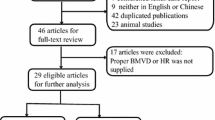
Abstract
Introduction
Gynecological malignancies are one of the leading causes of early mortality in women of all ages, whether they originate from any part of the female genital tract. Coming to demography, gynaecological malignancies account for around half of all cancers in women (45.2%). Cancer cervix accounts for 33.3% of all cancers, whereas fallopian tube cancer (0.15% of all gynaecological cancers) is the rarest of all gynaecological cancers. Gynaecological malignancies are more prevalent in rural India (76.5%). Since various tumors have variable amounts of angiogenic activity, corresponding with the development of gynaecological carcinomas, many specialists are intrigued by the possibility of blocking angiogenesis as a cancer therapy approach. Vascular endothelial growth factor (VEGF), also known as vascular permeability factor, stimulates the development of capillary channels around the tumor and works as a highly selective mitogen on endothelial cells. Intra-tumoural angiogenesis indicators include VEGF, basic fibroblast growth factor (bFGF), and microvessel density (MVD). MVD evaluation on excised tumor sections using particular antibodies that stain endothelial cells can be used to study angiogenesis.
Method
Our aim was to identify and quantify angiogenesis in paraffin-embedded histopathological sections of gynaecological malignancies by using an immune histochemical marker (CD105:BIOGENEX QD 400-60KE) to predict association of MVD with premalignant and malignant lesions of cervical, ovarian, and endometrial origin. The study design was observational descriptive over a sample size of 65.
Result
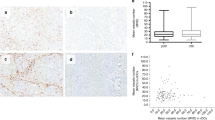
The mean of MVD origin in cancer cervix, ovary, and endometrium was 66.54 ± 28.76, 55.64 ± 39.76, and 54.3 ± 20.68, respectively. The mean difference was statistically insignificant (p = 0.3550). The inter-observer variability by pathologist-1 and pathologist-2 was excellent with reliable agreement (0.8717). Poorly differentiated carcinoma had a higher MVD than those more well differentiated. Consequently, angiogenesis may have a negative impact on the prognosis and survival of gynaecological cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gynecological malignancies are one of the leading causes of early mortality in women of all ages, whether they originate from any part of the female genital tract. Gynecological malignancies can be classified into two groups based on the iretiology. Cervical, vaginal, and vulvar cancers are all connected to high-risk human papillomavirus infection and have established premalignant phases before invasive malignancy arises, while ovarian, tubal, and corpus tumors have no such association. Ovarian cancer has a hereditary susceptibility that is linked to the BRCA1, BRCA2, and Lynch type 2 genes [1].
Gynecological malignancies account for around half of all cancers in women (45.2%). Cancer cervix accounts for 33.3% of all cancers, whereas fallopian tube cancer (0.15% of all gynecological cancers) is the rarest of all gynecological cancers. They are more prevalent in rural India (76.5%) and poor India (72%), respectively [2].
Ovarian carcinoma has a significant death rate despite the introduction of surgery and chemotherapy. Unfortunately, there is significant delay in detection since they initially shown on specific symptoms such as bloating, pelvic discomfort, and appetite loss [1]. A number of recent contributory variables have also been brought forward like obesity, high-fat diet, and BMI > 35. In postmenopausal women who are not on hormone replacement therapy, obesity is directly linked to an increased risk of ovarian cancer. In western society, smoking (a significant cause of cervical and ovarian carcinoma), alcohol, and coffee consumption (a major cause of ovarian cancer) are all prevalent practices that function as gynecological risk factors. The oral contraceptive pill (OCP) appears to protect against invasive ovarian and endometrial cancer, with a lower increase in the risk of liver, breast, and cervical cancer [3].
