Abstract
The increased incidence of severe opportunistic fungal and bacterial infections is an important problem. Therefore, there is a great need in the determination of new classes of natural products that may be effective against bacteria and fungi. Plant extracts represent a continuous effort to find new compounds with the potential to act against multi resistant bacteria. The aim of this investigation was to carry out the analysis of antibacterial and antifungal activities of different parts of Calotropis gigantea. Antimicrobial activity of ethanol, methanol and chloroform extracts from flower, leaves and roots of C. gigantea, was examined against six species of pathogenic and non-pathogenic microorganisms: Escherichia coli, Bacillus subtilis, Klebsiella pneumonia, Staphylococcus aureus, Aspergillus niger and Candian albican using disc diffusion method. The maximum level of antibacterial activity was observed in flower extract against S. aureus (34 ± 0.43 mm) at the concentration of 20 mg of chloroform extract and there was less activity observed against A. niger (02 ± 0.10 mm) at the concentration of 5 mg of ethanol extract. The methanol extract of root showed highest antibacterial activity against S. aureus (28 ± 0.30 mm) at the concentration of 20 mg of extract, but less activity was observed in the chloroform extract against K. pneumonia (9 ± 0.01 mm). Methanol leaf extract of C. gigantea showed more active against K. pneumonia (12 ± 0.44 mm) at the concentration of 20 mg and less activity was observed in chloroform extract against E. coli. Hence, the isolation of antimicrobial compounds from flower, root and leaf of C. gigantea with possible mechanism would be a better option for the synthesis of new antimicrobial drugs to treat infectious diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The prevalence of severe opportunistic bacteria and fungus infection is a crucial problem. Hence, there is a high essential for novel class of natural product that may provide effective counter against bacteria and fungi. The plant extract represents a continuous attempt to find a novel compound which is potentate to act against multi drug resistant microbes. 20 % of the plants found in the world have been acknowledged for pharmacological or biological test and a substantial number of novel antibiotics are popularised in the market, are obtained from natural or semi synthetic resources (Mothana and Lindequist 2005). Plants are reported to have antimicrobial, anticancer, anti-inflammatory, antidiabetic, haemolytic, antioxidant, larvicidal properties, etc.
The Plant Calotropis gigantea belongs to the family of Apocynaceae is an ayurvedic plant with high medicinal potent. Its vegetation is native to Cambodia but it is frequent in major parts of Asian subcontinent such as Indonesia, Malaysia, Philippines, China, Thailand, Nepal, Pakistan and India and tropical Africa. In India due to high frequency of availability, it is regarded as a waste land weed but the plant parts has very high ethano medicinal value such as leaves, stem, flower and root. The wide folk medicinal use is very common for fever, cough, cold, eczema indigestion, diarrhoea, vomiting, asthma and rheumatism. There are also some scientific reports of C. gigantea, for anti-Candida activity, cytotoxicity activity, anti pyretic activity and wound healing activity (Chitme et al. 2005; Wang et al. 2008; Subramanian and Saratha 2010; Kumar et al. 2010). Shoot, leaf, roots flowers and latex extracts are reported to have antibacterial and antifungal properties by researchers (Kumar et al. 2010; Suresh Babu and Karki 2012; Gouri sankar et al. 2014).
To the best of our knowledge, the antimicrobial activity of this plant against certain microbes is investigated but the information about antimicrobial activity of flower and complete comparative antimicrobial analysis of different parts of this plant is poorly reported. In this study a comparision of antimicrobial activity of three different extracts of three different parts of the plant was reported. The efficacy of ethanol, methanol and. Chloroform extract against four bacterial and two fungal species is reported.
2 Materials and Methods
The fresh plants of C. gigantea are collected from Orissa University Agriculture and Technology Campus, Bhubaneswar, Odisha. The plant specimens are further identified by Prof. Baladev Khuntia the Ex-reader in Botany, College of Basic Science and Humanity, OUAT, Odisha.
3 Preparation of the Extract
3.1 Ethanolic Extract
The leaf, flower and roots were separated from the whole plant and each part is washed thoroughly especially the roots. The parts are cut into small pieces and are allowed for shade dry at room temperature and for 15 days. The parts are finely powdered separately and 100 gm of each part is taken differently for extraction in absolute ethanol by Soxhlet apparatus (Borosil Glass Work Limited, Worli, Mumbai, India) at 40 °C for 18–20 h. The extract was filtered through Whatmen no.1 filter paper and concentrated at room temperature. All these extracts are stored at 4 °C till further analysis.
