Abstract
A simultaneous solid-phase preconcentration and spectrophotometric determination method based on adsorption of rhodamine B (RB) and Brilliant Blue FCF (BB) synthetic dyes onto Chromosorb 108 resin was developed. Solution pH, sample, eluent flow rates and eluent type were optimized. RB and BB concentrations were determined at 556 and 630 nm, respectively. Matrix effects of interference components were examined. Preconcentration factors and detection limits of the dyes were calculated. Adsorption isotherms of method, surface micrographs and pHzpc of resin experiments were performed. Limit of detections of the method were determined 0.22 and 0.31 µg L−1 for RB and BB, respectively. The method was successfully applied to determine RB and BB in industrial, foodstuff, cosmetics and environmental water samples. RB contents were found between 2.11 and 5.89 µg mL−1 for liquid samples and 17.55 and 370 µg g−1 for solid samples. BB concentrations in investigated solid samples were between 3.00 and 53.38 µg g−1. BB contents of liquid samples were determined between 1.57 and 9.66 µg mL−1. In order to test applicability of the method, analyte addition/recovery technique was also used. Quantitative recovery values ranging from 95 to 103% were obtained from the analyte addition/recovery tests.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Rhodamine B (RB) is highly water soluble, synthetic, a kind of xanthene dye and extensively used to dye final products in various industries such as food, cosmetic, plastic, textile, petroleum and automotive (Pourreza et al. 2008). Due to its stable characteristics and florescence feature, it has been preferred by manufacturers and widely used in different industries (Li et al. 2009; Hu et al. 2013) and their products such as lipstick, antifreeze and soap. It has four aromatic benzene rings, a color index of 45,100 and IUPAC name [9-(2-carboxyphenyl)-6-diethylamino-3-xanthenylidene]-diethylammonium chloride (Unsal et al. 2014a). Carcinogenic and toxic effects of RB on human and animal have been proved previously with experimental reports (Liu et al. 2013).
Brilliant Blue FCF (BB) is denoted by E number E133, a synthetic, triarylmethane dye and widely used in petroleum, oil, cosmetic, textile, food and automotive industries as a coloring agent. It is used also in soaps, energy drinks, lubricants, inks and antifreezes (Pourreza and Ghomi 2011). It has IUPAC name disodium 3-[N-ethyl-N-[4-[[4-[N-ethyl-N-(3-sulfonatobenzyl)-amino]phenyl] (2-sulfonatophenyl) methylene]-2,5-cyclohexadiene-1-ylidene] ammoniomethyl] benzene sulfonate, a color index of 42,090 and five aromatic rings which may have harmful effects on environment and also human health (Guo et al. 2013). Carcinogenicity and toxicity of BB have been well known and reported by science community (Bişgin et al. 2015a).
The use of harmful dyes in industrial products such as soaps is considered a great threat to human health. Both of dyes cause human health problems such as irritation to eyes and skin (Stachová et al. 2016). These harmful effects also depend on concentration of BB when consumed excessive amount of dye through foodstuffs. According to WHO (World Health Organization), the acceptable daily intake amount of BB is 6 mg/kg per day on a basis of human body weight (Wang et al. 2015). On the other hand, RB is illegal food dye and it has not been allowed to use in foodstuffs; therefore, it has no allowable concentration value (Fu et al. 2015). Because of harmful features of both dyes, toxicological considerations and carcinogenicities, these dyes cannot be considered safe (Zhai et al. 2017). Due to these reasons, great efforts have been made and various instrumental techniques including spectrophotometry (Dil et al. 2016), chromatography (Qi et al. 2016), voltammetry (Gao et al. 2016), electrochemistry (Gómez et al. 2016), capillary electrophoresis (Chen et al. 2015) and fluorometry (Fu et al. 2015) have been used for detection and determination of the dyes. Spectrophotometry is the most used and attractive in these techniques because it is simplistic and has lower operational cost than other instrumental techniques (Long et al. 2016).
