Abstract
This work aimed to investigate the combustion characteristics of rubberwood sawdust pellet (RSP), teak sawdust pellet (TSP), eucalyptus bark pellet (EBP), cassava rhizomes pellet (CRP), and their corresponding raw biomass. The experiments were performed in a thermogravimetric (TG) analyzer and a fluidized-bed combustor (FBC). Thermogravimetric analysis (TGA) was conducted in the temperature range of 30–1000 °C at a heating rate of 10 °C/min in a dry air atmosphere. The combustion experiments were conducted in a twin-cyclone FBC at 120 kilowatts (kWth) heat input with three values of excess air (EA): 40, 50, and 60%. The combustion reactivity of the pellets was lower than raw biomasses, as indicated by higher values of peak, ignition, and burnout temperatures, as well as a lower comprehensive performance index value. The activation energies of the biomass pellets were greater than the as-received biomasses, indicating that the biomass pellets required higher energy and temperature and a longer time for complete combustion. The pellet fuels had a higher residence time and better mixing of the fuel and bed particles, leading to higher combustion intensity in a fluidized bed. Carbon monoxide (CO), hydrocarbon (CxHy), and nitric oxide (NO) emissions of the combustor when firing the pellets were lower compared to the burning of as-received biomasses. When firing the pellets at optimal EA (about 40%), the combustor operated at high combustion efficiency of 99.0–99.8%. This study indicated that the biomass pellets have desirable combustion characteristics that could be used as alternative fuels for sustainable energy production.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The recent energy and environmental crises have driven the energy sector to develop technologies using renewable and sustainable resources instead of using less environmentally friendly fossil fuels. Biomass is a green and clean renewable energy source. It can be considered as one of the few viable replacement options for fossil fuels due to its high availability, carbon dioxide neutrality, and low sulfur content (Rokni et al. 2018). Using biomass as fuel allows rapid technological adaptation of existing coal installations. Among the main thermochemical processes of energy conversion, combustion is the primary method for biomass energy conversion and accounts for over 90% of the world's bio-energy production (Malico et al. 2019).
Thailand has a strong agro-industry base that generates substantial wastes and residues from local agricultural and forest industries, which can be utilized as renewable energy sources (biomass fuel) for producing heat and electricity. This country’s total energy generation capacity from solid biomasses accounted for 1480 petajoules (PJ), as reported in 2020 (DEDE 2020). Some biomasses include rice husks, oil palm residues, wood ship and pellets, sugarcane residues (Martsri et al. 2021), and rubberwood (Wongsapai et al. 2020), already been utilized for heat production and power generation in Thailand. Other biomasses, including rubberwood sawdust (RS), teak sawdust (TS), eucalyptus bark (EB), and cassava rhizomes (CR), are also vital bioenergy resources and show potential for use as fuel in direct combustion systems. These biomasses have not been used as fuel yet because of their inherent physicochemical properties, such as non-uniform physical properties, excessive moisture content, low calorific value, and energy density. Therefore, these feedstocks need pretreatment to improve their quality for efficient conversion and utilization. If fully utilized, energy about 155 PJ can be recovered in Thailand (DEDE 2020).
One of the promising ways to improve the quality of the low-quality feedstocks is pelletization. This process transforms the raw or as-received biomasses into homogeneous solid biofuel with higher bulk and energy densities, consequently leading to better handling, transportation, storage, and combustion efficiency. Tumuluru (2018) reported that the biomass pellets have low moisture content (below 10%), a calorific value of about 15–20 MJ/kg, and bulk density up to 750 kg/m3. The biomass pellets are generally made under mechanical pressure in an atmospheric environment. Mian et al. (2020) showed that the biomass’s chemical structure and composition underwent insignificant changes after pelletization, but the physical structure, such as the biomass’s porosity and surface morphology, changed substantially. As a result, the biomass pellet's thermal characteristic is expected to differ from the as-received feedstock.
Thermogravimetric analysis (TGA) is a helpful tool widely used for investigating thermal degradation behaviors and combustion characteristics of biomass feedstocks (Kongto et al. 2022; Saikia et al. 2018). Numerous research studies performed TGA to assess thermal degradation behaviors and combustion characteristics of different solid fuels, such as wood biomass, oat, sewage sludge (Magdziarz and Wilk 2013), waste capsicums, sawdust (Wang et al. 2012), cotton, forest residues, wood char, lignite, hard coal char (Kastanaki and Vamvuka 2006), palm shell, palm fiber, empty fruit bunches, sub-bituminous coal and their blends (Idris et al., 2012), peanut shells and tamarind shells (Kuprianov and Arromdee 2013), corn straw, corn straw pellet, pine and pine pellet (Mian et al. 2020), sludge pellet, camphor pellet, and sludge–camphor pellet (Jiang et al. 2015). Ninduangdee et al. (2015) found that biomass combustion in the TGA system was divided into three stages: (i) water evaporation, (ii) volatile release and its combustion, and (iii) char combustion. TGA also provides essential information for determining combustion kinetic parameters, which are helpful for efficient design of the reactors, optimization, and control of thermochemical processes. A short review of the literature regarding combustion characteristics and kinetics of various solid fuels using TGA is summarized in Table 1. The current work is the first report on the investigation of thermal degradation and combustion kinetics of RS, TS, EB, CR, and their corresponding pellets.
Fluidized-bed combustor (FBC) is suitable technique for burning biomasses such as agricultural residues, municipal solid waste, and sewage sludge. The combustion intensity in FBC is more significant than in a conventional combustion system, for example, a fixed-bed grate-firing system (Ninduangdee and Kuprianov 2018). With intensive and uniform particles mixing between bed material and biomass fuel, FBC ensures low carbon monoxide (CO) and nitric oxide (NO) emissions, and high combustion efficiency for burning various biomass fuels. The combustion efficiency of FBC fired with biomasses can be high, up to 99%. Shimizu et al. (2006) showed that cotton stalk was fired effectively in a bubbling FBC with 99.5–99.8% combustion efficiency. As Quan et al. (2022) reported, FBC operated with 98–99% combustion efficiency when firing sewage sludge in the range of 800–850 °C. Duan et al. (2014) conducted a combustion experiment on burning pelletized peanut shells in a vortexing FBC. They found that the combustor operated at 98.0–98.6% combustion efficiency with low emissions of CO (less than 70 ppm) and NO (up to 114 ppm). Werther et al. (2000) found that CO emission from the FBC can be controlled by increasing excess, while NO emission can be reduced by lowering the amount of excess air. Experiments on firing various biomasses by Permchart and Kouprianov (2004) revealed similar effects of excess air on emissions of CO and NO. Ninduangdee and Kuprianov (2014) reported that the combustion and emission performances of the FBC depended on fuel properties, operating conditions, and bed material type. Chirone et al. (2008) combusted biomass pellets in a bench-scale batch FBC. They observed that the pellets had a longer fuel devolatilization time than the original loose fuel constituents. This positive effect enhanced the combustion characteristics of FBC firing pellet fuels. Ninduangdee and Kuprianov (2018) investigated the combustion of rice husk pellet in a conical FBC. They observed that the rice husk pellet with heavy particles mainly combusted in a fluidized bed region, leading to high burnout and combustion efficiency.