3.2 Methanolic Extract
The leaf, flower and roots were separated from the whole plant and each part is washed thoroughly especially the roots. The parts are cut into small pieces and are allowed for shade dry at room temperature and for 15 days. The parts are finely powdered separately and 100 gm of each part is taken differently for extraction in methanol by Soxhlet apparatus (Borosil Glass Work Limited, Worli, Mumbai, India) at 40 °C for 18–20 h. The extract was filtered through Whatmen no.1 filter paper and concentrated at room temperature. All these extracts are stored at 4 °C till further analysis.
3.3 Chloroform Extract
The leaf, flower and roots were separated from the whole plant and each part is washed thoroughly especially the roots. The parts are cut into small pieces and are allowed for shade dry at room temperature and for 15 days. The parts are finely powdered separately and 100 gm of each part is taken differently for extraction in Chloroform by Soxhlet apparatus (Borosil Glass Work Limited, Worli, Mumbai, India) at 40 °C for 18–20 h. The extract was filtered through Whatmen no.1 filter paper and concentrated at room temperature. All these extracts are stored at 4 °C till further analysis.
3.4 Test Microorganism and Growth Media
Bacteria strains Bacillus subtilis (B. subtilis), Escherichia coli (E. coli), Klebsiella pneumonia (K. Pneumonia), Staphylococcus aureus and two fungal strains; Aspergillus niger (A. niger) and Candida albicans (C. albicans) were chosen based on their clinical and pharmacological importance. The bacterial strains obtained from the Department of Microbiology, College of Basic Science and Humanities, OUAT Bhubaneswar, were used for evaluating antimicrobial activity. The bacterial and fungal stock cultures were incubated for 24 h at 37 °C on nutrient agar and potato dextrose agar (PDA) medium (Himedia), respectively, following refrigeration storage at 4 °C. The bacterial strains were grown in Mueller–Hinton agar (MHA) plates at 37 °C (the bacteria were grown in the nutrient broth at 37 °C and maintained on nutrient agar slants at 4 °C), whereas the fungal strains were grown in Sabouraud dextrose agar and PDA media, respectively, at 28 °C. The stock cultures were maintained at 4 °C.
4 Antimicrobial Test
The antimicrobial activities of ethanolic extracts of C. gigantea were determined by filter paper disc diffusion method.
4.1 Disc Diffusion Method
A sterile filter disc (diameter 4 mm, Whatman paper No. 3) was placed in Petri dishes (diameter 90 mm) filled with Mueller–Hinton agar and seeded with 0.3 ml of the test organism. The disc was impregnated with test concentrations (5, 25, 50, 100 mg/ml) of the compounds investigated dissolved in DMSO. The zones of growth inhibition around the discs were measured after 24 h of incubation at 37 °C. Each microorganism was tested in triplicate and the solvent (DMSO) was used as a control, while streptomycin was used as a positive control.
4.2 Minimum Inhibitory Concentration
Different concentrations of the flower, leaves and root extract of C. gigantea were prepared to obtain 2.5, 5.0, 7.5 mg/ml. Three drops of overnight broth culture of the test organisms were inoculated into the dilutions and incubated at 37 °C for 24 h. The lowest concentration of the extracts that inhibited the growth of the test organisms was recorded as the minimum inhibitory concentration (MIC).
4.3 Statistical Analysis
Results obtained were reported as mean ± SD of triplicate measurements. Significance differences for multiple comparisons were determined by one-way analysis of variance (ANOVA) followed by Duncan test with po0.05 using SPSS (version 19).