Spectrophotometric determination of dyes could be problem because of low levels of dyes and interference effect of matrix in real samples (Bişgin et al. 2015b). Therefore, various extraction methods including solid-phase extraction (SPE) (Soylak et al. 2011), cloud point extraction (Sürme et al. 2016), dispersive liquid–liquid microextraction (Unsal et al. 2015a), membrane filtration (Unsal et al. 2015b), microextraction (Yigit et al. 2016) and liquid–liquid extraction (Unsal et al. 2014a, b) have been developed for preconcentration and separation of the dyes. Among these methods, SPE is inexpensive, simple and useful. The main advantages of this procedure are high preconcentration factor and low detection limit. Moreover, SPE resins can be regenerated and reused many times (Soylak et al. 1996; Bişgin et al. 2016).
In the present study, a combination method based on the simultaneous SPE and UV–Vis spectrophotometric determination of trace levels of RB and BB in different industrial and environmental water samples was developed. According to our best literature survey to date, simultaneous column solid-phase extraction and spectrophotometric determination of RB and BB dyes with using Chromosorb 108 resin have not been performed.
2 Materials and Methods
2.1 Chemicals
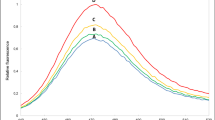
The chemicals used in the experiments were of analytical grade and purchased from Merck (Merck, Darmstadt, Germany) and Sigma (Sigma–Aldrich, Munich, Germany). RB and BB stock solutions were prepared with distilled water. Each of 1000 µg mL−1 stock dye solutions was stored in a refrigerator at 4 °C. Working standard solutions were prepared daily by mixing and diluting appropriate amounts of the stock dye solutions. The chemical structures of the dyes are given in Fig. 1 with their UV–Vis spectra.
Binary buffer chemicals H3PO4//NaH2PO4 for pH 2–3, HAc//NaAc for pH 4–5 and NaH2PO4//Na2HPO4 for 6–8 were used in the experiments. Buffer solutions were prepared in distilled water by adding calculated amounts of binary chemicals and controlling with a pH meter.
Chromosorb 108 is a synthetic and acrylic ester polymeric resin and has 100–200 m2 g−1 surface area and 60–80 mesh pore size. The resin possesses high stability in strong acidic and basic media; therefore, it can be used as stable in entire working pH range (Tuzen and Soylak 2006).
2.2 Instruments
A double-beam Shimadzu UV-160/A model UV–Vis spectrophotometer (Shimadzu Corporation, Japan) was used for UV–Vis measurements and determinations of the dyes. A Hanna HI-2211 model digital pH meter (Hanna Instruments, USA) was used to measure the pH values of the buffer solutions and control the pH of the aqueous solutions. An Olympus SZ-61 model stereomicroscope (Olympus Corporation, Japan) equipped with a Nikon D-90 model camera with a high zoom objective (Nikon Corporation, Japan) was used to take surface micrographs of the resin. Mini chromatographic glass columns (Çalışkan Glass and Technique, Ankara, Turkey) which have 1 cm in diameters, 10 cm longs and 500 mL reservoirs with Teflon stopcocks were used for extraction and separation procedures.
2.3 Solid-Phase Extraction Procedure
Twenty-five milliliters of model solution containing 2.5 µg RB and 3.75 µg BB was buffered to pH 4 with HAc//NaAC binary buffer chemicals. The solution was poured into reservoir of the column and permitted to flow through the column under gravity at a flow rate of 4 mL min.−1. After ending the flow of the sample solution, Chromosorb 108-loaded column was rinsed with 5 mL deionized water. Adsorbed RB and BB dyes on the sorbent were eluted to 5 mL of final volume with 0.5 mol L−1 HNO3 in ethanol solution at an eluent flow rate of 4 mL min−1. RB and BB concentrations were determined by a spectrophotometer at 556 nm and 630 nm, respectively.
2.4 Preparation of Real Samples
River, lake and wastewater samples were collected in polyethylene plastic tubes, and they were stored in a refrigerator at 4 °C until the analysis. Environmental water and wastewater samples were filtered through quantitative blue band filter paper after pH adjustments. Filtered water samples were directly subjected to the method.
