Abstract
Nowadays, Ni-Resist austenitic cast irons are widely used in industry. Because of the high amount of nickel (about 30 wt%), the production cost of these cast irons is high. This study aims to reduce the amount of nickel in these cast irons while increasing the amount of manganese. Manganese is well known for its ability to stabilize the austenite phase like nickel; it is also less expensive than nickel. After preparing as-cast iron samples, scanning electron microscopy was utilized to evaluate the microstructural characteristics of the samples. Microstructural observations showed increasing manganese and decreasing nickel caused the formation of LTF regions. Also, the mechanical behavior of specimens revealed that adding equal amounts of nickel and manganese simultaneously improves the strength and flexibility; however, adding 15 wt% manganese, causes the formation of Mn7C3. As for the final stage, electrochemical results obtained by potentiodynamic polarization analysis showed substituting nickel with manganese did not worsen corrosion resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gray cast irons are superior to steels in casting due to their low melting point and high flowability. Austenitic cast irons have been widely used in both low and high temperatures. Adding nickel stabilizes austenite even at low temperatures in as-cast conditions, but the main issue is that nickel is six times more expensive than its alternative elements. The alternative elements must be able to stabilize austenite, maintain corrosion resistance, and not jeopardize the mechanical properties of cast iron. Manganese, among these elements, is a cost-effective element and a reliable austenite stabilizer.1,2 Furthermore, it has been reported that manganese is capable of improving mechanical properties like wear resistance and hardness by inducing the formation of specific carbides.3,4
Rashidi and Ahmad reported that adding 10% nickel and manganese resulted in forming an as-cast austenite matrix. When the manganese content exceeded the nickel content, carbide manganese formed; as a result, these carbides reduced mechanical properties, particularly strength, and elongation, while increasing hardness.5,6,7,8,9
It has been suggested that copper, like manganese, can promote as-cast austenite by increasing the nickel equivalent, which is a necessary term in forming austenite. The wear rate of the austenite phase could be similar to that of the martensite phase due to the presence of manganese, which increases the hardness value by itself. Adding less than 1% Cr, Mo, and Al did not change the stability of austenite, but the hardness and corrosion resistance increase, when the concentration of these elements goes up.10,11,12,13,14,15
Li et al. reported that manufacturing austenite cast irons via powder metallurgy not only does not improve the hardness and corrosion resistance value compared to casting but also exacerbates these two items. The more manganese content, the more the corrosion resistance is increased because the electrical potential difference between matrix and carbide is lower than between matrix and graphite.16
As stated by Roger, increasing stress and temperature put austenite cast iron at risk of stress corrosion cracking (SCC) in seawater. Initiation of SCC could occur at the matrix/carbide interface due to casting imperfections. He claimed that stress-relieving castings do not suppress SCC but can postpone the SCC. SCC failure may take years or months. It is related to the rate of crack propagation. SCC even can happen at low concentrations of chloride and low temperatures.17,18
Rickard and his colleagues investigated the application of Ni-Resist cast Irons in low temperatures. They showed that the addition of alloying elements delays the martensite start temperature (Ms), but none of them is as effective as Mn, such that adding 0.1% Mn lowers the Ms by 3.3 °C, while Cr, Ni, Si, and Mo decrease the Ms by 2.2, 1.7, 1.1 and 1.1 °C, respectively.19
Dawson et al. showed that the limited number of metal carbides changes their properties drastically. Meanwhile, metal carbides of V and Ti are hard and used as refractory, but other metal carbides such as copper, nickel, and zinc are extremely unstable and have weak properties. Manganese carbides are between these two types of carbides but have different properties. They are hard but fragile. Their thermal stability is in the range of 1000–1100 °C. Only three types of manganese carbides (Mn23C6, Mn5C2, and Mn7C3) are stable at room temperature, and among them, Mn23C6 has high thermal stability. These carbides are ferromagnetic. In addition to the low thermal and chemical stability compared to other metal carbides, these types of carbides are not pure; that is, they tend to dissolve a large amount of oxygen and nitrogen in themselves, so they are hydrated in an aqueous environment or moisture conditions, which causes alterations in their properties.20,21,22
As stated by Hatano, gray Ni-Resist cast irons could have good mechanical properties and high damping capacity by adding a moderate amount of nickel (15%) and other elements.23
Rickard stated that austenitic high-alloy cast irons have 12 to 20 times better growth resistance compared to non-alloy cast irons and 5 to 20 times enhanced oxidation resistance than non-alloy cast irons. The adhesion of the oxide layer in these alloys is very high, so the risks of hot gases penetrating the cast irons are minimized. Also, due to the increased adhesion of the oxide layer, these types of cast irons have high wear resistance at high temperatures of 815 °C; even though this wear resistance is higher than that of steels, the reason for this is the presence of graphite in cast irons.24
According to ASTM A436, there are 8 types of austenitic gray cast irons. Four of which are designed to be used in elevated-temperature applications and four in applications requiring corrosion resistance. This study looks at how different amounts of manganese affect the microstructure, mechanical properties, and corrosion resistance of Ni-Resist gray cast irons.
