Abstract
The present investigation focuses on improvement in mechanical properties of AlSi5Cu3 aluminum alloy by in situ synthesis of TiB2 reinforcement particles. Stochiometrically calculated amount of potassium tetrafluoro borate and potassium hexafluoro titanate were used for the development of 3 and 6 wt% particles of TiB2 in the liquid metal. The melt having TiB2 particles was allowed to solidify naturally in the sand mold. X-ray diffraction (XRD) and scanning electron microscopy (SEM) revealed the formation of hexagonal TiB2 particles within the matrix. Microstructural studies concluded the formation of micron size TiB2 particles and reduction in grain size. Ultimate tensile strength increased from 21 to 64% and hardness increased from 30 to 50% compared to AlSi5Cu3 alloy due to the formation of 3% and 6% TiB2 particles, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum alloys are the most commonly used materials in automobile components like engine blocks, transmission cases, pistons, inlet manifolds, heads, closures, wheels etc., on account of their high strength to weight ratio. In 2012, European cars had around 350 pounds of Al castings and this is expected to increase by 100% in the year 2025.1
AlSi5Cu3 is the most commonly used in automobile components and other general purpose engineering applications like crankcase, gearbox, tool handles, etc. due to good mechanical properties, machinability and corrosion resistance.2 Sokolowski et al.3 investigated the effect of the single stage and the two-stage solution treatment (< 12 h) on mechanical properties of AlSi5Cu3 alloy. They observed that tensile strength increases from 190 to 215 MPa and hardness increases from 85 to 87 Hv after the single stage and the two-step solution treatments, respectively. Mahmudi et al.4 investigated the effect of Zr addition on wear behavior and tensile properties of AlSi5Cu3 alloy. They observed that with 0.15 wt% Zr addition, tensile strength and hardness are increased by 2% and 7%, respectively, compared to AlSi5Cu3 alloy. Poria et al. 5 investigated the effect of micron size TiB2 particles addition in AlSi5Cu3 alloy and they observed that as reinforcement percentage increases, friction and wear rate decreases. Poria et al.6 studied the effect of TiB2 and nano-graphene particles addition on wear and friction behavior of AlSi5Cu3 alloy, concluding that the combined effect of TiB2 and nano-graphene increases hardness to 82 Hv compared to 63 Hv of AlSi5Cu3 alloy. They also observed that wear resistance improves as reinforcement percentage increases. Pazhouhanfar et al.7 developed Al/TiB2 composites by adding various wt% of TiB2 particles in the aluminum alloy AlMg1SiCu. They have introduced K2TiF6 flux in the melt to increase the wettability of the TiB2 particles and also varied various process parameters like particle preheating temperature, stirring speed and stirring duration to achieve the homogeneous distribution of particles in the melt. Results show that tensile strength of developed composites increases with increment in the percentage of TiB2 particles.
In the previous works to improve mechanical and tribological properties of AlSi5Cu3 alloy, researchers have used either secondary heat treatment processes or externally addition of elements/compounds (ex situ composites). In the ex situ method, major concerns are agglomeration of particles, homogeneous distribution of particles, thermal instability of particles and weak interface between particles and matrix due to poor wettability.8 Researchers have explored various mixing processes to overcome these problems, but consistency in the quality of composite slurries is difficult to achieve.9,10,11,12,13,–14
In the last three decades, researchers have come up with the idea to overcome these problems by developing the in situ composites,17,18,19,20,21,22,–23 where reinforcement particles like TiB2, TiC and ZrB2 are synthesized during the process. Moreover, TiB2 has been used as the reinforcement in aluminum matrix composites because of unique combination of properties like low density, high Young’s modulus, high hardness and high wear resistance (Kennedy et al.15 and Akbari et al.16). Samuel et al.17 investigated the influence of Al–B and Al–Ti as the grain refiner in the presence of strontium (Sr)-based modifier. Ultimate tensile strengths of the alloy modified with Sr increases from 180.99 to 186.65 Mpa, after the addition of Al–B and Al–Ti-based refiners, respectively. Yang et al.18 synthesized TiB2 particles by addition of titanium and boron powders in aluminum. They observed the formation of reactive compound when Ti:B ratio was 1:2 in mole percentage. Complete formation of TiB2 was observed without any reaction compound when Ti:B ratio was 1:4, leading to improvement in mechanical properties. Emamy et al.19 synthesized TiB2 particles in Al matrix using Al–4B and Al–8Ti master alloy. Problem associated with the master alloy method is the formation of TiAl3 which is not stable as TiB2, leading to unfavorable reactions.20 The formation of TiAl3 can be eliminated via salt route method. Han et al.21 fabricated Al/TiB2 composite by the salt reaction method and observed that the distribution of TiB2 particles was homogeneous and reported that in situ fabricated TiB2 particles can stay in suspended form for the longer time, minimizing the settlement of TiB2 particles in crucible due to the gravity effect. Liu et al.22 developed Al–4.5Cu/TiB2 composites via addition of halide salts. Ultrasound-assisted re-melting and diluting approach was used to further enhance the mechanical properties. Due to the presence of TiB2 particles and the effect of high-intensity ultrasonic treatment, large sized dendrites (325.4 µm) were transformed to small spherical (37 µm) shapes. After ultrasonic treatment, ultimate tensile strength (UTS) and yield strength of Al–4.5Cu/5 wt% TiB2 were increased by 39.9% and 39.7% compared to pure Al–4.5Cu, respectively. Changizi et al.23 synthesized TiB2 by in situ reactions between H3BO3, TiO2 and Na3AlF6. They observed that TiB2 particles were spherical in shape having the average diameter of 1 micron.