Commercially available red and blue antifreeze samples were purchased from Turkish shops selling car care products. The method was applied to different brands of red and blue antifreeze samples which are known as engine coolant and heat transfer fluid in automobile industry and mixtures of water and either ethylene glycol or propylene glycol. RB and BB contents of the antifreeze samples were determined after necessary dilutions of the samples with and without analyte addition technique.
Certain volumes of liquid soaps were taken from different brands of red, blue and green soap samples and dried in an oven at 50 °C until the dryness. Solid soap samples were weighed and dissolved in water. Colorful soap solutions were applied to the developed method after pH adjustments and filtration process using a blue band filter paper.
Blue energy drink sample containing BB was directly analyzed using the given method after being filtered through a PTFE membrane filter (0.45 μm) and necessary dilutions.
Solid foodstuff samples containing BB were weighted and then dissolved with deionized water. Mixtures were filtered with a membrane filter (PTFE) of 0.45 μm pore size by using a syringe. The extraction procedure, as given above, was applied to filter samples.
Lipstick samples were accurately weighed and dissolved with CCl4. RB dyes of organic phase were extracted to water phase by 25 mL of 0.1 M NaOH solution using a separatory funnel. The pH values of these samples were adjusted to 4, and then, procedure was applied to extract sample.
2.5 pHzpc Experiments
The point of zero-charge experiments for Chromosorb 108 resin were performed between pH 2 and 10, at standard room temperature (25 °C), by contacting 50 mg of resin to 25 mL of each aqueous solution. NaCl was used as an inert electrolyte. 0.1 mol L−1 HCl and 0.1 mol L−1 NaOH solutions were used for pH adjustments. Adjusted initial pHs and containing 0.01 mol L−1 NaCl solutions were kept for 48 h. Final pHs of the solutions were measured with a pH meter after filtering the suspensions. Finally, pHzpc value of the sorbent was calculated by plotting initial pH versus ΔpH (final pH–initial pH).
2.6 Adsorption Experiments
Column adsorption experiments were performed separately for each of the dyes at the constant room temperature (25 °C) by applying optimum parameters of the method. Column was loaded with 300 mg Chromosorb 108 resin. Twenty-five milliliters sample solutions containing different dye concentrations were prepared. pHs and concentrations adjusted dye solutions were subjected to the method. RB and BB concentrations of the elution solutions were determined at 556 and 630 nm, respectively.
3 Results and Discussion
Red and blue colors were used in all graphs to symbolize RB and BB, respectively. Colors of the graphs were chosen according to original colors of the dyes and mixture of dyes. UV–Vis spectra of the dyes and their mixture are given in Fig. 1 which shows any interference effect to each other.
3.1 Influence of pH
pH is the most important parameter for quantitative extractions and preconcentration of RB and BB in developed SPE method. pH of the sample solution has provided the optimum retention conditions between dye molecules and sorbent surface. Influence of pH was investigated between pH 2 and pH 8. Testing range of pH was chosen as acidic to avoid precipitation of metal ions in the solution. Results are given in Fig. 2a with standard deviations. Quantitative extractions of RB and BB were obtained between pH 2–7 and pH 2–6, respectively. Quantitative recoveries were not observed at basic pH 8. pH 4 which was provided simultaneous quantitative extraction was chosen as optimum and working pH. Consequently, all further extraction studies were performed at pH 4.