5 Results and Discussion
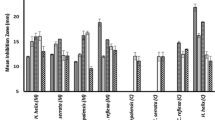
The present study revealed that the tested extracts of different parts of C. gigantea possess antibacterial activity against E. coli, B. subtilis, K. pneumonia, S. aureus, A. niger and C. albican. The extracts of different parts of C. gigantea showed different degrees of antibacterial activity and the results are represented in Tables 1, 2 and 3 as well as in Figs. 1, 2 and 3. The maximum level of antibacterial activity was observed in flower extract against S. aureus (34 ± 0.43 mm) at the concentration of 20 mg of chloroform extract and there was less activity observed against A. niger (02 ± 0.10 mm) at the concentration of 5 mg of ethanol extract. The methanol extract of root of C. gigantea showed highest antibacterial activity against S. aureus (28 ± 0.30 mm) at the concentration of 20 mg of extract, but less activity was observed in the chloroform extract against K. pneumonia (9 ± 0.01 mm). Methanol leaf extract of C. gigantea showed more active against K. pneumonia (12 ± 0.44 mm) at the concentration of 20 mg and less activity was observed in chloroform extract against E. coli.
Medicinal plants are being probed as an alternate source to get therapeutic compounds based on their medicinal properties. Calotropis gigantea is easily available in most of the agricultural and non agricultural fields and the usage of this plant for medicinal purpose was reported by several researchers. Gouri sankar et al. (2014) screened C. gigantea for its antimicrobial and phytochemical activities. The Acetone extract of C. gigantea showed more activity against fungus like C. albicans zone of diameter 14.5 mm, and bacteria like E. coli zone of diameter 17.5 mm, when compared to other solvent extracts. Kumar et al. (2010) screen leaves of C. gigantea for the antimicrobial activity against clinical isolates of bacteria. The extract showed maximum zone of inhibition against E. Coli (17.6 ± 1.15), whereas, lowest against K. pneumoniae (12.6 ± 1.52). Crude extract showed maximum relative percentage inhibition against B. cereus (188.52 %) and lowest relative percentage inhibition against M. luteus (24.92 %). Kori and Alawa (2014) reported. The root and latex of C. gigantea were screened for its antimicrobial and phytochemical activities. The aqueous, ethanolic and acetone extract of root of C. gigantea shows inhibition against all the test microbe ranging from 10- to 16-mm-diameter inhibitory zone.
The leaves extract of C. gigantea were screened for its antimicrobial and phytochemical activities by Senthil Kumar et al. (2012). The Ethanol extract of C. gigantea showed more activity against fungus like C. albicans zone of diameter 15.06 ± 0.11, Candida tropicalis zone of diameter 13.30 ± 0.26 and bacteria like Proteus mirablis zone of diameter 12.16 ± 0.15 and Pseudomonas aeruginosa zone of diameter 8.0 ± 0.00 when compared to other solvent extracts. Seniya et al. (2011) reported Ethyl Acetate leaves extract exhibited maximum zone of inhibition. Ethyl Acetate leaves extract was found to be most effective with MIC value also ranging from 0.25 to 1.0 mg/ml. Antimicrobial activities of flowers of C. gigantea reported by Rajamohan et al. (2014). The activity of the plant against both bacteria and fungus may be indicative to the presence of broad spectrum antibiotic compounds or simply general metabolic toxins in the plant, in addition to the plant (flower, leaves and root) content of pharmacological active metabolites.
Antimicrobial activity of different solvent extracts of C. gigantea showed varying degrees of antibacterial and antifungal activity against all microoraganisms tested. (Murti et al. 2010; Subramanian and Saratha 2010). There are many reports of plants in the family Asclepiadaceae possessing antimicrobial activity. (Sukanya et al. 2009; Okiei et al. 2009). From this study it can be said that ethanol and methanol shade dried leaf extract of C. gigantea showed wide range of antibacterial and antifungal activity can be used and administered in the ethno medical practice (Juncker et al. 2009; Yesmin et al. 2008). The present study has shown a spectrum of antimicrobial activities which provides a support to some traditional uses of these few medicinal plants (Kuta 2008; Mei et al. 2007). But the effective biomolecules which act as antimicrobial have to be identified isolated and subjected to extensive scientific and pharmacological screening that can be used as sources for new drugs.
6 Conclusions
The present investigation clearly indicates that the different solvent extracts of root, flower and leaf of C. gigantea exhibited antibacterial activity. Hence, the isolation of antimicrobial compounds from root, flower and leaf of C. gigantea with possible mechanism would be a better option for the synthesis of new antimicrobial drugs to treat infectious diseases caused by microorganisms. This may prove to be a right replacement for many of the synthetic drugs in future. Hence we conclude that C. gigantea represents a rich source of valuable medicinal compounds.