Amidst many prognostic factors, angiogenesis or the emergence of new blood vessels, has been linked to tumor growth, metastasis, and progression in a variety of malignancies [4]. According to research, angiogenesis is thought to have a key role in the development of gynecological disorders, including endometriosis and malignant tumors. Folkman et al. [5] established the fundamental idea of angiogenesis in 1971, hypothesizing that tumor development and metastasis are dependent on angiogenesis, suggesting that inhibiting angiogenesis might be a technique for slowing disease progression. Folkman also argued that neovascularization is an important step in metastatic spread because it allows malignant cells to enter the bloodstream quoting that once a tumor reaches a size of 1–2 mm, it has to obtain access to vasculature in order to continue growing Fig. 1.
Since various tumors have variable amounts of angiogenic activity, corresponding with the development of gynecological carcinomas, many specialists are intrigued by the possibility of blocking angiogenesis as a cancer therapy approach [6]. Vascular endothelial growth factor (VEGF), also known as vascular permeability factor, stimulates the development of capillary channels around the tumor and works as a highly selective mitogen on endothelial cells Fig. 2.
Intra-tumoral angiogenesis indicators include vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and microvessel density (MVD) [7]. Microvessel density (MVD) evaluation on excised tumor sections using particular antibodies that stain endothelial cells can be used to study angiogenesis Fig. 3.
According to the literature, endothelial cells from tumor-associated neo-vasculature grow 20–2000 times faster than those from normal tissues [8]. The density of intra-tumoral microvessels (MVD) measured by staining endothelial antigens on histological sections can be used to quantify angiogenesis. Immunohistochemistry can detect small blood veins and capillaries by using a variety of antigens. Factor VIII-related antigen (von Willebrand factor) has been utilized in several investigations, whereas markers including CD-31, CD-34, and CD105 have been employed in others [9]. While CD 34 is still a neo-angiogenic marker, it does not distinguish betweenimmature and mature blood vessels since it stains all of them. When it comes to proliferating endothelial cells, CD 105 shows up more frequently. Despite these drawbacks, the hotspot technique has proved to be an accurate predictor of MVD numbers [10]. A poor prognosis has been linked to high levels of microvessel density, VEGF, and the expression of VEGF receptors in several investigations [10]. However, there are conflicting findings too, suggesting an improved prognosis [11]. It is derived that increased blood flow to tumors helps cyto reductive drugs and oxygen get to the tumor more easily. However, there are studies that show no connection between microvessel density and prognosis [12].
Method
The aim was to identify and quantify angiogenesis in paraffin-embedded histopathological sections of gynaecological malignancies by using an immune histochemical marker and to predict association of MVD with premalignant and malignant lesions of cervical, ovarian, and endometrial origin. The study design was observational descriptive over a sample size of 65. Patients of clinically suspected gynecologic malignancy with histopathological evidence who signed consent to participate in the study were included, while patients on adjuvant therapy and/or not willing to participate were excluded. Ethical clearance was obtained from the institute before starting the study.
Steps:
1. Surgical tissue was processed and stained by hematoxylin and eosin.
2. Histological categorization of the cases was done by H &E.
3. IHC CD105 (Biogenex QD 400-60KE) primary antibody and secondary antibody (Biogenex SS Polymer-HRP detection kit, DAB QD400-60KE Batch QD4000318, HSN/SAC 3822/30002) were applied on section with maximum tumor volume
4. Evaluation of ‘hot spots’ or proliferating microvessels—The first step was identification by light microscopy of the area of highest neovessel density, the so-called hot spot, by scanning the whole tumoral section at low power, then individual microvessels were counted at a higher power in an adequate area. This technique of counting hot spots is very similar to that for finding mitotic counts. Each single count is expressed as the highest number of microvessels identified at the hot spot. Average of four most vascular areas was taken.
5. Definition of microvessel—Any stained endothelial cell or clusters separate from adjacent vessels were counted as a single microvessel, even in the absence of vessel lumen. Vessels with muscular walls were not counted. Vessel lumen and red cells were not used to define a microvessel.