The microstructure of the samples was examined by an optical and a Scanning Electron Microscope (SEM) equipped with EDS correspondingly. Hardness and tensile tests were performed to determine the value of mechanical properties, and the XRD test was used to analyze phases. Also, to investigate the corrosion behavior, a potentiodynamic polarization analysis was conducted on samples.
Experimental Procedure
Material Preparation
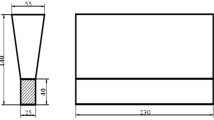
An Induction furnace with a capacity of 1 kg was used in this study. In addition, due to the low capacity of the furnace, the standard Y-Block mold was replaced with a 20 mm-thickness rectangular plate mold (shown in Figure 1). The samples were molded using green sand, sodium silicate, and carbon dioxide gas. The combination of this gas and sodium silicate hardens the sand, increasing its strength. Table 1 lists the chemical composition of small gray cast iron ingots used with nickel pellets and low-carbon Ferro-manganese. Because nickel has a high melting point, nickel pellets were first added to the crucible, followed by cast iron ingots, and finally Ferro-manganese. The melting time was about 20 minutes, and the pouring the temperature was approximately 1430 °C, and also post inoculation has not been performed.
Identifying Chemical Composition and Microstructural Analysis
After casting, the spectrometer test was performed with a GNR machine at 22 °C and 40% humidity to identify the concentration of each element in as-cast samples. That is, small size of samples from test casting were remelted and chill-cast according to ASTM E1010-16 practices. The results are shown in Table 2.
The microstructure of as-cast samples is evaluated using an Olympus SZX9 optical microscope. A grinding machine was used to cut these samples into 22 cm squares, which were then molded in circular molds with a diameter of 5 cm using a cold mounting method. After polishing and grinding to 2500 grit abrasive paper, the samples were etched for 30 seconds with glyceregia solution (3 ml hydrochloric acid + 2 ml glycerin + 1 ml nitric acid).
the X-ray diffraction test was carried out to investigate the phases in various samples. XRD was used in the Brucker-Axs Advance 8D model. A 1.54 Å wavelength Cu-K ray lamp was used. The step size was 0.05 degrees, and the exposure time between steps was one second. The range of diffraction angles was set between 20 and 100 degrees. The XRD patterns obtained for each sample were then analyzed using the X’pert HighScore and Maud software. Finally, the SEM equipped with EDS was utilized to determine the percentage of elements at any point in the samples and observation of the type of segregation, a CAM SCAN MV2300 scanning electron microscope with a secondary electron detector at a voltage of 20 kV was used.
