e46989. https://doi.org/10.3897/BDJ.8.e46989
Otte D, Spearman LA (2005) Mantida species file, catalog of the mantids of the world. Association of Insect Diversity, Philadelphia
Perez B (2005) Calling behaviour in the female praying mantis, Hierodula patellifera. Physiol Entomol 30:42–47
Raut GA, Gaikwad SM (2016) Observations on the life cycle, mating and cannibalism of Mantis religiosa religiosa linnaeus, 1758 (Insecta: Mantodea: Mantidae). J Entomol Zool Stud 4(6):478–482
Raut GA, Bhawane GP, Gaikwad SM (2014) Laboratory studies on the life history of Hierodula ventralis Giglio-Tos, 1912 (Mantodea: Mantidae). J Entomol Zool Stud 2(6):147–152
Robert RA (1937) Biology of the bordered mantid, Stagmomantis limbata Hahn(Orthoptera, Mantidae). Ann Entomol Soc Am 30:97–109
Schwarz CH, Roy R (2019) The systematics of mantodea revisited: an updated classification incorporating multiple data sources (Insecta: Dictyoptera). Annales de la Société entomologique de France (N.S.) 55(2):101–196
Vanitha K, Bhat PS, Raviprasad TN, Srikumar KK (2016) Biology and behaviour of Ephestiasula pictipes (wood-mason) (Hymenopodidae: Mantodea) under captive breeding. Int J Pest Manag 62:308–318. https://doi.org/10.1080/09670874.2016.1195026
Acknowledgements
The authors would like to express their sincere gratitude to people whose contribution made this work possible. Many thanks go to Dr. H. Lotfalizadeh for his aid in identifying the parasitoid wasp. We are also grateful to Prof. Dr. T. Schmitt and Dr. M. Wiemers for their comments on the manuscript and our colleagues M. Barmeshori, M. J. Ghasempour, D. Hashemi, and N. Sanjarani for their aid in the fieldworks.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by ZM. The first draft of the manuscript was written by ZM, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there are no conflict of interest and no financial and proprietary interest in the writing of this article.
Rights and permissions
About this article
Cite this article
Mirzaee, Z., Sadeghi, S. & Battiston, R. Biology and Life Cycle of the Praying Mantid Hierodula tenuidentata Saussure, 1869 (Insecta: Mantodea). Iran J Sci Technol Trans Sci 46, 1163–1169 (2022). https://doi.org/10.1007/s40995-022-01325-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-022-01325-2