6. CX 33 Olympus microscope and MAGCAM DC5 5.1MP ½0.5 micron CHOS SENSOR MAGNUS were used for photography.
7. MVD was statistically correlated with tumor type, grade, recurrence, and other relevant clinical parameters.
8. Inter-observer variability was recorded in Fig. 4.
Results were analyzed using SPSS software (SPSS Inc., Chicago, IL, USA) for Windows program (21.0 version). The continuous variables were evaluated by mean (standard deviation) or range value when required. The dichotomous variables were presented in number/frequency. To compare the means between the two groups, analysis by Student t test and ANOVA (one way) with 95% confidence interval was used. Correlation was done using Spearman correlation analysis, and p value of < 0.05 or 0.001 was regarded as significant.
Results
The majority of patients had cervical origin (n = 39(60.00%)) followed by ovarian (n = 14(21.54%)) and endometrium (n = 10(15.38%)], respectively. On basis of specimen size, majority of small biopsies are of cervical origin (n = 39(60.00%)) followed by endometrium (n = 4(6.15%)) and vault (n = 2(3.98%)), respectively. Similarly, majority of large resections had ovarian origin (n = 14(21.54%) followed by endometrium (n = 6(9.23%)). In cervical neoplasms, majority of patients aged between 51–60 years [n = 12(30.77%)] followed by 41–50 years (n = 11(28.21%)], respectively. The majority of patients with ovarian neoplasms were above 60 years [n = 8(57.14%)] followed by 41–50 years (n = 3(21.43%)], respectively. Endometrium neoplasm patients were: above 60 years of age n = 4(40.00%)] followed by 51–60 years (n = 3(30.00%)], respectively. Whereas, patients with vault neoplasm were aged between 41–50 years [n = 2(100.00%)].
The majority of cervical cancers were in IIB [n = 17(43.59%)] FIGO stage of malignancy with MVD mean of 61.22 ± 26.78 followed and stage IIIB [n = 2(5.13%)] with MVD mean of 40 ± 10.00. The mean of MVD of cervical neoplasm in malignant and premalignant (CIN) was 59.39 ± 26.72 and 16 ± 2.646, respectively. The mean difference was statistically significant (p = 0.0092).
In cervical biopsy, the majority of cases showed SCC (moderately differentiated) histological grade [n = 24(61.54%)] followed by SCC (well differentiated) [n = 4(10.26)]. The mean MVD was higher in SCC (poorly differentiated) grade [82.75 ± 12.21]. While analyzing spearman correlation analysis of cervical malignancy with MVD, a statistically significant correlation was found between them (p = 0.0049). The mean MVD in ovarian neoplasm was 82.5 ± 57.5 and 46.33 ± 29.75, with the presence and absence of lymph node involvement [n = 2(25%)] and [n = 12(75%)], respectively. The mean difference was statistically insignificant (p = 0.2633). The mean MVD in ovarian lesions in malignant and premalignant was 0.63 ± 36.99 and 19 ± 5.29, respectively. The mean difference was statistically significant (p = 0.0447).
Discussion
It has been suggested that increased blood supply to the tumors enhances the access of the cytoreductive agents and oxygen to the tumor. There are also studies where a correlation between microvessel density and prognosis was not found [13]. With the above background, our research question is, “Does angiogenesis favor poor prognosis and reduced survival of gynecological cancer patients?” seems appropriate! Sixty-five samples were collected from different gynecological regions like cervix [n = 39], ovary [n = 14], endometrium [n = 10], and vault [n = 2]. The age group of our study population varied between 31 and 91 years.
Morre et al., 1998, found that immune staining for CD31 and CD34 enhanced microvessel density in both high and average vessel density regions of mucinous tumors when compared to serous and benign tumors. A dense microvessel network has been associated with both a shorter and a longer progression-free survival. A high value of microvessel density was related with a poor prognosis, and the most relevant antibody was identified as one against CD34. There is an increase in MVD from premalignant condition (19) to malignant (70.63) in ovarian tumors. Cases with palpable lymph nodes were (82.5) as compared to those without lymph nodes (46.33). Thus, our results relate with Morre et al. [14].