Specimens and Mechanical Properties Tests
The tensile test was performed at room temperature by an AI-7000-L5 GOTECH Taiwan universal testing machine 50kN under the ASTM: E-8M standard. Proof strength, tensile strength, and elongation are determined using tensile samples. To determine proof stress, the 0.2% offset method is usually stated in terms of strain. In this method a line is drawn parallel to the linear part of a stress-strain curve, but displaced to the right by 0.2% strain. The stress where this line intersects the stress-strain curve is then deemed to be the proof stress. Tensile plain samples were prepared on a lathe, and gauge diameter, gauge length, and total length were 6 mm, 13 mm, and 46 mm respectively, according to the BS EN 1561 standard (Figure 2). Furthermore, the strain rate was \(3\times {10}^{-4}\) s−1 until fracture. Hardness tests were conducted under two conditions. The first condition was tested at the macro level, while the second was tested at the micro level. The samples were divided into 2 cm2. Vickers hardness with 30 Kg load and 15 s time duration was used to perform macro hardness with DHT HBRVS-187.5 High Accuracy Brinell Rockwell Vickers Universal Hardness Tester. The DVRB.M model hardness tester with a 300-gram load was used to conduct the microhardness test. Five hardness measurements were taken from different regions at a distance of 10 mm from another region using the Vickers method. The ROELL AMNLER GERMANY model machine was used to perform the Charpy impact test to determine toughness in ambient conditions. The impact sample was prepared using a wire-cut machine in accordance with the ASTM-E23 standard (Figure 3).
Electrochemical Measurements
The samples were electrochemically tested in a 3.5 wt% NaCl solution using a potentiostat corrosion test system model ZIVE SP2. A standard three-electrode cell was used for this experiment. The reference electrode was Ag/AgCl (3M) KCI, and the counter electrode was platinum. Working electrodes were cut from as-cast samples with an exposure area of 1 cm2. Samples were mounted and ground on 3000 grit abrasive paper before being degreased in alcohol and washed with distilled water. The test was carried out at room temperature, and the potentiodynamic polarization curves were obtained at a scan rate of 1 mV S−1.
Results and Discussions
Carbon equivalents was calculated using equations 1. Table 2 shows that sulfur has decreased from alloy 1 to 4, and even in samples 3 and 4, the amount of sulfur is lower than the standard (the average amount is 0.05–0.1). Because manganese reacts quickly with sulfur to form manganese sulfide (MnS), it increases the chill depth.25,26,27,28 Besides, the content of Ceq in Table 2, illustrates that all the alloys, except alloy 4, are substantially hypereutectic, which is significantly higher than C of ASTM A436 alloys. (3% C max).
Figure 4 depicts the microstructure of as-cast AGI. In Figure 4a and b, the graphite patterns are less dense and far apart due to lower manganese, whereas in Figure 4c and d, the graphite is more compact and closer to each other due to the formation of manganese carbide (Mn7C3) around graphite. To elaborate, during the solidification of iron, manganese moves away from the solid phase (graphite) which is first nucleate and solidifies. Manganese is the last element that solidifies after the nucleation of graphite. It has been grouped and segregated away from the graphite. So, carbides suppress the existence of free graphite. An X-ray analysis pattern and EDS have been used to investigate the possibility of Mn carbide formation.26,27,28,29
Figure 5 shows the microstructure of the 17% Ni–15% Mn sample with white spots caused by the addition of manganese. These areas are known as LTF (Last To Freeze), meaning they were solidified later than other areas. Its chemical composition and mechanical properties will be discussed and investigated further to identify this segregation. According to Figure 5, the microstructure of the 17% Ni–15% Mn sample has the most LTF regions because manganese is more abundant in this sample than in other samples, so adding manganese increases the solidification range and the slope of heat transfer, resulting LTF regions.7,8,30,31
Figure 6 indicates XRD patterns of samples. As can be seen in Figure 6, the samples 30% Ni and 25% Ni–5% Mn contain more graphite than the others because the first peak in the above samples is stronger than in % 25 Ni–%10 Mn and %17 Ni–%15 Mn. In the last graphite-related samples, only minor peaks emerged, additionally, according to Figure 6, the first peak of austenite related to the %17 Ni–%15 Mn sample has a lower intensity when compared to the rest of the samples. On the other hand, the intensity of the second peak of its austenite is higher than in the other samples because the %17 Ni–%15 Mn sample has more Manganese and less nickel than the others. Peak intensity changes can indicate changes in solidification, grain orientation, and the presence of more LTF regions in the %17 Ni–%15 Mn sample compared to the other samples.6,7,8