The knowledge regarding thermal degradation and combustion characteristics, as well as combustion kinetic parameters of rubberwood sawdust pellet (RSP), teak sawdust pellet (TSP), eucalyptus bark pellet (EBP), and cassava rhizome pellet (CRP), has been still very limited. This information is crucial prior to using biomass pellets for thermochemical processes. There is also limited information regarding the combustion characteristics of these biomass pellets in a practical combustion system, particularly in FBC.
The purpose of this work was to investigate the combustion characteristics of RSP, TSP, EBP, and CRP in a thermogravimetric (TG) analyzer and a pilot-scale FBC. The TGA was performed to assess the pelletized biomass's thermal decomposition under combustion condition. The characteristic temperatures of combustion, such as ignition (Tig), peak (Tp), and burnout (Tb), comprehensive performance index values (Si), and kinetic parameters were determined with the objective of assessing the thermal and combustion reactivity of the proposed biomasses. The combustion experiment was also conducted in FBC for a specified range of excess air (EA). Special attention was given to combustion behaviors inside the combustor and the effects of fuel properties and EA on major gaseous emissions and the combustor’s combustion efficiency. The novelty of the present work is knowledge of (i) the thermal degradation behaviors, characteristic temperatures of combustion, and kinetic parameters of RSP, TSP, EBP, and CRP and (ii) combustion characteristics and performance of the twin-cyclone FBC when firing the proposed biomass pellets in the specified operating conditions.
2 Materials and methods
The working hypothesis for this study was that the pelletized biomasses (RSP, TSP, EBP, and CRP) could be efficiently utilized as a fuel in a combustion system. Two experimental series: (i) TGA and (ii) combustion tests in the twin-cyclone FBC were performed in order to test the hypothesis. In the first stage, the thermal decomposition, combustion characteristics, and kinetics of the proposed fuels were investigated by employing TGA. In the second series, RSP, TSP, EBP, and CRP were burned in a twin-cyclone FBC (Ninduangdee et al. 2020) for a specified range of the operating condition (i.e., EA) to investigate the combustion characteristics and performance of the combustor. The TGA and combustion experiments were also performed for the corresponding as-received biomasses to facilitate an interpretation of the main results. Materials and experimental facilities, and methods are explained in the following section.
2.1 Properties of feedstock
The selected biomass pellets: RSP, TSP, EBP, and CRP, used in this study were made by using a flat-die pellet pelletizer at room temperature. As-received biomasses: RS, TS, EB, and CR; were shredded in a shredder to reduce particle size and then pulverized by a grinding machine, ensuring a particle size of up to 3 mm. During pellet making, no binders, chemicals, or additives were used. Each ground biomass, with a specified amount of water (10–15% by weight), was put into a 75 kW pelletizer which pressed the ground biomass into densified cylindrical pellets of 6 mm in diameter and up to 60 mm in length. After cooling by ambient air, the pellets were kept in fabric sacks and stored at room temperature to ensure stable fuel quality. The solid density of the pelletized biomasses was 1450–1650 kg/m3.
Table 2 shows the combustion-related properties, as well as the chemical structure of the raw feedstock and biomass pellets used in this study. The proximate analysis was determined following ASTM standards: ASTM E871 for fuel moisture (W), ASTM E872 for volatile matter (VM), and ASTM E1755 for ash content (A), whereas fixed carbon (FC) was calculated by difference. The ultimate analysis of biomass components: carbon (C), hydrogen (H), nitrogen (N), and sulfur (S); were determined based on ASTM E777 (for C and H), ASTM E778 (for N), and ASTM E775 (for S), while oxygen content (O) was calculated by difference. The chemical structure of the fuels was obtained according to the method presented by Van Soest et al. (1991). From the data in Table 2, all the pelletized biomasses had improved fuel properties, e.g., lowered W content and VM, while increased FC, compared to the corresponding raw biomasses. The ultimate analysis indicated that C content in the pellets increased, whereas the O and H content decreased due to a reduction of moisture during the pelletization of the biomass. As a result, the lower heating value (LHV) of all biomass pellets was relatively higher than that of the raw feedstock. From Table 2, the S content in all biomasses was insignificant. The chemical constituents of both raw and pelletized biomasses were quite similar. This fact indicated a minor impact of pelletization on the fuel’s chemical structure. The LHV of RSP, TSP, and CRP were relatively high at about 17–18 MJ/kg, while the LHV of EBP was about 15 MJ/kg. Note that the calorific values of tested biomasses were measured using an oxygen bomb calorimeter (1341 Plain Jacket Bomb Calorimeter equipped with a Parr 6775 digital thermometer, Parr Instrument Company, USA) following the ASTM D2015 standard test method. The LHV was determined from the higher heating value by subtracting latent heat of evaporation of water formed from fuel moisture and hydrogen content. All test data was repeated three times to ensure accuracy and reproducibility.
2.2 Thermogravimetric analysis
The TGA was performed in a thermogravimetric analyzer (TGA/DSC1-Mettler Toledo, Switzerland). The measurement precision for temperature was ± 0.5 °C, while the sensitivity of the microbalance was less than 1.0 μg. Before the tests, an individual sample was carefully prepared from a cylindrical pellet to create a tablet-like shape (5 mm in diameter, 4 mm in height, and about 120 mg in mass). In each test, the individual sample was put in a 900 μL aluminum crucible (pan) and heated from 30 to 1000 °C at a constant heating rate of 10 °C/min in a combustion environment. Dry air at a flow rate of 30 mL/min was used as a furnace medium. The thermal degradation behavior of the biomass sample was represented by the TG and differential thermogravimetric (DTG) curves. The TG curve shows the sample mass loss versus furnace temperature, whereas the DTG curve represents the time derivative of the sample mass loss.
Note that the TGA for the original biomasses was also conducted with the same heating and airflow rates as the main experiments. The as-received biomasses were ground to ensure fine particle sizes of about 200 μm, with approximately 20 mg of sample used in each test. The obtained results were used to assist in an interpretation of the main results of this study. Triplicate tests were conducted for each fuel option to ensure the reproducibility of the findings.
The comprehensive performance index (Si) value was determined and then compared between the fuel options to evaluate the combustion performance of selected fuels. The index Si is an integrated solid fuel ignition, combustion, and burnout characteristics. The greater value of Si indicates better combustion characteristics and faster burnout of the feedstock. The index Si can be calculated as follows (Niu et al. 2016; Kongto et al. 2022):
where DTGmax is the maximum burning rate (%/min); DTGmean is the average weight loss rate (%/min); Tig and Tb are the ignition and burnout temperatures (ºC). The corresponding parameters in Eq. (1) were determined from the TG/DTG curves. Note that Tig and Tb were determined according to the method recommended by Kuprianov and Arromdee (2013).
2.3 Kinetic models
The combustion kinetic parameters of the raw biomass feedstock and their pellets during combustion were analyzed using the methodology recommended by Coats and Redfern (1964). Following this method, the parameters of thermal events such as activation energy (Ea), pre-exponential factor (A), and reaction order (n) can be calculated.
From the TG curve, the mass conversion ratio (α) can be defined as follows:
where m0 is the initial mass of the sample, mτ is the actual mass (at time τ) of the sample, and mf, is the final mass of the sample.
The kinetic equation describing the biomass decomposition with respect to time can be written as:
where R is the universal gas constant (R = 8.314 J/mol·K) and T is absolute temperature (K).