The mean MVD for cervical malignancy is 66.54 ± 28.56, which is higher as compared to that observed by Kluz et al., [15] 2020. They documented relationship between the budding tumor index (TBI) and microvessel density (MVD) as well. Between malignant and non-malignant endometrial lesions, they reported significant differences in MVD. The histological grade of the tumour was found to be significantly linked with MVD by CD34, and it was substantially greater in high-grade malignancy (G3; MVD, CD34, 24.9) than in grade G1 or G2 lesions, MVD by CD34, 14 and 18.6, respectively. FIGO clinical stage was not linked with MVD CD34 in low or high stage lesions (MD, 18.4 for FIGO stage I/II lesions; MD, 17.6 for FIGO stage III/IV lesions). These observations sync with ours.
The density, proportion, size, and quantity of microvessels stained with endoglin did not attain statistical significance as a prognostic predictor. However, there was a trend toward a worse prognosis with more profuse microvessels. On the other hand, chemotherapy agents may be less effective when delivered to the tumor via immature arteries. The disparate results obtained with CD34 and endoglin staining suggest that they quantify unique vascular types. Additionally, contrary to other investigations, this study could not assert the advantage of one or the other antibody in predicting ovarian cancer prognosis. We observed higher MVD in poorly differentiated as compared to well-differentiated carcinoma.
Fourteen cases of ovarian origin, with a mean age of 48.25 ± 16.47 years, have mean MVD of 55.64 ± 39.76. Ovarian malignancies were also segregated on the basis of premalignant and malignant lesions. We were not able to statistically correlate MVD with Lymph node infiltration as in the majority of cases, lymph nodes were not submitted. However, one of the submitted cases with positive Lymph nodes showed maximum MVD (82.5) in studied samples favoring positive correlation of MVD with poor prognosis. Davidson et al. [16] in 1999 found that microvessel counts for ulexlectin (mean 6.84.8/field) and CD31 (mean 8.7 ± 5.3/field) were comparable, and both indicators had a positive correlation with tumor stage. A progressive increase in microvessel density was observed, ranging from a mean of 28 vessels in normal tissue to 57 vessels in SCC.
They concluded that as the cervix transitions from a normal to a malignant phenotype, PDECGF expression and MVD increase. Our study also showed increasing tendency of MVD from premalignant to malignant conditions as in CIN (16) and SCC (59.39). In our study, there is progressive increase in MVD from lower stage and well differentiated (35.09), to moderately differentiated (58.45) and poorly differentiated (82.75) as in CIN stages. These findings were similar with results of Stephen P Dobbs et al. and Ben Davidson et al. [16]. Ozalp et al. [17] in 2003 sought to establish the predictive value of microvessel density (MVD) in endometrial carcinoma (EC) and normal endometrium during the proliferative and secretory phases, as well as its prognostic value in cases of EC. They observed a mean age of 58.3 ± 1.4 for 43 endometrial cancer cases. According to reports, MVD was prevalent among EC patients. The presence of MVD was associated with advanced surgical stage, cervical involvement, adnexal involvement, lympho-vascular space involvement, pelvic and para-aortic lymph node metastases, and positive peritoneal cytology. With an MVD cut-off value of 81/0.739 mm2, surgical stage, LVSI, retroperitoneal lymph node involvement, adnexal metastases, peritoneal cytology, and MVD count appeared to be independent predictors of survival in univariate analysis. They concluded that angiogenesis occurs throughout both the initial and subsequent stages of tumor development. In EC patients, MVD appears to have a significant prognostic value, advocating our finding of higher MVD in poorly differentiated as compared to well-differentiated gynecological malignant tissue.
CD105/endoglin antibody binds preferentially to proliferating endothelial cells in areas involved in angio genesis. They compared the performance of anti-CD34 and anti-CD105 in women with benign and malignant endometrial alterations in order to quantify angiogenesis. They observed good correlation between endometrial histology and MVD detected in "hot regions" of tumoral tissue with a high density of microvessels. Significant differences were also observed in cases of CD105 MVD between women with benign and malignant endometrial alterations (CD105 MVD = 11.8, vs. 6.4). Both CD34 and CD105 MVD were substantially linked with menopausal state, but not with clinical stage or histological grading [18]. They concluded that microvessel density alterations appear to be associated with the transition from endometrial hyperplasia to endometrial cancer. Their findings showed that endoglin (CD105), which stains proliferating microvessels, may be a more specific and sensitive marker for tumor neo-angiogenesis than the more widely used marker (CD34).