According to Figure 7, which is the result of EDS in the LTF regions (blue line). a slight negative segregation was occurred, the amount of manganese shown in Figure 7 is nearly 17 wt%, which is higher than the average amount of 15 wt%. The amount of nickel is meager and is about 12 wt%, which is much less than the average amount of 17 wt%, and also, EDS analysis was carried out to ensure that there were carbides in the 17% Ni–15% Mn sample. According to Figure 7, the carbon content in the LTF region in the 17% Ni–15% Mn sample is greater than 7 wt%, while manganese content is roughly 17 wt%, which was led to formation of Mn7C3.6,7,8
In Figure 8, the EDS analysis was performed to indicate that compacted graphite is pure and was made up of graphite. This selected analysis is 10 elemental analyses from the above sample, and the amount of carbon in all 10 compressed graphite analyses is greater than 99 wt%, indicating that manganese carbide prevented the formation of free graphite.6,7,8
Many mechanical properties can be extracted from a stress-strain curve (Figure 9), including tensile strength, proof stress, and elongation. Figures 10, 11 and 12 show that from alloy 1 to 4 tensile strength, proof stress, and elongation have all improved due to the two main reasons; (i) Size, thickness, and space between graphite in alloy 1 and alloy 2 are less than in alloy 3 and alloy 4. Meaning that, graphite may play a vital role in determining strength and ductility of austenite gray cast irons. (ii) This may the solid solution mechanism’s strengthening. The amounts of nickel and manganese are approaching each other from alloy 1 to alloy 4. These two elements could dissolve easily in the austenite phase; combining them simultaneously enhances strength and elongation.32,33,34
Among the other samples, the 25% Ni–5% Mn sample had the lowest strength and elongation. With a decrease in nickel and an increase in manganese, austenite could not be stable compared to the 30% Ni because there was insufficient manganese in the austenite matrix to combine with nickel. In other words, solid solution strengthening could not occur in a sample containing 25% Ni–5% Mn. In terms of the 30% Ni sample, Ni only helps austenite to be chemically stable at ambient temperature; In comparison, it performs better than the 25% Ni–5% Mn sample in terms of mechanical properties; it is inferior to the other two samples. The 17% Ni–15% Mn and 25% Ni–10% Mn samples have higher strength and elongation than the others, and the values are close.
The 30% Ni sample has the lowest macro hardness due to a lack of manganese, as shown in Figure 13. The addition of manganese led to the formation of carbides around graphite, in other words, the more manganese content a sample has, the highest value of hardness it has. Due to the high amount of carbide.6,7,8
The microhardness test determines the hardness of the LTF regions. Due to the higher amount of manganese and the more significant number of LTF regions, this test was only performed on samples containing 25% Ni–10% Mn and 17% Ni–15 Mn (Figure 14). According to the microhardness result, the average numerical value of this microhardness in the 25% Ni–10% Mn sample is 205 Vickers, which is higher than the sample’s macro hardness. Because of the high manganese content, the value of this hardness in percent of the 17% Ni–%15 Mn sample is roughly twice (400Vickers) that of the previous sample.6,7,8
The impact energy of the samples is close to each other due to the presence of austenite in all four samples, as shown in Figure 15; the matrix is the most important determining factor in the impact energy, followed by the alloy elements. In general, nickel increases the impact of energy. Nickel is more abundant in the 30% Ni sample than in the other samples, but the impact energy is higher in the 25% Ni–5% 5 Mn and 25% Ni–10% Mn samples. The reason for this is the high amount of austenite in these samples compared to the 30% Ni sample, as demonstrated by the x-diffraction pattern test analysis.32,35,36
According to Table 3 and Figure 16, thermodynamically, the sample of %30 Ni has a lower tendency to be corroded than the other samples because its potential is higher. The potential decreased in the remaining samples as nickel was reduced and manganese was increased, from 30% Ni to 17% Ni–15% Mn, respectively. The amount of nickel in the sample % 25 Ni–% 10 Mn is the same as the amount of nickel in the sample % 25 Ni–% 5 Mn, but the amount of manganese is different. Because these changes in Mn did not affect the potential, nickel plays a more critical role in determining corrosion behavior than Mn. Because of the lower amount of nickel in the % 17 Ni–% 15 Mn sample, the potential of this sample is lower than the others.