AlSi5Cu3 alloy is one of the most commonly used metal in the automobile industries on account of good mechanical properties and favorable casting characteristics. Researchers have explored external addition of elements/compounds and secondary heat treatment processes in AlSi5Cu3 alloy to enhance mechanical properties. These approaches have inherent limitations. In the present paper, research work on AlSi5Cu3 alloy is further extended to enhance the mechanical properties by in situ synthesis of TiB2 particles using the salt route method. Among all methods explored by researchers, the salt route method offers better control over formation of unwanted TiAl3 and other intermetallic phases. Similar effort has not been reported in the literature for AlSi5Cu3 alloy.
Materials and Methods
Materials
In this study, the matrix material used was aluminum alloy AlSi5Cu3. Table 1 shows the chemical composition derived using spectroscopic analysis. Two types of halide salts K2TiF6 and KBF4 were used for the synthesis of TiB2 particles.
Methodology
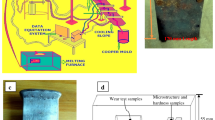
Aluminum alloy AlSi5Cu3 was melted at 800 °C in the specially designed modular set up as shown in Figure 1. Modular system comprises three units: (1) melting unit for melting of metal, (2) pouring unit with the provision to place mold and the direct pouring of metal into mold cavity via bottom pouring arrangement, and (3) stirring unit for the alloying of metals and the development of metal matrix composites. Resistance furnace having cylindrical muffle is designed and developed for melting and superheating of metal up to 1200 °C. Specially designed graphite crucible with stopper rod type of arrangement is used for the bottom pouring of metal. Figure 2 shows crucible with stirrer and stopper rod arrangement. Electrical actuator is attached at stopper rod to control the flow rate of metal. Stoichiometrically calculated amounts of K2TiF6 and KBF4 were preheated at 300 °C in resistance heater for 40 min, and then these halide salts were added into molten metal at 800 °C. 150 (300) gm of K2TiF6 and 189 (378) gm of KBF4 were added in the liquid metal to form 3 (6) wt% of TiB2. Melt temperature was measured and controlled using flexible K-type thermocouple which was placed inside the stopper rod. Manual stirring has been carried out for 60 min with zirconia-coated SS rod at 10-min interval (3 min stir then 10 min rest, and repeat) to facilitate occurrence of exothermic reactions. Several reactions take place between the halide salts and aluminum in the following manner.24
Subsequently, melt was degassed using hexa-chloro-ethane tablet and after removal of dross pouring has been carried out in the sand mold (Figure 3a) having cavity size 125 × 110 × 13 mm (casted specimen shown in Figure 3b). A flowchart of the process followed is shown in Figure 4. Castings with 3% and 6% weight fractions of TiB2 particles were developed.
Extraction of TiB2 Particles
In order to identify the size and presence of TiB2, extraction of particles was carried out. Small segment (20 mm × 20 mm × 10 mm) was cut from the fabricated AlSi5Cu3/TiB2 composites. After cleaning with sand paper, segment was added into beaker containing 20 wt% sodium hydroxide solution (in water) for dissolving aluminum matrix. After complete dissolution, suspension was packed in centrifuge tube and centrifuged in microprocessor-controlled centrifuge (REMI R-24) at 6500 rpm for 15 min. After centrifugation, TiB2 particles were collected from the bottom of the tube and washed with deionized water. Finally, TiB2 particles were dried in the oven at 80 °C for 60 min.