In a similar study that compared CD31 and CD105 in endometrial cancer, more intensive staining of microvessels was observed for endoglin when compared to CD31. Saad et al. [19] found that in normal endometrial tissue, only approximately 20% of all microvessels could be identified with CD105 staining. Thus, in women with endometrial cancer, the average numbers of microvessels per high power field (HPF) stained with CD31 and CD105 were 308/HPF and 3/HPF, respectively. The authors noted that women with high CD-105 MVD had a significantly worse prognosis than patients with low MVD assessed with this marker.
Traditionally, angiogenesis has been quantified by examining the samples' most densely vascularized regions, dubbed hot spots, and determining the microvessel density. Earlier investigations found anywhere between one and eleven hot sites. Three hotspots were chosen here, like in Raspollini's 2005 research [20]. Vieira et al. [21] (2005) observed that the total number of microvessels stained per case ranged between 48 and 200. However, they found that the median vascular density in undifferentiated carcinoma was 10.8 while 9.3 in differentiated carcinoma, a statistically significant difference. As a result of these investigations, it can be concluded that as the tumor's invasive potential or differentiation grade decreases, the MVD increases dramatically. In present study, it was observed that patients of poorly differentiated carcinoma had a higher MVD than those who were more well differentiated. Consequently, angiogenesis may have a negative impact on the prognosis and survival of gynecological cancer patients. However, additional research is necessary to determine the relevance of vessels in gynecological cancer patients and to quantify the impact of antiangiogenic therapy on microvessel parameters in gynecological malignancies. The small sample size and the single centric study were the limitations of the present study. Authors recommend further multicentric studies to increase the reliability and generalizability of the result.
Conclusion
Angiogenesis in tumors is thought to be a predictive factor for tumor growth and metastasis. Microvessel density (MVD) assessment using widely used endothelial markers such as CD34/CD105 shows to have an effect on prognosis in patients with various gynecological cancers. Tendency of MVD from premalignant CIN to SCC (WD) to SCC (MD) and SCC (PD) increases significantly. MVD is relatively higher in cervical lesions as the cervix is prone to different infections leading to inflammation. Patients of poorly differentiated carcinoma show a higher MVD than those more well differentiated. Consequently, angiogenesis may have a negative impact on the prognosis and survival of gynecological cancer patients.
References
Iyoke CA, Ugwu GO. Burden of gynaecological cancers in developing countries. World J Obstet Gynecol. 2013;2(1):1–7. https://doi.org/10.5317/wjog.v2.i1.1.
Chhabra S, Sonak M, Prem V, et al. Gynaecological malignancies in a rural institute in India. J Obstet Gynaecol. 2002;22(4):426–9. https://doi.org/10.1080/01443610220141434.
Rieck G, Fiander A. The effect of lifestyle factors on gynaecological cancer. Best Pract Res Clin Obstet Gynaecol. 2006;20(2):227–51. https://doi.org/10.1016/j.bpobgyn.2005.10.010.
Jilaveanu LB, Puligandla M, Weiss SA, et al. Tumour microvessel density as a prognostic marker in high-risk renal cell carcinoma patients treated on ECOG- ACRIN E2805. Clin Cancer Res. 2018;24(1):217–23. https://doi.org/10.1158/1078-0432.CCR-17-1555.
Folkman J. Tumour angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. https://doi.org/10.1056/NEJM197111182852108.
Folkman J. What is the evidence that tumours are angiogenesis dependent? J Natl Cancer Inst. 1997;82:4–6. https://doi.org/10.2147/vhrm.2006.2.3.213.
Nicol D, Hii SI, Walsh M, Teh B, Thompson L, Kennett C, Gotley D. Vascular endothelial growth factor expression is increased in renal cell carcinoma. J Urol. 1997;157:1482–6. https://doi.org/10.1016/S0002-9440(10)64016-3.