Since the corrosion behavior of all four samples is close to each other and has slight differences, the corrosion tendency of all four samples is kinetically similar. Because of their lower corrosion current density (icorr), the samples of %25 Ni–%10 Mn and % 25 Ni–% 5 Mn have the highest corrosion resistance compared to the other samples. The %17 Ni–%15 Mn sample has the lowest corrosion resistance.37,38,39,40
Conclusions
-
Increasing manganese causes the graphite to grow compactly and close to each other due to the formation of manganese carbide.
-
According to the microscopic observation, increasing manganese and decreasing nickel cause the formation of LTF regions.
-
The increase of manganese for the 17% Ni–15% Mn sample compared to the sample of 30% Ni has caused an increase in hardness.
-
The elemental analysis of late to freeze areas (White areas in Figure 7) shows that the amount of manganese is more than nickel, so the microhardness of these points is twice the amount of macro hardness and the reason for the high hardness in the sample of 17% Ni–15% Mn.
-
By decreasing the content of Ni and increasing of content of Mn, Ecorr decreased; however, the corrosion rates of all samples are approximately identical in a 3.5 wt% NaCl solution.
References
C. Changhong, S. Renbo, L. Shuai, F. Yifan, P. Zhongzheng, Wear behavior and subsurface layer work hardening mechanism of Fe-24.1 Mn-1.21 C-0.48 Si steel, International Conference on the Technology of Plasticity, Cambridge, United Kingdom, (2017). https://doi.org/10.1016/j.proeng.2017.10.990
F. Shi, P. Tian, N. Jia, Z. Ye, Y. Qi, C. Liu, X. Li, Improving intergranular corrosion resistance in a nickel-free and manganese-bearing high-nitrogen austenitic stainless steel through grain boundary character distribution optimization. Corros. Sci. 107, 49–59 (2016). https://doi.org/10.1016/j.corsci.2016.02.019
A. Janus, Forming of castings structure of austenitic cast iron Ni-Mn-Cu (Editorial Office of Wroclaw University of Technology, Wroclaw, Poland, 2013)
A. Roula, G. Kosnikov, Manganese distribution and effect on graphite shape in advanced cast irons. Mater. Lett. 62(23), 3796–3799 (2008). https://doi.org/10.1016/j.matlet.2008.04.056
A. Muzafar, M. Rashidi, I. Mahadzir, Z. Shayfull, Effect on Mechanical Properties of Heat Treated High Manganese Austenitic Cast Iron, MATEC Web of Conferences, EDP Sciences, (2016), p. 01081. https://doi.org/10.1051/matecconf/20167801081
M.M. Rashidi, M.H. Idris, The effects of solidification on the microstructure and mechanical properties of modified ductile Ni-resist iron with a high manganese content. Mater. Sci. Eng., A 597, 395–407 (2014). https://doi.org/10.1016/j.msea.2013.12.070
M.M. Rashidi, M.H. Idris, Microstructure and mechanical properties of modified ductile Ni-resist with higher manganese content. Mater. Sci. Eng. A 574, 226–234 (2013). https://doi.org/10.1016/j.msea.2013.02.038
M.M. Rashidi, M.H. Idris, Effect of inoculation on microstructure, mechanical and corrosion properties of high manganese ductile Ni-resist alloy. Mater. Des. 51, 861–869 (2013). https://doi.org/10.1016/j.matdes.2013.04.064