Results and Discussion
Characterization of Composites
X-ray diffraction (XRD) analysis was carried out using D2 PHASER (BRUKER) on polished specimens. Figure 5 shows the XRD result of the fabricated composites, confirming the formation of TiB2 particles in AlSi5Cu3 matrix. K2TiF6 and KBF4 react with liquid aluminum and form TiAl3 and AlB2, respectively. Subsequently, reaction happens between the atoms of B and Ti on the TiAl3 surface, forming TiB2 particles. TiAl3 compounds were completely diluted due to fragmentation and natural cracking behavior and only TiB2 particles remain present on completion of reaction.20 Researchers showed that TiB2 is thermodynamically stable and the presence of reactive compound like TiAl3 reduces the mechanical properties of composites due to its lower stability compared to TiB2.25,26 Davidson et al.27 and Natarajan et al.28 also reported that thermodynamically stable reinforcements restrict the growth of reactive compound like TiAl3 at the interface between matrix and particle. Figure 6 shows the XRD spectra of extracted TiB2 particles that confirm the formation of TiB2 without any other reactive compound. During the addition of salts to liquid metal, an exothermic reaction occurs which increased the temperature of the metal from 800 to 910–930 °C. At this temperature, boron atoms encounter the loss, impacting the formation of AlB2 particles. Thus, for the completion of reactions and to avoid the presence of unreacted TiAl3 particles, 20% more KBF4 by molar mass was added.
Optical Microscopy
Figure 7 shows the optical micrographs of pure AlSi5Cu3 and their composites with TiB2 captured at 100X magnification using Carl Zeiss (Axio Vert. A1MAT) inverted metallurgical microscope. Metallographic samples were prepared according to ASTM E3. The microstructures of in situ composite reveal the homogeneous distribution of TiB2 particles in matrix material. TiB2 particles act as nucleating sites and refine the grain structure of AlSi5Cu3 due to heterogeneous nucleation, leading to the reduction in average grain size.
Figure 8 shows the reports of grain size analysis performed using BIOVIS Material plus software. Grain size number (G) for pure AlSi5Cu3 is 9 and for AlSi5Cu3/TiB2 composite is 9.5, indicating that the number of grains/unit area is increased from 256 to 362.04 grains/in2 in the developed composite compared to pure AlSi5Cu3 according to ASTM E 112 standard. The average size of TiB2 particles in composite is 42 microns. The micrographs as shown in Figure 7b and c reveals that the dendrite region of matrix material is concentrated with TiB2 particles and particles are at grain boundaries. During solidification, development of α Al phase is hindered by the presence of TiB2 particles and as the percentage of TiB2 increases, number of nucleation sites increases, offering the resistance to the grain growth and resulting in grain refinement.29 The higher freezing range (100 °C) of AlSi5Cu3 alloy facilitates aluminum grains to solidify over TiB2, and low-density difference (2 g/cm3) between TiB2 and aluminum allows the reinforcement particles to stay in suspended form for longer time, leading to the homogeneous distribution of particles.21
SEM and EDAX Analysis
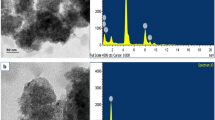
SEM micrographs (Figure 9) show the minimal agglomeration of particles and distinct bonding between TiB2 particles and aluminum alloy AlSi5Cu3. SEM micrographs reveal the formation of polygonal-shaped TiB2 particles (having three to six sides) and most of the particles are hexagonal in shape. Researches have reported that the in situ formed TiB2 particles have various shapes such as spherical, cubical, and hexagonal.29,30,–31 SEM micrographs shown in Figure 9b and d also reveals clear and reaction-free interface between the matrix and particles, indicating improvement in the mechanical properties of composites. Philips make (SEM, EDAX XL-30) scanning electron microscope equipped with ultra-thin window was used to carry out EDAX spectra analysis and to confirm the presence of light element like boron. Figure 10 shows the EDAX spectra of pure AlSi5Cu3 and composites, confirming the presence of titanium and boron in the developed composites. In AlSi5Cu3/3%TiB2 (Figure 10b), wt% of Ti and B are 1.41 and 2.81, respectively, and in AlSi5Cu3/6%TiB2 (Figure 10c), wt% of Ti and B are 2.31 and 4.64, respectively. Small amount of oxygen is also observed in the developed composites due to reaction of aluminum with environmental oxygen.