a Effect of pH [SFR: 4 mL/min., EFR: 4 mL/min., eluent: 0.5 mol L−1 HNO3 in ethanol (5 mL), SV: 25 mL, RA: 300 mg] (N = 3); b effect of sample flow rate [pH: 4, EFR: 4 mL/min., eluent: 0.5 mol L−1 HNO3 in ethanol (5 mL), SV: 25 mL, RA: 300 mg] (N = 3); c effect of eluent flow rate [pH: 4, SFR: 4 mL/min., eluent: 0.5 mol L−1 HNO3 in ethanol (5 mL), SV: 25 mL, RA: 300 mg] (N = 3); d effect of sample volume [pH: 4, SFR: 4 mL/min., EFR: 4 mL/min., eluent: 0.5 mol L−1 HNO3 in ethanol (5 mL), RA: 300 mg] (N = 3)
3.2 Influence of Sample Flow Rates
A sample flow rate is an important parameter in the development of column solid-phase extraction method for simultaneous quantitative retention of RB and BB target dyes. Therefore, sample flow rates of the target dyes were investigated in the range of 1 and 10 mL min−1. In order to develop less time-consuming method, testing range of sample flow rate was chosen at fast flow rates. Quantitative recoveries were obtained for both dyes between 1 and 5 mL min.−1. Maximum 5 mL min.−1 sample flow rate was too enough for quantitative adsorption of dye molecules. Results of the experiments are given in Fig. 2b with standard deviations. According to these results, sample flow rates of the target dyes were chosen as 4 mL min.−1 and optimum flow rate; therefore, all subsequent extraction studies were performed by applying the optimum sample flow rate.
3.3 Influence of Eluent Flow Rates
Eluent flow rates of the target dyes are as important as sample flow rate because both dyes could be eluted simultaneously as quantitative. Eluent flow rates of the dyes were investigated simultaneously between 1 and 10 mL−1 min. Testing range of eluent flow rate was chosen at fast flow rates to apply fast extraction and determination. Working range of flow rate was too enough to obtain quantitative extractions. Quantitative elutions for the target dyes were obtained in the range of 1 and 5 mL min.−1. Results are given in Fig. 2c with standard deviations. As a result, 4 mL min−1 eluent flow rate was employed all further extraction studies.
3.4 Influence of Sample Volume
In order to obtain lower detection limits, sample volume is an important parameter. Effect of sample volume on the simultaneous extraction of the dyes was examined with constant dye amounts by varying sample volumes in the range of 25 and 800 mL. In order to obtain high preconcentration factor, extraction studies were performed by applying as high as possible volume. Quantitative extraction values were obtained up to 700 mL of sample volume for both dyes. Results are given in Fig. 2d with standard deviations. According to the highest sample volume which was obtained quantitative recoveries and final elution volume, the preconcentration factor of the method was determined as 140 for both dyes.
3.5 Concentration of Buffer Solution
Influence of ionic strength on the simultaneous extraction of RB and BB was investigated by varying buffer concentrations in the range of 0.1 and 0.5 mol L−1. Lower than 0.1 mol L−1 concentration was not employed to keep buffer solution stability. On the other hand, higher concentration than 0.5 mol L−1 was not performed to use less chemicals because 0.2 mol L−1 ionic strength of buffer solution is too enough to fix pH of aqueous solution. No considerable effects were observed in terms of recovery values with increasing buffer concentration. Recovery values of both dyes reached 100–101% from 97 to 98% with increasing buffer concentration from 0.1 to 0.5 mol L−1. Therefore, ionic strength of buffer solution was chosen as 0.2 mol L−1, and all further extraction studies were performed by applying 0.2 mol L−1 ionic strength.
3.6 Influence of Eluent Type
Type of eluent is another important parameter on the simultaneous extraction and elution processes for simultaneous elution of the dyes from the resin as quantitative. Therefore, various elution solvents and their mixtures were used in elution step of the method. Elution solutions were chosen according to polarities of the organic solvents, acids and their mixtures. When used 1 mol L−1 HNO3 in ethanol, 0.5 mol L−1 HCl in ethanol, 0.5 mol L−1 HNO3 in methanol or ethanol and 1 mol L−1 HCl in methanol or ethanol, both dyes were simultaneously eluted as quantitative. Results are given comparatively in Fig. 3. In view of economic, less toxicities and availabilities of the chemicals, 0.5 mol L−1 HNO3 in ethanol was chosen as optimum elution solution and it was used in all further extraction studies.
3.7 Influence of Matrix Components
Influence of potential interference ions and dyes which could be found in water, soap and antifreeze samples on the simultaneous extraction of RB and BB was investigated. Results are given in Table 1 with standard deviations. Any interfering effects were observed in the presence of examined dye and ions which have certain concentrations given in Table 1.