K. Ahmad, M. Maarof, M. Ishak, M. Huzairi, Microstructure and mechanical properties of austenitic compacted cast iron with additive manganese, MATEC Web of Conferences, EDP Sciences, 2016, p. 00009. https://doi.org/10.1051/matecconf/20167400009
D. Medyński, A. Janus, Effect of nickel equivalent on structure and corrosion resistance of nodular cast iron Ni-Mn-Cu. Arch. Foundry Eng. 15(1 spec.), 69–74 (2015)
D. Medyński, A. Janus, Effect of austenite transformation on abrasive wear and corrosion resistance of spheroidal Ni-Mn-Cu cast iron. Arch. Foundry Eng. 16(3), 63–66 (2016). https://doi.org/10.1515/2Fafe-2016-0051
D. Medyński, A. Janus, Effect of chemical composition on structure and corrosion resistance of Ni-Mn-Cu cast iron. Arch. of Foundry Eng. 16(3), 59–62 (2016). https://doi.org/10.1515/2Fafe-2016-0050
D. Medyński, A. Janus, Effect of Cr, Mo and Al on structure and selected mechanical properties of austenitic cast iron. Arch. Foundry Eng. (2019). https://doi.org/10.24425/afe.2019.129627
D. Medyński, A. Janus, B. Samociuk, J. Chęcmanowski, Effect of microstructures on working properties of nickel-manganese-copper cast iron. Metals 8(5), 341 (2018). https://doi.org/10.3390/met8050341
D. Medyński, B. Samociuk, A. Janus, J. Chęcmanowski, Effect of Cr, Mo and Al on microstructure, abrasive wear and corrosion resistance of Ni-Mn-Cu cast iron. Materials 12(21), 3500 (2019). https://doi.org/10.3390/ma12213500
X. Li, J. Liu, J. Xiong, L. Yang, Q. Gou, X. Song, Z. Guo, T. Hua, M. Liang, Wear and corrosion resistant Mn-doped austenitic cast iron prepared by powder metallurgy method. J. Market. Res. 9(3), 6376–6385 (2020). https://doi.org/10.1016/j.jmrt.2020.03.099
R. Francis, G. Byrne, Stress corrosion cracking of austenitic cast iron in chloride-containing waters. Corrosion 78(6), 563–569 (2022). https://doi.org/10.5006/4097
M. Daud, G. Venkateswaran, K. Venkateswarlu, Electrochemical corrosion characteristics of Ni-Resist type 2 in sea water containing ferrous sulphate. Br. Corros. J. 25(4), 303–308 (1990). https://doi.org/10.1179/000705990799156418
A. Rickard, Development of a special ‘Ni-Resist’cast iron for low temperature use. Cryogenics 3(2), 86–95 (1963)
X. Chong, Y. Jiang, R. Zhou, J. Feng, First principles study the stability, mechanical and electronic properties of manganese carbides. Comput. Mater. Sci. 87, 19–25 (2014). https://doi.org/10.1016/j.commatsci.2014.01.054
W. Dawson, F. Sale, Enthalpies of formation of chromium carbides. Metall. Trans. A 8(1), 15–18 (1977). https://doi.org/10.1007/BF02677258
W. Dawson, F. Sale, Enthalpy of formation of manganese carbide, Mn23C6. Metall. Trans. A 11(11), 1849–1852 (1980). https://doi.org/10.1007/BF02655100
S.-I. Hatano, T. Matsuda, T. Okamoto, K.I. Matsushita, High-damping-capacity grey cast iron with a tensile strength of 200 MPa. Cast Metals 3(1), 49–53 (1990). https://doi.org/10.1080/09534962.1990.11819019
R.J. Greene, F.G. Sefing, Cast irons in high temperature service. Corrosion 11(7), 43–49 (1955). https://doi.org/10.5006/0010-9312-11.7.43
R.B. Ross, Metallic materials specification handbook, (Springer Science & Business Media 2013), p. 197
S. Dawson, I. Hollinger, M. Robbins, J. Daeth, U. Reuter, H. Schulz, The effect of metallurgical variables on the machinability of compacted graphite iron. SAE Trans. (2001). https://doi.org/10.4271/2001-01-0409