Tensile Behavior of AlSi5Cu3/TiB2 Composites
The tensile specimens were prepared according to ASTM E8 standard having 25 mm gauge length, 6 mm thickness and 6 mm gauge width as shown in Figure 11a. Three samples have been prepared for each composition to get an average value and tests have been carried out on the universal testing machine (TINIUS OLSEN/L-series H50KL) as shown in Figure 11b. Significant improvement in the ultimate tensile strength (UTS) of in situ composites as compared to pure AlSi5Cu3 is observed as shown in Figure 12. The UTS of in situ composites increased by 21% and 64% due to the formation of 3% and 6% TiB2 particles, respectively. The most of TiB2 particles are present at grain boundaries (refer Figure 7b and c), leading to reduction in the mobility of dislocation and ultimately restrict the crack propagation. In addition, the grain refinement due to TiB2 particles provides more grain boundary area resisting the dislocation, resulting in the improvement in strength.30
Hardness Analysis of AlSi5Cu3/TiB2 Composites
Hardness analysis has been carried out on the samples according to ASTM E18. Hardness was measured on Rockwell hardness tester at 100 kgf load, applied for 10 s using 1/16″ (1.588 mm) tungsten carbide ball. The hardness of in situ composites increased considerably due to the formation of TiB2 particles. Homogeneously distributed TiB2 particles lead to the Orowan strengthening of grains, leading to increase in the load-bearing capacity of composite.32 Clear and reaction-free interface resists the detachment of TiB2 particles from matrix material, resulting in increased hardness (Rajan et al.29). The average Rockwell hardness (HRB) of in situ composites increased by 30% and 50% due to the formation of 3% and 6% TiB2 particles, respectively, as shown in Figure 13.
Conclusions
In present investigation, specially designed modular setup consisting of melting unit, pouring unit and stirring unit is developed and in situ AlSi5Cu3/TiB2 composites were prepared by the exothermic reactions of melt with salts. Major conclusions derived from the work are:
EDAX analysis confirms the presence of titanium and boron phase in the material and XRD analysis confirms the formation of TiB2 particles in liquid melt.
Microstructures of in situ composite reveal the homogeneous distribution of TiB2 particles in matrix material. Polygonal-shaped TiB2 particles were observed, having the sides from three to six.
Number of grains/unit area is increased from 256 grains/in2 to 362.04 grains/in2 in developed composites.
SEM micrographs show clear and reaction-free interface between the matrix and particles, leading to the improvement in mechanical properties.
Ultimate tensile strength (UTS) of in situ composites having 3% and 6 % TiB2 particles has been increased by 21% and 64% compared to pure AlSi5Cu3.
Hardness of in situ composites increased by 30% and 50% due to the formation of 3% and 6% TiB2 particles, respectively.
References
North American Light Vehicle Aluminum Content Study. (Ducker Worldwide Study, 2016), http://www.ducker.com/news-insights/ducker-worldwide-studyaluminum-content-cars-public-summary. Accessed 31 Aug 2017
A.S.M. Handbook, Properties and selection: nonferrous alloys and special-purpose materials. ASM Int. 2, 597–599 (1990)
J.H. Sokolowski, M.B. Djurdjevic, C.A. Kierkus et al., J. Mater. Process. Technol. 109, 174 (2001). https://doi.org/10.1016/S0924-0136(00)00793-7
R. Mahmudi, P. Sepehrband, H.M. Ghasemi, Mater. Lett. 60, 2606 (2006). https://doi.org/10.1016/j.matlet.2006.01.046