3.8 Influence of Resin Amount
In the optimization experiments, amounts of target analytes and other variables were kept steady. One variable was changed at one time. Influence of Chromosorb 108 amount was investigated between 100 and 600 mg of resin. Results are given in Fig. 4a. When column was loaded less than 200 mg resin, quantitative retention and extraction of the dyes were not obtained. On the other hand, when used more than 500 mg resin, the dye molecules remained on the resin and simultaneous quantitative elution was not observed. Therefore, column was loaded with 300 mg of sorbent and the optimum amount of resin was used in all further extraction studies.
3.9 Surface Micrographs
Commercially original and colorful surface micrographs of the Chromosorb 108 resin were also taken and are given in Fig. 4b. The resin which has white color is commercial pure original resin. Red- and blue-colored materials are RB and BB adsorbed states of the resins, respectively. Violet-colored material is mixture of RB and BB dyes adsorbed state of the resin. Colorful surface micrographs of the resins showed that RB and BB dyes were well adsorbed onto Chromosorb 108.
3.10 Point of Zero Charge
Point of zero-charge experiments support and facilitate to clarify the adsorption mechanisms of the dyes. According to our experiments, the adsorbent surface is charged as positive at lower pHs than pHpzc value. When the solution pH is equal to pHpzc, the adsorbent charge is neutral. When the pH value of the solution is higher than pHpzc, adsorbent surface is charged negatively (Bazrafshan et al. 2015). The pHpzc value of Chromosorb 108 resin was determined to be 6.8. Results are given in Fig. 4c. According to pHpzc and working pH values, sorbent surface was charged positively.
On the other hand, pKa values of RB and BB were 3.7 and 6.6, respectively (Ghoreishi et al. 2011). The analyte dyes and sorbent surface were charged positively at the working pH 4. These positive charges mean that strong adsorption of analyte dyes takes place onto Chromosorb 108 resin because of hydrophobic interactions between dye molecules and resin and hydrogen bonding through hard–hard interaction (Ghaedi et al. 2011). The hard–hard interaction is largely electrostatic, between ions or strongly dipolar molecules. They can approach each other closely with increasing electrostatic attraction. Therefore, strong adsorption is observed with increasing electrostatic attraction.
3.11 Isotherms and Mechanisms of Adsorption
Adsorption isotherms help to describe the interaction between sorbent and dye molecules and are useful to determine adsorption behaviors of the method. Adsorption isotherms related to retentions of RB and BB onto Chromosorb 108 resin were investigated. Obtained results were performed to fit Langmuir and Freundlich adsorption models. Linear forms of Freundlich adsorption isotherms with high correlation coefficients were obtained for both dyes. Calculated results are given in Fig. 4d.
Freundlich adsorption isotherm or equation is a curve relating the concentration of a solute on the surface of a solid phase, to the concentration of the solute in the liquid phase with which it is in contact. The basic Freundlich model is specified with x/m = k.C1/n0. In this equation, x is amount of adsorbed analyte, m is sorbent amount, x/m (q) is concentration of adsorbed analyte, C0 is initial or equilibrium concentration of analyte solution and k and n are empirical constants. This equation could be rearranged into linear form which was ln q = lnk + 1/n ln C0. Freundlich adsorption isotherms have been observed for a wide range of heterogeneous surface (Ng et al. 2002).
The Freundlich model was found to be the most appropriate to describe the adsorption process of RB and BB dyes on Chromosorb 108, suggesting that multilayer adsorption process occurs on the heterogeneous distribution of active sites onto Chromosorb 108 surface. The results related to adsorption of the dyes have not fitted to Langmuir adsorption model; therefore, adsorption capacity of the resin could not be calculated.
3.12 Applications of Real Samples
In order to test and prove the reliability of the developed method, natural water and wastewater samples were subjected to the suggested procedure with and without analyte addition technique. Results are given in Table 2 with standard deviations. Concentrations of the dyes in all analyzed real water samples are below detection limit of the reported method. Quantitative recovery values ranging from 95 to 101% were obtained from the analyte addition/recovery tests. These quantitative results supported the applicability of the method to different matrix.