R. Gundlach, M. Meyer, L. Winardi, Influence of Mn and S on the properties of cast iron part III—testing and analysis. Int. J. Metalcast. 9(2), 69–82 (2015). https://doi.org/10.1007/BF03355617
H.M. Muhmond, H. Fredriksson, Relationship between inoculants and the morphologies of MnS and graphite in gray cast iron. Metall. Mater. Trans. B. 44(2), 283–298 (2013). https://doi.org/10.1007/s11663-012-9768-6
D. Yousefpuor, M. Abbasi, M. Javahery, Effect of manganese content on the as cast microstructure of nodular cast iron. Found. Res. J. 4(4), 203–213 (2021). https://doi.org/10.22034/FRJ.2020.205051.1102
C. Selig, A. Lacaze, Study of microsegregation buildup during solidification of spheroidal graphite cast iron. Metall. Mater. Trans. B. 31(4), 827–836 (2000). https://doi.org/10.1007/s11663-000-0119-7
Y. Shen, S. Yang, J. Liu, H. Liu, R. Zhang, H. Xu, Y. He, Study on micro segregation of high alloy Fe–Mn–C–Al steel. Steel Res. Int. 90(5), 1800546 (2019). https://doi.org/10.1002/srin.201800546
H. Bayati, R. Elliott, Relationship between structure and mechanical properties in high manganese alloyed ductile iron. Mater. Sci. Technol. 11(3), 284–293 (1995). https://doi.org/10.1179/mst.1995.11.3.284
R.K. Dasgupta, D.K. Mondal, A.K. Chakrabarti, A.C. Ganguli, Microstructure and mechanical properties of as-cast ductile irons alloyed with manganese and copper. J. Mater. Eng. Perform. 21(8), 1728–1736 (2012). https://doi.org/10.1007/s11665-011-0058-2
R. Srivastava, B. Singh, K.K. Saxena, Influence of S and Mn on mechanical properties and microstructure of grey cast iron: an overview. Mater. Today: Proc. 26, 2770–2775 (2020). https://doi.org/10.1016/j.matpr.2020.02.577
M.N. Ahmadabadi, T. Ohide, E. Niyama, Effects of successive-stage austempering on the structure and impact strength of high-Mn ductile iron. Cast Metals 5(2), 62–72 (1992). https://doi.org/10.1080/09534962.1992.11819094
H. Chen, D. Zhao, Q. Wang, Y. Qiang, J. Qi, Effects of impact energy on the wear resistance and work hardening mechanism of medium manganese austenitic steel. Friction 5(4), 447–454 (2017). https://doi.org/10.1007/s40544-017-0158-6
F. Gall, The corrosion of centrifugal pumps in aqueous environments. In Pumps Compressors Conference, (2013), pp. 1-29.
C.-H. Hsu, M.-L. Chen, Corrosion behavior of nickel alloyed and austempered ductile irons in 3.5% sodium chloride. Corrosion Sci. 52(9), 2945–2949 (2010). https://doi.org/10.1016/j.corsci.2010.05.006
S. Kumar, R.B. Singh, Corrosion behavior of newly developed heat treated 10Mn-2Ni-3.5 Cr-1.5 Cu alloy. Mater. Res. Express 6(11), 1165c4 (2019). https://doi.org/10.1088/2053-1591/ab4ec8
A. Malik, S. Basu, I. Andijani, N. Siddiqi, S. Ahmad, Corrosion of Ni-Resist cast irons in sea water. Br. Corros. J. 28(3), 209–216 (1993). https://doi.org/10.1179/000705993798318614
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azimijam, A., Khalil-Allafi, J., Mehrvarz, A. et al. Effect of Manganese on Microstructural, Mechanical, and Electrochemical Properties of Ni-Resist Gray Cast Irons. Inter Metalcast 18, 1486–1495 (2024). https://doi.org/10.1007/s40962-023-01128-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-023-01128-0