Abulafia O, Sherer DM. Angiogenesis of the endometrium. Obstet Gynecol. 1999;94:148–53. https://doi.org/10.1016/s0029-7844(99)00262-8.
Olszanecki R, Gebska A, Korbut R, et al. Production of prostacyclin and prostaglandin E2 in resting and IL-1beta-stimulated A549, HUVEC and hybrid EA.HY 926 cells. J Physiol Pharmacol. 2006;57(4):649–60.
He L, Wang Q, Zhao X, et al. Microvessel density as a prognostic factor in ovarian cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2015;16(3):869–74. https://doi.org/10.7314/apjcp.2015.16.3.869.
Ruscito I, Castillo-Tong DC, Vergote I, et al. Characterisation of tumour microvessel density during progression of high-grade serous ovarian cancer: clinic-pathological impact (an OCTIPS Consortium study). Br J Cancer. 2018;119:330–8. https://doi.org/10.1038/s41416-018-0157-z.
Shen GH, Ghazizadeh M, Kawanami O, et al. Prognostic significance of vascular endothelial growth factor expression in human ovarian carcinoma. Br J Cancer. 2000;83:196–203. https://doi.org/10.1054/bjoc.2000.1228.
Czekierdowski A, Czekierdowska S, Czuba B, et al. Microvessel density assessment in benign and malignant endometrial changes. J Physiol Pharmacol. 2008;59(Suppl 4):45–51. https://doi.org/10.1111/j.1600-0463.2003.
Orre M, Lotfi-Miri M, Mamers P, Rogers PA. Increased microvessel density in mucinous compared with malignant serous and benign tumours of the ovary. Br J Cancer. 1998;77(12):2204–9. https://doi.org/10.1038/bjc.1998.367.
Kluz T, Tozinski T, Czekierdowska S, et al. Tumor budding index and microvessel density assessment in patients with endometrial cancer: a pilot study. Oncol Lett. 2020;20(3):2701–10. https://doi.org/10.3892/ol.2020.11811.
Davidson B, Goldberg I, Gotlieb W, et al. Macrophage infiltration and angiogenesis in cervical squamous cell carcinoma: clinic-pathologic correlation. Acta obstet et gynecol Scand. 1999;78(3):240–4.
Ozalp S, Yalcin OT, Acikalin MU, et al. Microvessel density (MVD) as a prognosticator in endometrial carcinoma. Eur J Gynaecol Oncol. 2003;24(3–4):305–8. https://doi.org/10.1097/MS9.0000000000000939.
Salvesen HB, Gulluoglu MG, Stefansson I, et al. Significance of CD 105 expression for tumour angiogenesis and prognosis in endometrial carcinomas. APMIS. 2003;111:1011–8. https://doi.org/10.1111/j.1600-0463.2003.apm1111103.x.
Saad RS, Jasnosz KM, Tung MY, et al. Endoglin (CD105) expression in endometrial carcinoma. Int J Gynecol Pathol. 2003;22:248–53. https://doi.org/10.1097/01.pgp.0000070852.25718.37.
Raspollini MR, Amunni G, Villanucci A, et al. Microvessel density in ovarian carcinoma: computer image analysis in patients with shorter and longer survival. Int J Gynecol Cancer. 2005;15:844–9. https://doi.org/10.1111/j.1525-1438.2005.00146.x.
Vieira SC, Silva BB, Pinto GA, Vassallo J, Moraes NG, et al. CD34 as a marker for evaluating angiogenesis in cervical cancer. Pathol Res Pract. 2005;201:313–8. https://doi.org/10.1016/j.prp.2005.01.010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
By the institutional committee (ECR/262/Inst/UP/2013/RR-19; Ref code 102nd ECM IIB- Thesis/P85).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aslahuddin, M., Jaiswal, R., Qayoom, S. et al. Microvessel Density Assessment in Gynecological Malignancies. Indian J Gynecol Oncolog 22, 78 (2024). https://doi.org/10.1007/s40944-024-00852-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-024-00852-7