References
Chitme HR, Chandra R, Kaushik S (2005) Evaluation of antipyretic activity of Calotropis gigantean (Asclepiadaceae) in experimental animals. Phytother Res 19:454–456
Gouri Sankar K, Amaralinga Reddy B, Sathya Chaitanya B, Satish Kumar B, Sivaiah C (2014) Anti-microbial investigation and phytochemical analysis on organic solvent leaf extracts of Calotropis gigantea. Int J Biol Pharm Res 5(4):308–312
Juncker T, Schumacher M, Dicato M, Diederich M (2009) UNBS1450 from Calotropis procera as a regulator of signaling pathways involved in proliferation and cell death. Biochem Pharmacol 78:1–10
Kori P, Alawa P (2014) Antimicrobial activity and phytochemical analysis of Calotropis gigantea root, latex extracts. IOSR J Pharm 4(6):7–11
Kumar G, Karthik L, Bhaskara Rao KV (2010) Antibacterial activity of aqueous extract of Calotropis gigantea leaves—an in vitro study. Int J Pharm Sci Rev Res 4(2):141–144
Kuta FA (2008) Antifungal effect of Calotropis procera stem bark on Epidermophyton flocosum and Trichophyton gypseum. Afr J Biotechnol 7:2116–21118
Mei WL, Gan YJ, Dai HF (2007) Advances in studies on chemical constituents of Antiaris toxicaria and their pharmacological activities. Tradit Chin Herb Drugs 39:151–154
Mothana RA, Lindequist U (2005) Antimicrobial activity od some medicinal plant of the island Soqotra. J Ethanopharmacol 96(1–2):177–181
Murti Y, Yogi B, Pathak D (2010) Pharmacognostic standardization of leaves of Calotropis procera (Ait.) R. Br. (Asclepiadaceae). Int J Ayurveda Res 1:14–17
Okiei W, Ogunlesi M, Ofor E, Osibote EAS (2009) Analysis of essential oil constituents in hydro-distillates of Calotropis procera (Ait.) R.Br. Res J Phytochem 3(3):44–53
Rajamohan S, Kalaivanan P, Sivagnanam I, Rajamanickam M (2014) Antioxidant, antimicrobial activities and GC-MS analysis of Calotropis gigantea white flowers. J Phytopharmacol 3(6):405–409
Seniya C, Trivedia SS, Verma SK (2011) Antibacterial efficacy and phytochemical analysis of organic solvent extracts of Calotropis gigantea. J Chem Pharm Res 3(6):330–336
Senthil Kumar S, Sivamani P, Baskaran C, Jamal Mohamed M (2012) Evaluation of anti microbial activity and phytochemical analysis of organic solvent extracts of Calotropis gigantea. IOSR J Pharm 2(3):389–394
Subramanian SP, Saratha V (2010) Evaluation of antibacterial activity of Calotropis gigantea latex extract on selected pathogenic bacteria. J Pharm Res 3(4):32–45
Sukanya SL, Sudisha J, Hariprasad P, Niranjana SR, Prakash HS, Fathima SK (2009) Antimicrobial activity of leaf extracts of Indian medicinal plants against clinical and phytopathogenic bacteria. Afr J Biotechnol 23:6677–6682
Suresh Babu AR, Karki SS (2012) Wound healing activity of Calotropis gigantea leaves in albino wistar rats. Int J Pharm 2(1):195–199
Wang Z, Wang M, Mei W, Han Z, Dai H (2008) A new cytotoxic pregnanone from Calotropis gigantea. Molecules 13(12):3033–3039
Yesmin MN, Uddin SN, Mubassara S, Akond MA (2008) Antioxidant and antibacterial activities of Calotropis procera. Am Eurasian J Agric Environ Sci 4(5):550–553
Acknowledgments
The authors are grateful to the Vice-Chancellor and Dean of Orissa University of Agriculture and Technology for providing all facilities, financial support and encouragement throughout.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There is no conflict of interest among the authors.
Rights and permissions
About this article
Cite this article
Kar, D., Kuanar, A. & Pattanaik, P.K. Antimicrobial Activities of Different Parts of Calotropis gigantea: a Naturally Occurring Prophylactic Medicinal Shrub. Iran J Sci Technol Trans Sci 42, 1057–1062 (2018). https://doi.org/10.1007/s40995-016-0079-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-016-0079-7