Red antifreezes and red liquid soaps which are containing RB were analyzed with and without analyte addition technique. The analyzed results are given in Table 3. Results are quantitative for all analyzed real samples. Recovery values of RB are ranging from 97 to 103%. These results show that the method could be applied reliably to analyzed industrial samples.
Blue antifreezes, windshield washer fluid, blue and green liquid soaps samples containing BB were analyzed to determine BB concentrations. Analyte addition technique was also applied to these samples, and results are given in Table 4. Quantitative extraction results were obtained in the range of 97–101%. Quantitative analyzed results show that method is applicable for industrial samples containing BB dye.
RB and BB contents of the analyzed real samples are given in Table 5 with standard deviations. Dye contents of dried liquid soap samples were also determined. The dye concentrations of the real samples were determined as µg mL−1 and µg g−1 according to their physical (liquid or solid) form. RB contents were found between 2.11 and 5.89 µg mL−1 for liquid samples and 17.55 and 370 µg g−1 for solid samples. BB concentrations in investigated solid samples were between 3.00 and 53.38 µg g−1. BB contents of liquid samples were determined between 1.57 and 9.66 µg mL−1.
3.13 Analytical Characteristics and Performance of the Method
Analytical specificities of the method were determined, and they were also compared with other previously reported studies in Table 6. A 140 preconcentration factor (PF), 0.22 and 0.31 µg L−1 of detection limits (LOD) and 0.57 and 0.82 µg L−1 of quantitation limits (QOD) were determined for RB and BB, respectively. LOD was calculated with using (ATB + 3SD)/m/PF formula where ATB is average of 21 blank solutions, SD is standard deviation of blank solutions (n = 21) and m is the slope of calibration curve. Relative standard deviations of the method were calculated as 4.8% and 5.1% for RB and BB dyes, respectively. Linear dynamic ranges which could be plotted simultaneously were determined between 0.08 and 2.50 µg mL−1 RB and 0.11–3.75 µg mL−1 BB. A = 0.2503 C + 0.0005 (R2 = 0.9999) and A = 0.1414 C–0.0007 (R2 = 0.9990) equations with correlation coefficients were obtained from the linear calibration curves of the dynamic ranges for RB and BB, respectively.
Reusability test for the solid-phase resin was also investigated, and only 300 mg of Chromosorb 108 can be used and regenerated at least 50 times by using the mentioned chemicals in the proposed method.
4 Conclusions
The proposed separation and preconcentration method offers safe, rapid, easy, low-cost and simple analyses for simultaneous extraction and determination of RB and BB. In addition, colorful surface micrographs proved that both of the dyes simultaneously and separately well adsorbed to sorbent surface.
The developed method has served higher preconcentration factors and lower detection limits than other methods for both dyes. Developed method has the highest PF in the methods which are given in Table 6. Relative standard deviations of the method are comparable with other methods. Among the methods in Table 6, the developed method has permitted the best LOD values for the both dyes. Chromosorb 108-loaded column has shown good analytical characteristics and wide-range applications for the simultaneous and single determinations of RB and BB in industrial and environmental samples.
RB contents were found between 2.11 and 5.89 µg mL−1 for liquid samples and 17.55 and 370 µg g−1 for solid samples. BB concentrations in investigated solid samples were between 3.00 and 53.38 µg g−1. BB contents of liquid samples were determined between 1.57 and 9.66 µg mL−1. In order to test applicability of the method, analyte addition/recovery technique was also used. Quantitative recovery values ranging from 95 to 103% were obtained from the analyte addition/recovery tests. These quantitative results supported the applicability of the method to different matrix.
When used 1 mol L−1 HNO3 in methanol as an eluent, only BB dye could be eluted and only RB could be eluted from the resin with using methanol or ethanol. Therefore, developed method has exhibited that single and simultaneous determinations of the RB and BB dyes could also be performed.