For a constant heating rate (\(\beta = \frac{{{\text{d}}T}}{{{\text{d}}\tau }}\) = const.) TG experiments, Eq. (3) can be rewritten as:
For the Coats and Redfern method, it is assumed that the value of 2RT/Ea is much less than 1.0 in the combustion reactions and the value of Ea is constant. Thus, integration of Eq. (4) results:
From Eqs. (5) and (6), one can observe the linear correlation if the left-hand side of Eqs. (5) and (6) are plotted versus 1/T. The best fit with the highest correlation coefficient can be achieved when the n value is appropriately selected. Then, the kinetic constants: Ea and A can be calculated from the slope of the fitted line and the intercept term (i.e., ln(AR/βEa)), respectively.
2.4 Combustion study on firing raw and pelletized biomasses in FBC
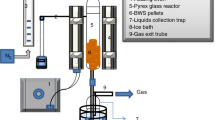
A combustion study on burning the pelletized biomasses and their as-received biomasses was performed in a twin-cyclone FBC, as depicted in Fig. 1. The combustor's design and geometrical details have been described in the previous studies (Ninduangdee et al. 2020). This combustor had a compact design, allowing high-intensive burning of various kinds of solid fuel with different particle sizes and fuel properties. It comprised two cyclonic combustion chambers, with an identical configuration and geometrical characteristics, arranged co-axially and connected in series by a pipe with a 0.2 m inner diameter. The lower combustion chamber used a swirl motion from the annular spiral air distributor to ensure high-intensive burning of the biomass fuel, whereas the upper chamber was used to ensure complete combustion of the fuel burned and mitigate the major gaseous pollutants. A diesel oil-firing burner was used to preheat the bed material during the combustor start-up mode. Once the bed temperature reached approximately 500 ºC, which was higher than the ignition temperature of the selected fuels obtained from TGA, the burner was turned off. Then, the biomass was supplied into the lower combustor at a specified fuel feed rate using a screw-type fuel feeder at 0.9 m above the air distributor.
All test series were performed at a similar heat input of approximately 120 kWth to the combustor with three specified amounts of excess air (EA): 40%, 50%, and 60%. An actual value of excess air was estimated according to Basu et al. (2000). During the trial under specified operating conditions, concentrations of O2 and major gaseous pollutants (CO, CxHy, and NO) were measured using a portable flue gas analyzer (Testo-350, Testo, Germany) at the stack. The measurement accuracies of the gas concentrations were: ± 0.2 vol.% for O2, ± 5% for CO within the range of 200–2000 ppm, ± 10% for CO higher than 2000 ppm, ± 10% for CxHy (as CH4), and ± 5% for NO. The temperature and O2 concentration were measured along the radial direction in the combustor for the test at 50% excess air at five levels (Z) above the air distributor: 0.45 m, 0.95 m, 1.30 m, 1.85 m, and 2.35 m. The combustion efficiency of the combustor was assessed for each test using the heat-loss method provided in Appendix A.
Quartz sand, with a particle size of 0.3–0.5 mm and a solid density of 2500 kg/m3, was used as the bed material in the reactor. During all combustion tests, the bed material was maintained at a 20 cm height (under static conditions). For the selected bed material and bed height, the value of the minimum velocity of full fluidization (umff) was quantified as 0.8 m/s, ensuring a fully bubbling fluidized-bed regime with partial swirl motion (Ninduangdee et al. 2020). According to the methods recommended by Ninduangdee and Kuprianov (2018), the range of the superficial air velocity at the air distributor exit (u) in the combustor when firing different fuel options at the lowest amount of excess air (i.e., EA = 40%) was predicted to be 1.2–1.6 m/s, which was higher than the above-mentioned umff value. Therefore, sustainable bed fluidization can be achieved during combustion of the selected biomasses for the range of excess air levels.
3 Results and discussion
3.1 Thermal decomposition of raw and pelletized biomasses
Figure 2 shows TG and DTG curves of the raw biomasses: RS, TS, EB, and CR; under an air atmosphere and heating rate of 10 °C/min. When increasing the furnace temperature, the mass loss of the samples was due to the consecutive thermal decomposition of the biomass structural components, exhibiting some specific features at distinct temperatures determined using the boundary temperatures corresponding to the two neighbor minimums on the DTG curve. Haykiri-Acma et al. (2010) reported that the decomposition of hemicellulose occurs at relatively low temperatures (160–360 °C), followed by volatilization of the cellulose (typically observed at 240–400 °C), and decomposition of lignin at 150–900 °C. These reference temperatures facilitated the characterization of different stages during biomass degradation.
The analysis can be made based on the TG/DTG curves that thermal decomposition of raw biomass feedstock occurred mainly in three sequential stages associated with: (i) biomass dewatering and (ii) devolatilization and volatile oxidation, followed by (iii) char oxidation. Note that the effect of biomass dewatering on thermal degradation of the sample was negligible, and it was therefore excluded from this study’s discussion.
Within the temperature range of 180–390 °C, chemical bonds in the biomass began to break, followed by releasing the lightest volatile compounds. Following the temperature regions of major structural compounds mentioned above, this stage was associated with the endothermic volatilization of hemicellulose and cellulose and partial lignin. The rate of mass loss in DTG profiles of RS, EB, and CR exhibited two peaks and corresponding two peak temperatures, related to the volatilization of hemicellulose and cellulose. However, only one peak (attributed to cellulose decomposition) with a small shoulder was observed in the DTG curve for TS, which can be explained by the limited hemicellulose content (1.8 wt.%) in this biomass. As shown in Fig. 2, the biomass degradation in this stage resulted in the sample's 60–70% weight loss.
At temperatures above 400 °C, a broad reduction of the sample mass was found in the DTG curves, mainly induced by the decomposition of the remaining lignin, accompanied by its conversion into fuel char, and afterward, char oxidation in the analyzer furnace. The remaining material of the fuel degradation was ash, represented by a constant line in the TG curve.
Figure 3 shows the TG/DTG profiles of the biomass pellets tested under similar operating conditions to those in Fig. 2. The thermal degradation behavior during the devolatilization of pellet samples was similar to that of their original biomasses, exhibiting the same number of peak(s) on the DTG profiles. However, the location of decomposition (peak) temperatures for the pelletized biomasses shifted to higher values compared to the locations for the corresponding raw feedstock, indicating the lower thermal reactivity of the biomass pellets. During the char combustion stage (at temperatures greater than 400 °C), a gradual decrease of the sample mass can be observed over a relatively broad temperature range of DTG profiles with no apparent peaks (except for EBP), pointing to a lower oxidation/combustion rate of biomass pellets in this region. These findings can be explained by the effects of pelletization causing a denser morphology and lowered pore volume, as well as by the pore size of the pellets compared to the as-received materials (Mian et al. 2020; Niu et al. 2017), consequently leading to limited mass and heat diffusion during the devolatilization and char combustion stages of pellet combustion in the TGA system. It can be generally concluded that biomass pellets' thermal and combustion reactivity was lower than that of the raw biomasses.
3.2 Combustion characteristics of raw and pelletized biomasses
Table 3 summarizes the combustion characteristics (Tp, Tig, Tb, and Si) of the raw and pelletized biomasses obtained from the combined analysis of the TG/DTG curves in Figs. 1 and 2, as well as from Eq. (1).