S. Poria, P. Sahoo, G. Sutradhar, Silicon 8, 591 (2016). https://doi.org/10.1007/s12633-016-9437-5
S. Poria, G. Sutradhar, P. Sahoo, Mater. Res. Express 5, 056509 (2018). https://doi.org/10.1088/2053-1591/aac0df
Y. Pazhouhanfar, B. Eghbali, Mater. Sci. Eng. A 172, 180 (2018). https://doi.org/10.1016/j.msea.2017.10.087
S.C. Tjong, Z.Y. Ma, Mater. Sci. Eng. R 29, 49 (2000). https://doi.org/10.1016/S0927-796X(00)00024-3
S.M.Y. Kaku, A.K. Khanra, M.J. Davidson, J Alloys Compd 666, 675 (2018). https://doi.org/10.1016/j.jallcom.2018.03.088
R. Gecu, A. Karaaslan, Inter. Metalcast. 1, 9 (2018). https://doi.org/10.1007/s40962-018-0253-0
S. Soltani, R.A. Khosroshahi, R.T. Mousavian et al., Rare Met. 36, 581 (2017). https://doi.org/10.1007/s12598-015-0565-7
S. Agrawal, A.K. Ghose, I. Chakrabarty, Mater. Des. 113, 195 (2017)
J.M. Mistry, P.P. Gohil, Compos Part B Eng 190, 204 (2019). https://doi.org/10.1016/j.compositesb.2018.10.074
R. Mohammadi Badizi, M. Askari-Paykani, A. Parizad et al., Inter. Metalcast. 12, 565 (2018)
A.R. Kennedy, A.E. Karantzalis, S.M. Wyatt, J. Mater. Sci. 34, 933 (1999). https://doi.org/10.1023/A:1004519306186
M.K. Akbari, H.R. Baharvandi, K. Shirvanimoghaddam, Mater. Des. 66, 150 (2015). https://doi.org/10.1016/j.matdes.2014.10.048
A.M. Samuel, H.W. Doty, S. Valtierra et al., Inter. Metalcast. 11, 305 (2017). https://doi.org/10.1007/s40962-016-0075-x
B. Yang, Y.Q. Wang, B.L. Zhou, Metall. Mater. Trans. B 29, 635 (1998). https://doi.org/10.1007/s11663-998-0098-7
M. Emamy, M. Mahta, J. Rasizadeh, Compos. Sci. Technol. 66, 1063 (2006). https://doi.org/10.1016/j.compscitech.2005.04.016
B.S. Murty, S.A. Kori, M. Chakraborty, Int. Mater. Rev. 47, 3 (2002). https://doi.org/10.1179/095066001225001049
Y. Han, X. Liu, X. Bian, Compos. A 33, 439 (2002). https://doi.org/10.1016/S1359-835X(01)00124-5
J. Liu, Z. Liu, Z. Dong et al., J. Alloys Compd. 1008, 1017 (2018). https://doi.org/10.1016/j.jallcom.2018.06.303
A. Changizi, A. Kalkanli, N. Sevinc, J. Alloys Compd. 509, 237 (2011). https://doi.org/10.1016/j.jallcom.2010.08.089
P. Davies, J.L.F. Kellie, D.P. Patron, J.V. Wood, Metal Matrix Alloys. U.S. Patent 6,228,185, 2001
S. Kumar, V.S. Sarma, B.S. Murty, Mater. Sci. Eng. A 476, 333 (2008). https://doi.org/10.1016/j.msea.2007.04.113
L. Lü, M.O. Lai, Y. Su et al., Scripta Mater. 45, 1017 (2001). https://doi.org/10.1016/S1359-6462(01)01128-9
A.M. Davidson, D. Regener, Compos. Sci. Technol. 60, 865 (2000). https://doi.org/10.1016/S0266-3538(99)00151-7
S. Natarajan, R. Narayanasamy, S.K. Babu et al., Mater. Des. 30, 2521 (2009). https://doi.org/10.1016/j.matdes.2008.09.037
H.M. Rajan, S. Ramabalan, I. Dinaharan et al., Arch. Civil Mech. Eng. 14, 72 (2014). https://doi.org/10.1016/j.acme.2013.05.005
S. Kumar, M. Chakraborty, V.S. Sarma et al., Wear 265, 134 (2008). https://doi.org/10.1016/j.wear.2007.09.007
C.S. Ramesh, S. Pramod, R. Keshavamurthy, Mater. Sci. Eng. A 528, 4125 (2011). https://doi.org/10.1016/j.msea.2011.02.024
Z. Zhang, D.L. Chen, Mater. Sci. Eng. A 483, 148 (2008). https://doi.org/10.1016/j.msea.2006.10.184
Acknowledgement
The authors gratefully acknowledge the support from Department of Science and Technology (DST), New Delhi, sponsored SMART Foundry Project (DST/TSG/AMT/2015/332 dated 17/08/2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ayar, V.S., Sutaria, M.P. Development and Characterization of In Situ AlSi5Cu3/TiB2 Composites. Inter Metalcast 14, 59–68 (2020). https://doi.org/10.1007/s40962-019-00328-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-019-00328-x