Single extraction and determination method for RB and BB have already available in the literature, but simultaneous extraction and determination of mentioned binary dyes were performed as a first attempt.
These advantages mentioned above will make this method more useful for a wide range of future applications such as industrial, food and environmental.
References
Bazrafshan AA, Hajati S, Ghaedi M (2015) Synthesis of regenerable Zn(OH)2 nanoparticleloaded activated carbon for the ultrasoundassisted removal of malachite green: optimization, isotherm and kinetics. RSC Adv 5:79119–79128
Bişgin AT, Narin İ, Uçan M (2015a) Determination of sunset yellow (E110) in foodstuffs and pharmaceuticals after separation and preconcentration via solid-phase extraction method. Int J Food Sci Technol 50:919–925
Bişgin AT, Uçan M, Narin İ, Soylak M (2015b) A comparative study for separation, preconcentration and determination of tartrazine (E 102) in soft drink samples by two kinds of amberlite resins. Food Anal Methods 8:2141–2149
Bişgin AT, Sürme Y, Uçan M, Narin İ (2016) Simultaneous spectrophotometric determination and column solid-phase extraction of two Lanaset textile dyes in environmental water samples. J Ind Eng Chem 38:186–192
Chen L, Hu J, Zhang W, Zhang J, Guo P, Sun C (2015) Simultaneous determination of nine banned azo dyes in foodstuffs and beverages by high-performance capillary electrophoresis. Food Anal Methods 8:1903–1910
Dil EA, Ghaedi M, Asfaram A, Mehrabi F, Bazrafshan AA, Ghaedi AM (2016) Trace determination of safranin O dye using ultrasound assisted dispersive solid-phase micro extraction: artificial neural networkgenetic algorithm and response surface methodology. Ultrason Sonochem 33:129–140
Fu DS, Wu PP, Zhong XD, Liu Q, Luo HD, Li YQ (2015) A simple synchronous fluorescence approach for rapid and sensitive determination of rhodamine B in chilli products. Food Anal Methods 8:189–194
Gao Y, Xie Z, Zhang Y, Zou L, Ye B (2016) A simple and sensitive voltammetric method for the determination of orange II based on a functionalized graphene-modified electrode. J AOAC Int 99:1287–1294
Ghaedi M, Shokrollahi A, Tavallali H, Shojaiepoor F, Keshavarz B, Hossainian H, Soylak M, Purkait MK (2011) Activated carbon and multiwalled carbon nanotubes as efficient adsorbents for removal of arsenazo(III) and methyl red from waste water. Toxicol Environ Chem 93:438–449
Ghoreishi SM, Behpour M, Golestaneh M (2011) Simultaneous voltammetric determination of Brilliant Blue and Tartrazine in real samples at the surface of a multi-walled carbon nanotube paste electrode. Anal Methods 3:2842–2847
Gómez M, Arancibia V, Aliaga M, Núñez C, Rojas-Romo C (2016) Determination of Sudan I in drinks containing Sunset yellow by adsorptive stripping voltammetry. Food Chem 212:807–813
Guo J, Wu H, Du L, Fu Y (2013) Determination of Brilliant Blue FCF in food and cosmetic samples by ionic liquid independent disperse liquid–liquid micro-extraction. Anal Methods 5:4021–4026
Heydari R, Hosseini M, Alimoradi M, Zarabi S (2016) A simple method for simultaneous spectrophotometric determination of brilliant blue FCF and sunset yellow FCF in food samples after cloud point extraction. J Chem Soc Pak 38:438–445
Hu L, Yang F, Lu W, Hao Y, Yuan H (2013) Heterogeneous activation of oxone with CoMg/SBA-15 for the degradation of dye Rhodamine B in aqueous solution. Appl Catal B 134–135:7–18
Li R, Jiang ZT, Wang RX (2009) Solid phase extraction combined direct spectrophotometric determination of brilliant blue in food using β-cyclodextrin polymer. Food Anal Methods 2:264–270
Liu X, Zhang X, Zhou Q, Bai B, Ji S (2013) Spectrometric determination of Rhodamine B in chili powder after molecularly imprinted solid phase extraction. Bull Korean Chem Soc 34:3381–3386