The values of Tp1, Tp2, Tp3, Tig, and Tb of the raw biomass samples were 240–246 °C, 268–290 °C, 409–454 °C, 244–258 °C, and 550–593 °C, respectively. These characteristic temperatures of combustion were in agreement with the biomass chemical structure presented in Table 2 and similar to palm oil residues (Idris et al., 2012) and some shell-type biomasses (Kuprianov and Arromdee 2013). From Table 3, the values of Tp and Tig for RS were the lowest, while the value of combustion characteristic Si was the highest among the other samples, pointing to the highest thermal and combustion reactivity of this biomass fuel.
As seen in Table 3, the values of Tig, Tp, and Tb for all pelletized biomasses were higher than those for their corresponding raw biomasses, consistent with the TG/DTG profiles in Figs. 2 and 3. This result implies a lowered reactivity of the biomass pellets. The Tp1 and Tp2 for the pellets were 257–332 °C and 290–355 °C, respectively. In the case of EBP, the third peak temperature (Tp3) was found at 800 °C, which was likely to be associated with the thermal instability of lignin and the decomposition of the mineral carbonates contained in EBP at relatively high temperatures (Idris et al. 2010).
The Tig of the pellets in Table 3 ranged from 259 to 304 °C, 11 to 58 °C higher than the raw biomasses. This result was attributed to a limitation of heat transfer to the dense structure of the pellet samples during the devolatilization stage, leading to delayed ignition of the sample in the analyzer furnace. Note that Tig values for the biomass pellets found in this study were quite close to those of the shell-type biomass with hard structure reported in other studies (Kuprianov and Arromdee 2013; Ninduangdee et al. 2015). The burnout temperatures for the pellet samples showed a significant increase compared to those for the original biomass, mainly due to the decreased rate of char oxidation, as observed in TG/DTG curves in Fig. 3. Due to the delayed ignition and burnout, the Si index values for all biomass pellets in Table 3 were about 2–3 times lower compared to those for the original biomasses. Therefore, the integral combustion characteristics of ignition and burnout for biomass pellets were lower than that of raw biomasses. These findings agreed well with those reported in the literature (Wang et al. 2012; Niu et al. 2017).
Based on the TGA, the selected raw biomasses with a high level of desirable combustion characteristics (taking into account Tig, Tp, Tb, and Si) can be used as a fuel in (co-)combustion systems with expected high combustion efficiency. The pellet fuels with a dense structure and high density, consequently, heavy particles, are recommended for use in FBC, ensuring a high residence time and excellent mixing in the reactor (Kaewklum and Kuprianov 2010). Thus, the proposed biomass pellets fired in a fluidized bed at a typical operating temperature (800–900 °C) are expected to have more residence time and good mixing with bed particles in the reactor bottom, leading to a higher burnout rate and high combustion efficiency.
3.3 Kinetic parameters
Table 4 summarizes the combustion kinetic parameters (Ea, A, and n) of the raw and pelletized biomasses. For accurate fitting of the experimental data, the kinetic parameters of all biomasses were determined for two main stages: (i) devolatilization and volatile oxidation, and (ii) char oxidation, according to the distinct temperature regions of biomass degradation, shown in Figs. 2 and 3.
Activation energy is related to the material’s structure and reactivity. The material molecules can only complete the reaction once they have reached the top of the energy barrier corresponding to the Ea value. This parameter can be used to measure the reactivity of the fuel; lower Ea means higher reactivity (Edreis et al. 2014). High Ea reactions require a high temperature or a longer reaction time (Gil et al. 2010). Besides, this parameter is used for reactor design, simulation, optimization, and control of thermochemical processes (Cai et al. 2014). Data in Table 4 shows that the Ea values for as-received biomasses during the first (i.e., volatiles combustion) and second stages (i.e., char combustion) of combustion were in the range of 119.1–130.8 kJ/mol and 29.2–34.8 kJ/mol, respectively. The values of Ea found in this work were in the same ranges as those reported by other researchers. As reported by Vamvuka et al. (2003), the value of Ea was 90–125 kJ/mol for hemicellulose, 145–285 kJ/mol for cellulose, and 30–39 kJ/mol for lignin. Idris et al. (2012) found that Ea values for combustion of palm shell, palm fiber, and empty fruit bunch were 139 ± 49, 118 ± 49, and 105 ± 56 kJ/mol, respectively. Kastanaki and Vamvuka (2006) studied combustion kinetics of forest residue, cotton residue, and wood char and calculated Ea values to be 130, 150, and 145 kJ/mol, respectively.
The Ea value of RS was the lowest among the other raw biomasses, indicating that this biomass had the highest reactivity, consistent with the abovementioned combustion characteristics in Table 3. As noted in Table 4, the values of Ea for the volatiles combustion stage were significantly higher than those for the char combustion stage, which was in consistent with other researchers (Wang et al. 2012; Guo and Zhong 2017), who found that the value of Ea for the release and combustion of volatile compounds stage was higher than that of the char combustion stage. The result indicates that the combustion reaction was controlled by the combustion of volatiles rather than that of char.
From Table 4, the Ea values of the biomass pellets were greater than those of the as-received biomasses. Mian et al. (2020) observed that the fuel analyses and chemical compositions are the main factors that affect biomass combustion. However, the proximate and ultimate analyses and chemical composition and structure of the as-received biomasses and corresponding pellets were similar as seen in Table 2. The compact structure of the biomass pellet caused by pelletization suppressed the mass and heat diffusion and thus resulted in an elevated value of Ea for the combustion of pellets. The pellets had Ea values ranging from 159.3 to 167.8 kJ/mol for devolatilization and volatiles combustion stage, and 47.3 to 67.2 kJ/mol for char combustion stage. The increased Ea values indicated that the biomass pellets required higher energy and temperature, and a longer time for complete combustion. Therefore, biomass pellets’ combustion was expected to be more complicated than raw biomasses. Note that the value of r in Table 4 was relatively high (r > 0.91), showing the high correlation of the calculation.
3.4 Temperature and O 2 concentration in the twin-cyclone FBC
Figure 4 compares the radial profiles of temperature and O2 in the twin-cyclone FBC at five different levels (Z) above the air distributor when firing raw and pelletized biomasses at EA about 50%.
The results in Fig. 4a show important effects of fuel type on the behavior of temperature inside the combustor. The radial temperature for tests with both raw and pelletized biomasses in the lower combustion chamber of the reactor were rather uniform, indicating highly intensive heat-and-mass transfer in the gas–solid flow along the radial direction. The temperature profiles in the upper combustion chamber exhibited a slightly negative gradient along a radial direction, resulting in a noticeably lower temperature in the peripheral zone of the combustor compared to that at the combustor’s center (i.e., at r/R = 0). This phenomenon was attributed to heat loss across the combustor walls.
When firing as-received biomasses, the maximum temperature was observed in the vicinity of fuel injection (at Z = 0.95 m). Based on the TGA discussed above, substantial gaseous volatiles were generated during raw biomass combustion at temperatures of 180–390 °C. However, the temperatures inside the combustor during operation were 800–900 °C, substantially higher than the devolatilization temperature of biomass particles. Thus, the endothermic drying and devolatilization of biomass occurred rapidly once the as-received biomass was introduced into the combustor by the fuel feeder. Meanwhile, fine and light char particles were generated in the combustor during the combustion of raw biomasses, especially when firing dust-like biomasses (i.e., RS and TS). Significant portions of these fine char particles were carried out from the conical section and burned in the cylindrical section of the lower combustion chamber. It can be observed in Fig. 4a that temperatures inside the combustor decreased with combustor height because of the effect of heat loss across the combustor wall. At Z > 0.95 m, temperatures at all points inside the combustor burning raw biomasses were relatively higher than those for burning biomass pellets. This result can be explained by the carry-over of relatively light biomass/char from the lower combustion chamber into the upper chamber, contributing to a greater heat release in the higher combustor levels.
Despite the lower thermal and combustion reactivity of all biomass pellets compared to that of the original biomasses, as revealed by the TGA study, the bed temperatures (at Z = 0.45 m) when firing all pelletized biomasses were higher than burning the as-received feedstock at the same EA. At the fuel feeding point, the pellet particles released volatiles rapidly, however, at a lower rate than the as-received biomasses according to the thermal decomposition found in the TGA. Due to the dense structure of the pellets, larger and heavier char particles were formed in the combustor that fell and penetrated the fluidized bed. These char particles had a longer residence time in the combustor bottom, leading to greater combustion intensity in this dense bed zone. A large amount of combustion heat was released in the bed zone, resulting in the maximum temperature in the conical section of the lower combustion chamber (at Z = 0.45 m). Similar behaviors have been reported in the pioneering study (Ninduangdee and Kuprianov 2018).
As shown in Fig. 4a, the temperature at the combustor bottom when firing RSP was the highest compared to the other pellet fuels. This fact can be explained by this fuel's highest thermal and combustion reactivity. On the other hand, CRP, with the lowest thermal and combustion reactivity, had the lowest bed temperature among the selected fuels.
As shown in Fig. 4b, the radial O2 concentration profiles were relatively uniform. It can be explained by the intensive gas–solid and gas–gas mixing in the radial direction and by the uniform distribution of airflow across the bed, the latter ensured by the unique design of the twin-cyclone FBC providing a swirling gas–solid bed.
The concentrations of O2 in the combustor at Z = 0.45 m for the test runs with biomass pellets were substantially lower than those for burning raw biomasses. This result indicated that a more significant proportion of O2 was consumed for the oxidization of CO, CxHy, char-C, and other combustible species released from the pellets in the fluidized bed (i.e., in a conical section of the lower combustion chamber), resulting in a greater fuel burnout in this region. Meanwhile, O2 consumption was significant in the cylindrical part of the lower combustion chamber (at Z = 0.95 m) and the connecting pipe (at Z = 1.30 m) when firing raw biomasses. This phenomenon can be explained by the oxidation of fine char particles carried over from the dense bed zone (i.e., conical section). In the upper combustion chamber (at Z > 1.30 m), the rate of O2 consumption (and, accordingly, biomass oxidation) was substantially lower than in the lower chamber. At the reactor top, O2 correlated with the specified percentage of EA (i.e., at about 50%).
3.5 Emissions of CO , C x H y , and NO
Figure 5 depicts the CO, CxHy, and NO emissions from the twin-cyclone FBC (all on a dry gas basis and at 6% O2) tested with raw and pelletized biomasses at an identical heat input to the combustor for varying values of EA from about 40 to 60%. As shown in Fig. 5, the major gaseous pollutants were significantly influenced by the fuel properties and the amount of EA.
For most fuel options, except RS and TS, the CO and CxHy emissions can be mitigated by increasing EA, which was attributed mainly to the increased oxidation rates of CO and CxHy to CO2 by excessive O2. In the comparisons shown in Fig. 5a and b with similar amounts of EA, the CO and CxHy emissions for testing biomass pellets were noticeably lower than for raw biomass, despite the lower thermal and combustion reactivity of these fuels. During combustion of biomass pellets, oxidation of the volatile CO and CxHy, as well as of fuel chars, occurred substantially in the fluidized bed, as indicated by the temperature profile in Fig. 4. The oxidation of CO in chemical reactions involved O2 and OH, whereas the CxHy oxidation occurred via two groups of secondary reactions: (i) breakdown of CxHy to CO, and afterward (ii) oxidation of CO to CO2 (Turns 2006). The above-mentioned higher residence time of the fuel in the fluidized bed and the higher bed temperature enhanced the reactions for CO and CxHy oxidations. As a result, higher burnout (i.e., lower CO and CxHy emissions) can be achieved when burning biomass pellets. It can be further noticed from Fig. 5b that burning more highly reactive pellet fuels resulted in diminished CO and CxHy emissions from the combustor, which can be explained by the higher burnout rate of the fuel inside the reactor particularly in the bed region.
When firing RS and TS, the EA exhibited reverse effects on the CO and CxHy emissions. An increased EA led to an increase in these emissions, with this effect more significant when increasing amounts of EA to approximately 50–60%. This behavior can be explained by the lower burn rate of these fuels in the reactor, particularly in the lower combustion chamber, due to the carry-over mentioned above of fuel/char and lower residence time. The CO and CxHy emissions from testing with EB and CR exhibited similar effects of EA as for the tests with biomass pellets, however, to different extents.
Note that the CO emission levels from all the tests with biomass pellets were lower than the emissions limit in Thailand (740 ppm, at 6% O2 on a dry gas basis (PCD 2013)), whereas the emission levels were unacceptable when firing dust-like biomasses (i.e., RS and TS) at EA above 50%. Therefore, the combustor fired with RS and TS should be operated with EA lower than 50% to control CO emission within the above-mentioned emission standard.
Unlike CO and CxHy emissions, the NO emission from the twin-cyclone FBC can be reduced by decreasing EA, except when firing RS and TS showing the opposite effects of EA on this type of emission. During biomass combustion, NO emission can be reduced via heterogeneous reduction of NO by CO and light hydrocarbons on the surface of various catalysts (char, ash, and bed material particles), as well as (ii) homogeneous reactions of NO with CxHy (Winter et al. 1999). Due to the high CO and CxHy when testing at low EA, the contribution of CO, CxHy, and char particles to NO emission reduction was significant. NO was reduced by the above-mentioned catalytic reaction with CO and some hydrocarbons on the char surface and homogeneous with CxHy. Because of the high combustion intensity of the twin-cyclone FBC, the reactions for NO reduction can be enhanced in the fluidized bed region of the lower combustion chamber, particularly when burning pellet fuels. As shown in Fig. 5, burning CR and CRP with elevated fuel N content generated the highest NO emission, following the fuel-NO formation mechanism (Winter et al. 1999). When burning RS and TS with higher EA, the NO emission diminished mainly due to higher levels of CO and CxHy concentration in the combustor, attributed to the above-mentioned catalytic reduction of NO to N2. Note that the NO emission from all the tests was relatively low, showing values below Thailand’s emissions standards (214 ppm, at 6% O2 on a dry gas basis (PCD 2013)).
3.6 Combustion efficiency
The combustion-related heat losses (due to unburned carbon and incomplete combustion) and the combustion efficiency of the twin-cyclone FBC when firing raw and pelletized biomasses at variable EA are depicted in Fig. 6.