Long Z, Xu W, Lu Y, Qiu H (2016) Nanosilica-based molecularly imprinted polymer nanoshell for specific recognition and determination of rhodamine B in red wine and beverages. J Chromatogr B Anal Technol Biomed Life Sci 1029–1030:230–238
Ng C, Losso JN, Marshall WE, Rao RM (2002) Freundlich adsorption isotherms of agricultural by-product-based powdered activated carbons in a geosmin–water system. Biores Technol 85:131–135
Pourreza N, Ghomi M (2011) Simultaneous cloud point extraction and spectrophotometric determination of carmoisine and brilliant blue FCF in food samples. Talanta 84:240–243
Pourreza N, Rastegarzadeh S, Larki A (2008) Micelle-mediated cloud point extraction and spectrophotometric determination of rhodamine B using Triton X-100. Talanta 77:733–736
Qi F, Qian L, Liu J, Li X, Lu L, Xu Q (2016) A high-throughput nanofibers mat-based micro-solid phaseextraction for the determination of cationic dyes in wastewater. J Chromatogr A 1460:24–32
Soylak M, Elçi L, Doğan M (1996) Determination of some trace metal impurities in refined and unrefined salts after preconcentration onto activated carbon. Fresen Environ Bull 5:148–155
Soylak M, Unsal YE, Yilmaz E, Tuzen M (2011) Determination of rhodamine B in soft drink, waste water and lipstick samples after solid phase extraction. Food Chem Toxicol 49:1796–1799
Stachová I, Lhotská I, Solich P, Šatínský D (2016) Determination of green, blue and yellow artificial food colorants and their abuse in herb-coloured green Easter beers on tap. Food Addit Contam 33:1139–1146
Sürme Y, Bişgin AT, Uçan M, Narin İ (2016) Comparison of preconcentration and determination methods of a textile dye by spectrophotometry: cloud point extraction and solid-phase extraction. Desalin Water Treat 57:14965–14972
Tuzen M, Soylak M (2006) Chromium speciation in environmental samples by solid phase extraction on Chromosorb 108. J Hazard Mater B 129:266–273
Unsal YE, Soylak M, Tuzen M (2014a) Spectrophotometric detection of Rhodamine B after separation-enrichment by using multi-walled carbon nanotubes. J AOAC Int 97:1459–1462
Unsal YE, Tuzen M, Soylak M (2014b) Spectrophotometric determination of Sudan blue II in environmental samples after dispersive liquid–liquid microextraction. Quim Nova 37:1128–1131
Unsal YE, Soylak M, Tuzen M (2015a) Dispersive liquid–liquid microextraction– spectrophotometry combination for determination of rhodamine B in food, water, and environmental samples. Desalin Water Treat 55:2103–2108
Unsal YE, Tuzen M, Soylak M (2015b) Separation and preconcentration of Sudan blue II using membrane filtration and UV-visible spectrophotometric determination in river water and industrial wastewater samples. J AOAC Int 98:213–217
Wang M, Yang M, Sun Q, Gao Y, Zhao J (2015) Development of a facile sensor for the determination of Brilliant Blue FCF in beverages. Int J Environ Anal Chem 95:969–979
Yigit S, Tuzen M, Soylak M, Dogan M (2016) Supramolecular solvent microextraction of Sudan blue II in environmental samples prior to its spectrophotometric determination. Int J Environ Anal Chem 96:568–575
Zhai H, Huang L, Chen Z, Su Z, Yuan K, Liang G, Pan Y (2017) Chip-based molecularly imprinted monolithic capillary array columns coated GO/SiO2 for selective extraction and sensitive determination of rhodamine B in chili powder. Food Chem 214:664–669
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bişgin, A.T., Sürme, Y., Uçan, M. et al. Simultaneous Preconcentration and Determination of Rhodamine B and Brilliant Blue. Iran J Sci Technol Trans Sci 44, 695–705 (2020). https://doi.org/10.1007/s40995-020-00892-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-020-00892-6