The fuel type and EA substantially affected the heat losses and combustion efficiency of the combustor. Despite the higher thermal and combustion reactivity of RS and TS, the combustion efficiency of the combustor when firing these fuels was lower than other fuel options, strengthened by the amount of EA. Both types of heat loss increased substantially with an increased amount of EA, following the previously discussed carry-over of fuel particles and the behavior of CO and CxHy emissions.
The combustion efficiency of the combustor tested with the other two raw biomasses and pellet fuels exhibited an improvement when operated at a higher value of EA. Both heat losses decreased significantly with an increasing amount of EA within the specified range. From Fig. 6a and b, the heat loss owing to incomplete combustion in all tests with pellet fuels was lower than those for raw biomasses, following the characteristics of CO and CxHy emissions, as depicted in Fig. 5. This result indicated a higher burnout and more complete combustion of the pelletized fuels, owing to fuel char particles' more excellent residence time in a fluidized bed and a higher bed temperature. For the range of EA, the combustor operated at high combustion efficiency: 97.4–99.5% for firing as-received biomasses and 99.0–99.8% for firing biomass pellets.
Based on the combustion and emission characteristics discussed above, the amount of EA should be optimized to achieve the highest performance of the combustor. Compared to CO and CxHy, NO is a more harmful gas that adversely impacts the environment and human health. Therefore, it is suggested to operate the combustor at a minimum possible EA to minimize NO emission and heat loss with flue gas, while ensuring CO emission below the emission standard mentioned above. Based on such criteria, EA of about 40% can be regarded as the optimal option for firing all fuel options in the twin-cyclone FBC. Under the optimum EA, the twin-cyclone FBC can operate with more than 99% combustion efficiency and control the CO and NO emissions below the emission standard of Thailand. The CxHy emission is also maintained at low level.
Table 5 compares the CO, CxHy, and NO emissions and combustion efficiency of the twin-cyclone FBC fired with the proposed biomasses and other pilot-scale FBCs burning various biomasses for selected EA. Table 5 also includes important thermal characteristics of the combustor, including heat input and estimated combustion intensity in the main combustion zone. Based on the above-mentioned optimal EA for firing the selected fuel options, the cases have been selected for EA of about 40% for comparability of the results. As can be seen in Table 5, all FBCs operate at high combustion performance and efficiency (about 99%). The combustion efficiency seems more accurate when the effects of unburned carbon (in fly ash) and CO and CxHy in exhaust gas leaving the combustor are considered. Compared to other FBCs, the twin-cyclone FBC fired with the proposed biomasses emitted a relatively higher CO emission but lower NO emission. Table 5 shows that the NO emission from the optimally-operated twin-cyclone FBC firing pellet fuel was substantially lower than in other case studies. This fact can be resulted from high (975 kW/m3) combustion intensity in the lower combustor’s chamber compared to other FBCs. Considering the above characteristics of combustors shown in Table 5, the twin-cyclone FBC fired with biomass pellets has an advantage for high combustion efficiency and NO reduction.
Based on the findings from the combustion experiment in the twin-cyclone FBC, the combustion property of the as-received biomasses (particularly the dusk-like fuels) was enhanced by pelletization. Although this process led to diminishing thermal and combustion reactivity of the proposed fuel options, the physical combustion property of these biomasses significantly improved.
3.7 Practical applications and future work
The main significance of this study is information on the combustion of pelletized biomasses, including RSP, TSP, EBP, and CRP, that could be used as a fuel in heat and power generation. This information is essential for the large-scale combustion process. The characteristic combustion temperatures and activation energy found in this work can be used to analyze thermal behaviors, which are very helpful for efficient design, optimization, and control of combustion systems. Based on the combustion and emission characteristics of the pilot-scale twin-cyclone FBC, the proposed biomass pellets can be recommended for steam production of fluidized- bed boilers. The as-received biomass can also be co-fired with coal or other high-quality biomasses. In the future, further study on long-term combustion is essential to ensure a safe and reliable combustor operation. Although no evidence of bed agglomeration was observed during the combustion experiments, a detailed investigation of bed material characteristics is needed to assess this problem's tendency, especially when firing high-ash biomass (i.e., EBP and CRP). Furthermore, a co-combustion study of the proposed biomass pellets with some unconventional biomasses is needed to be conducted to extend the information on the use of biomass for energy production.
4 Conclusions
Combustion characteristics of raw and pelletized biomasses from rubberwood sawdust, teak sawdust, eucalyptus bark, and cassava rhizomes have been investigated in a thermogravimetric system and a pilot-scale twin-cyclone fluidized-bed combustor. Combustion of these biomasses can be divided into three stages: (i) biomass dewatering and (ii) devolatilization and volatile oxidation, followed by (iii) char oxidation. Pelletized biomasses have lower combustion reactivity that raw biomass, as higher ignition, peak, and burnout temperatures, and lower comprehensive performance index values. The activation energies of raw biomasses and pellets for devolatilization and volatiles combustion stage are 119.1–130.8 kJ/mol and 159.3–167.8 kJ/mol, whereas for char combustion are 29.2–34.8 kJ/mol and 47.3–67.2 kJ/mol, respectively. Fuel properties and excess air significantly impact the combustor’s combustion characteristics. The biomass pellets have higher residence time and excellent mixing with bed particles in a fluidized bed, leading to more significant combustion intensity in the bed region. This result contributes to higher fuel burnout, as lower CO and CxHy emissions. When raw biomasses are fired with high excess air, the carry-over of light char particles causes elevated CO and CxHy emissions and combustion heat losses, consequently lowering combustion efficiency. The combustor fired with pellet fuels emits relatively low NO emission due to the high combustion intensity in the fluidized bed. Excess air of about 40% is optimal for firing the selected biomass in this combustor, ensuring above 99% combustion efficiency and low emission characteristics. Therefore, the proposed biomasses in both forms have a high level of desirable combustion characteristics, which can be used as a fuel in practical (co-)combustion systems with expected high combustion efficiency and low emissions.
Abbreviations
- A:
-
Ash content in fuel (wt.%)
- A :
-
Pre-exponential factor
- C:
-
Carbon content in fuel (wt.%)
- Cfa :
-
Unburned carbon content in fly ash (wt.%)
- CO:
-
Carbon monoxide (ppm)
- CR:
-
Cassava rhizomes
- CRP:
-
Cassava rhizomes pellet
- CxHy :
-
Hydrocarbon (ppm)
- DTG:
-
Differential thermogravimetric
- DTGmax :
-
Maximum burning rate (%/min)
- DTGmean :
-
Average weight loss rate (%/min)
- E a :
-
Activation energy (kJ/mol)
- EA:
-
Excess air (%)
- EB:
-
Eucalyptus bark
- EBP:
-
Eucalyptus bark pellet
- FBC:
-
Fluidized-bed combustor
- FC:
-
Fixed carbon content in fuel (wt.%)
- H:
-
Hydrogen content in fuel (wt.%)
- LHV:
-
Lower heating value, kJ/kg
- m τ :
-
Actual mass (at time τ) of the sample (kg)
- m 0 :
-
Initial mass of the sample (kg)
- m f :
-
Final mass of the sample (kg)
- n :
-
Reaction order
- N:
-
Nitrogen content in fuel (wt.%)
- NO:
-
Nitric oxide, ppm
- O:
-
Oxygen content in fuel (wt.%)
- q ic :
-
Heat loss due to incomplete combustion (%)
- q uc :
-
Heat loss due to unburned carbon (%)
- r :
-
Correlation coefficient
- RS:
-
Rubberwood sawdust
- RSP:
-
Rubberwood sawdust pellet
- S:
-
Sulfur content in fuel (wt.%)
- S i :
-
Comprehensive performance index (%2/min2·°C3)
- T :
-
Absolute temperature (°C)
- T b :
-
Burnout temperature (°C)
- TG:
-
Thermogravimetric
- TGA:
-
Thermogravimetric analysis
- T ig :
-
Ignition temperature (°C)
- T p :
-
Peak temperature (°C)
- TS:
-
Teak sawdust
- TSP:
-
Teak sawdust pellet
- u :
-
Superficial air velocity at the air distributor exit (m/s)
- u mff :
-
Minimum velocity of full fluidization (m/s)
- V dg@6% O2 :
-
Volume of flue gas per 1 kg fuel on a dry basis and at 6% O2 (m3/kg)
- VM:
-
Volatile matter in fuel, wt.%, on as received basis
- W:
-
Moisture content in fuel, wt.%, on as received basis
- Z :
-
Level along the combustor height above the air distributor, m
- α :
-
Mass conversion ratio
- β :
-
Heating rate (ºC/min)
- η c :
-
Combustion efficiency (%)
References
Basu P, Kefa C, Jestin L (2000) Boilers and burners: design and theory. Springer, New York
Batcha MFM, Othaman MF, Sabudin S, Mohammed AN, Abdullah MK, Ali MFM (2020) Combustion and emission of pelletized empty fruit bunch and oil palm shell in a swirling fluidized bed combustor. Biomass Convers Biorefin 10(3):755–763. https://doi.org/10.1007/s13399-020-00903-x
Cai J, Wu W, Liu R (2014) An overview of distributed activation energy model and its application in the pyrolysis of lignocellulosic biomass. Renew Sust Energ Rev 36:236–246. https://doi.org/10.1016/j.rser.2014.04.052
Chirone R, Salatino P, Scala F, Solimene R, Urciuolo M (2008) Fluidized bed combustion of pelletized biomass and waste-derived fuels. Combust Flame 155:21–36. https://doi.org/10.1016/j.combustflame.2008.05.013
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201(4914):68–69. https://doi.org/10.1038/201068a0
DEDE (2020) Department of Alternative Energy Development and Efficiency, Thailand Alternative Energy Situation 2020. https://www.dede.go.th/download/stat63/Thailand%20Alternative%20Energy%20Situation%202020.pdf Accessed 10 Oct 2021
Duan F, Zhang JP, Chyang CS, Wang YJ, Tso J (2014) Combustion of crushed and pelletized peanut shells in a pilot-scale fluidized-bed combustor with flue gas recirculation. Fuel Process Technol 128:28–35. https://doi.org/10.1016/j.fuproc.2014.06.022
Edreis EMA, Luo G, Yao H (2014) Investigations of the structure and thermal kinetic analysis of sugarcane bagasse char during non-isothermal CO2 gasification. Anal Appl Pyrolysis 107:107–115. https://doi.org/10.1016/j.jaap.2014.02.010
Gil MV, Casal D, Pevida C, Pis JJ, Rubiera F (2010) Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Bioresour Technol 101(14):5601–5608. https://doi.org/10.1016/j.biortech.2010.02.008
Guo F, Zhong Z (2017) Experimental studies on combustion of composite biomass pellets in fluidized bed. Sci Total Environ 599–600:926–933. https://doi.org/10.1016/j.scitotenv.2017.05.026
Haykiri-Acma H, Yaman S, Kucukbayrak S (2010) Comparison of the thermal reactivities of isolated lignin and holocellulose during pyrolysis. Fuel Process Technol 91:759–764. https://doi.org/10.1016/j.fuproc.2010.02.009
Idris SS, Rahman NA, Ismail K, Alias AB, Rashid ZA, Aris MJ (2010) Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresour Technol 101(12):4584–4592. https://doi.org/10.1016/j.biortech.2010.01.059
Idris SS, Rahman NA, Ismail K (2012) Combustion characteristics of Malaysian oil palm biomass, sub-bituminous coal and their respective blends via thermogravimetric analysis (TGA). Bioresour Technol 123:581–591. https://doi.org/10.1016/j.biortech.2012.07.065
Jiang L, Yuan X, Li H, Xiao Z, Liang J, Wang H, Wu Z, Chen X, Zeng G (2015) Pyrolysis and combustion kinetics of sludge–camphor pellet thermal decomposition using thermogravimetric analysis. Energ Convers Manage 106:282–289. https://doi.org/10.1016/j.enconman.2015.09.046
Kaewklum R, Kuprianov VI (2010) Experimental studies on a novel swirling fluidized-bed combustor using an annular spiral air distributor. Fuel 89(1):43–52. https://doi.org/10.1016/j.fuel.2009.07.027
Kastanaki E, Vamvuka D (2006) A comparative reactivity and kinetic study on the combustion of coal–biomass char blends. Fuel 85(9):1186–1193. https://doi.org/10.1016/j.fuel.2005.11.004
Kongto P, Palamanit A, Ninduangdee P, Singh Y, Chanakaewsomboon I, Hayat A, Wae-hayee M (2022) Intensive exploration of the fuel characteristics of biomass and biochar from oil palm trunk and oil palm fronds for supporting increasing demand of solid biofuels in Thailand. Energy Rep 8:5640–5652. https://doi.org/10.1016/j.egyr.2022.04.033
Kuprianov VI, Arromdee P (2013) Combustion of peanut and tamarind shells in a conical fluidized-bed combustor: a comparative study. Bioresour Technol 140:199–210. https://doi.org/10.1016/j.biortech.2013.04.086
Madhiyanon T, Lapirattanakun A, Sathitruangsak P, Soponronnarit S (2006) A novel cyclonic fluidized-bed combustor (ψ-FBC): combustion and thermal efficiency, temperature distributions, combustion intensity, and emission of pollutants. Combust Flame 146(1):232–425. https://doi.org/10.1016/j.combustflame.2006.03.008
Magdziarz A, Wilk M (2013) Thermal characteristics of the combustion process of biomass and sewage sludge. J Therm Anal Calorim 114:519–529. https://doi.org/10.1007/s10973-012-2933-y
Malico I, Pereira RN, Gonçalves AC, Sousa AMO (2019) Current status and future perspectives for energy production from solid biomass in the European industry. Renew Sust Energ Rev 112:960–977. https://doi.org/10.1016/j.rser.2019.06.022
Martsri A, Yodpijit N, Jongprasithporn M, Junsupasen S (2021) Energy, economic and environmental (3E) analysis for sustainable development: a case study of a 9.9 MW biomass power plant in Thailand. Appl Sci Eng Prog 14(3):378–386. https://doi.org/10.14416/j.asep.2020.07.002
Mian I, Li X, Dacres OD, Wang J, Wei B, Jian Y, Zhong M, Liu J, Ma F, Rahman N (2020) Combustion kinetics and mechanism of biomass pellet. Energy 205:117909. https://doi.org/10.1016/j.energy.2020.117909
Ninduangdee P, Kuprianov VI (2014) Combustion of palm kernel shell in a fluidized bed: optimization of biomass particle size and operating conditions. Energ Convers Manage 85:800–808. https://doi.org/10.1016/j.enconman.2014.01.054
Ninduangdee P, Kuprianov VI (2018) Fluidized bed co-combustion of rice husk pellets and moisturized rice husk: the effects of co-combustion methods on gaseous emissions. Biomass Bioenergy 112:73–84. https://doi.org/10.1016/j.biombioe.2018.02.016
Ninduangdee P, Kuprianov VI, Cha EY, Kaewrath R, Youngyuen P, Atthawethworawuth W (2015) Thermogravimetric studies of oil palm empty fruit bunch and palm kernel shell: TG/DTG analysis and modeling. Energy Procedia 79:453–458. https://doi.org/10.1016/j.egypro.2015.11.518
Ninduangdee P, Arromdee P, Se C, Kuprianov VI (2020) Effects of (co-)combustion techniques and operating conditions on the performance and NO emission reduction in a biomass-fueled twin-cyclone fluidized-bed combustor. Waste Biomass Valori 11(10):5375–5391. https://doi.org/10.1007/s12649-020-01124-z
Niu S, Chen M, Li Y, Xue F (2016) Evaluation on the oxy-fuel combustion behavior of dried sewage sludge. Fuel 178:129–138. https://doi.org/10.1016/j.fuel.2016.03.053
Niu S, Chen M, Li Y, Lu T (2017) Combustion characteristics of municipal sewage sludge with different initial moisture contents. J Therm Anal Calorim 129(2):1189–1199. https://doi.org/10.1007/s10973-017-6262-z
PCD (2013) Pollution control department, ministry of natural resources and environment, Thailand, Air pollution standards for industrial sources. http://www.pcd.go.th/info_serv/reg_std_airsnd03.html. Accessed 26 Jan 2022
Permchart W, Kouprianov VI (2004) Emission performance and combustion efficiency of a conical fluidized-bed combustor firing various biomass fuels. Bioresour Technol 92:83–91. https://doi.org/10.1016/j.biortech.2003.07.005
Quan LM, Kamyab H, Yuzir A, Ashokkumar V, Hosseini SE, Balasubramanian B, Kirpichnikova I (2022) Review of the application of gasification and combustion technology and waste-to-energy technologies in sewage sludge treatment. Fuel 316:123199. https://doi.org/10.1016/j.fuel.2022.123199
Rokni E, Ren X, Panahi A, Levendis YA (2018) Emissions of SO2, NOx, CO2, and HCl from Co-firing of coals with raw and torrefied biomass fuels. Fuel 211:363–374. https://doi.org/10.1016/j.fuel.2017.09.049
Saikia M, Ali AA, Borah RC, Bezbarua MS, Saikia BK, Saikia N (2018) Effects of biomass types on the co-pyrolysis behaviour of a sub-bituminous high-sulphur coal. Energ Ecol Environ 3(5):251–265. https://doi.org/10.1007/s40974-018-0097-8
Shimizu T, Han J, Choi S, Kim L, Kim H (2006) Fluidized-bed combustion characteristics of cedar pellets by using an alternative bed material. Energy Fuels 20:2737–2742. https://doi.org/10.1021/ef0601723
Sirisomboon K, Charernporn P (2017) Effects of air staging on emission characteristics in a conical fluidized-bed combustor firing with sunflower shells. J Energy Inst 90(2):316–323. https://doi.org/10.1016/j.joei.2015.12.001
Sun Z, Jin B, Zhang M, Liu R, Zhang Y (2008) Experimental studies on cotton stalk combustion in a fluidized bed. Energy 33:1224–1232. https://doi.org/10.1016/j.energy.2008.04.002
Tumuluru JS (2018) Effect of pellet die diameter on density and durability of pellets made from high moisture woody and herbaceous biomass. Carbon Resour Convers 1(1):44–54. https://doi.org/10.1016/j.crcon.2018.06.002
Turns S (2006) An introduction to combustion. McGraw-Hill, Boston
Vamvuka D, Kakaras E, Kastanaki E, Grammelis P (2003) Pyrolysis characteristics and kinetics of biomass residuals mixtures with lignite. Fuel 82(15):1949–1960. https://doi.org/10.1016/S0016-2361(03)00153-4
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74(10):3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Wang X, Si J, Tan H, Niu Y, Xu C, Xu T (2012) Kinetics investigation on the combustion of waste capsicum stalks in Western China using thermogravimetric analysis. J Therm Anal Calorim 109(1):403–412. https://doi.org/10.1007/s10973-011-1556-z
Werther J, Saenger M, Hartge EU, Ogada T, Otara S (2000) Combustion of agricultural residues. Prog Energy Combust Sci 26:1–27. https://doi.org/10.1016/S0360-1285(99)00005-2
Winter F, Wartha C, Hofbauer H (1999) NO and N2O formation during the combustion of wood, straw, malt waste and peat. Bioresour Technol 70(1):39–49. https://doi.org/10.1016/S0960-8524(99)00019-x
Wongsapai W, Achawangkul Y, Thepsaskul W, Daroon S, Fongsamootr T (2020) Biomass supply chain for power generation in southern part of Thailand. Energy Rep 6:221–227. https://doi.org/10.1016/j.egyr.2019.11.066
Acknowledgements
The authors would like to sincerely acknowledge the financial support received from Thailand Science Research and Innovation (TSRI), Phetchaburi Rajabhat University, and the Department of Mechanical Engineering, Faculty of Engineering and Industrial Technology, Silpakorn University.
Author information
Authors and Affiliations
Contributions
PA: Designed and performed experiments, and undertook systematic review, statistical analysis, original drafting, and revision writing. PN: Undertook conceptualization, performed experiments, undertook data curation interpretation, writing, reviewing, revision writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A: Models for determining combustion-related heat losses and combustion efficiency
Appendix A: Models for determining combustion-related heat losses and combustion efficiency
The heat-loss method (Basu et al., 2000) was used to quantify combustion efficiency of the twin-cyclone FBC when firing the proposed biomass.
The heat loss owing to unburned carbon, quc (%LHV) was predicted by using the measured fraction of unburned carbon in fly ash carried over from the combustor (Cfa, wt%) determined using a "Perkin Elmer PE2400 Series II" CHNS/O elemental analyzer, the fuel-ash content (A, wt%, on as-received basis), and the fuel lower heating value (LHV, kJ/kg, on an as-received basis) as:
The heat loss owing to incomplete combustion, qic (%LHV), was quantified using the above-calculated quc and CO and CxHy (as CH4) emissions (in ppm, on a dry gas basis and at 6% O2) as:
where Vdg@6% O2 is calculated according to Kuprianov and Arromdee (2013) using the fuel composition on “as-received” basis. The combustion efficiency of the fluidized-bed combustors, \(\eta_{{\text{c}}}\) (%LHV), was then determined as:
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arromdee, P., Ninduangdee, P. Combustion characteristics of pelletized-biomass fuels: a thermogravimetric analysis and combustion study in a fluidized-bed combustor. Energ. Ecol. Environ. 8, 69–88 (2023). https://doi.org/10.1007/s40974-022-00263-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40974-022-00263-4