Abstract
Extrauterine endometrial stromal sarcomas are rare malignancies, with 181 cases reported so far. The basic predisposing factor is endometriosis, and ovary is the most common extrauterine site. Low-grade varieties are the most common subtypes encountered. Our patient is a young lady with low-grade extrauterine endometrial stromal sarcoma inoperable disease at presentation involving bilateral ovaries, omentum, peritoneum and hemorrhagic third space collections. The uterus was radiologically normal. She initially received palliative endocrine therapy and later underwent complete cytoreductive surgery. Currently, she is on an aromatase inhibitor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcomas of the uterus are rare entities, which amount to < 10% of all uterine malignancies [1]. They arise from the uterine endometrium and are diverse in nature. The sarcoma types differ as per histogenesis. They can be either pure mesenchymal in origin, like endometrial stromal sarcoma (ESS), leiomyosarcoma, smooth muscle tumor of unknown malignant potential or can be mixed with epithelial elements to form carcinosarcoma, adenosarcoma, etc. [1]. Extrauterine ESS is very rare, often originating from malignant transformation of endometriotic foci elsewhere. Here, we present a rare case of extrauterine ESS with generalized abdominal involvement and pleural effusion at presentation, its clinical course and highlight the importance of multimodality therapy.
Case Report
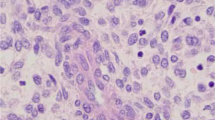
This 32-year-old, unmarried female was evaluated for bloating symptoms of three-month duration. She attained menarche at the age of 11 years and had dysmenorrhea for the past 5 years. She reported no comorbidities and was not on any drugs. There was no history of substance abuse or addictions. Her family history was unremarkable. At presentation, she was of Eastern Cooperative Oncology Group performance status 2, with ascites and peritoneal deposits. The tumor marker values were normal (βHCG- < 2 mIU/mL, AFP 1.9 ng/ml, CEA- < 0.5 ng/mL, CA-125 183 U/mL, CA 19–9-6.7 U/mL). Computed tomography (CT) abdomen and pelvis showed bilateral hemothorax, hemoperitoneum, multiple large omental and peritoneal deposits largest measuring 10 × 7 cm, right adnexal mixed density mass of 7 × 5 cm, a similar left ovarian mass of 10 × 7 cm and non-significant tiny retroperitoneal nodes (Fig. 1a). The uterus was anteverted and normal sized (9 cm in long axis), with empty cavity and no focal myometrial lesions. Endometrium measures 12 mm in thickness and was normal. Biopsy of the peritoneal deposit revealed low-grade ESS (Fig. 2). The tumor consisted of sheets of short spindly cells, and the MIB1 labeling index was 3–4%. The tumor cells were immunoreactive for CD10 & PR and were immunonegative for h-caldesmon and desmin.
a Gross specimen of solid mass with areas of necrosis and hemorrhage. b, c Microscopy showing neoplasm composed of sheets of atypical cells with scant cytoplasm, uniform ovoid nuclei with delicate network of arterioles (H&E X100, H&EX200). d Tumor cells show diffuse strong nuclear positivity for PR (IHCX200). e Tumor cells show CD10 positivity (IHCX200)
While on work up, she deteriorated with symptomatic anemia (Hb-5.4gm%) and hypoalbuminemia. She was optimized with transfusions and supportive care. In view of extensive inoperable disease, she was started on gonadotropin releasing hormone (GnRH) analogues (leuprolide depot formulation 11.25 mg intramuscular injections once in every three months) after multidisciplinary tumor board discussion. She became symptomatically better at 1 month. At third month, she had no palpable disease. After 6 months of treatment, CT of the abdomen and pelvis showed partial response (Fig. 1b). The pleural effusion and ascites had resolved. The largest omental deposit and bilateral adnexal lesions had reduced in size, and there were no new lesions. She got clinical benefit from GnRH analogues for one and half years. Later, she presented with clinical features of torsion of the adnexal mass. CT scan was suggestive of progressive intra abdominal disease with features of adnexal mass torsion (Fig. 1c). She underwent emergency laparotomy. Intra-operatively, there were bilateral adnexal masses, ascites, multiple deposits in pouch of douglas, omental deposits and ovarian surface deposits. The uterus was grossly normal. She underwent complete cytoreduction including bilateral salpingo oophorectomy, omentectomy, appendectomy and excision of all residual disease. Histopathology showed low-grade ESS with ascites, and all excised specimens were positive for tumor deposits. There were areas of necrosis and cystic degeneration; however, pathologists could not demonstrate endometriotic focus in the specimen (Fig. 2). Postoperative period was uneventful, and she was started on letrozole (2.5 mg once daily) as adjuvant therapy.
Discussion
ESS is the 2nd most common uterine sarcoma and accounts for 0.2% of female genital tract malignancies [2]. They are made up of cells which are similar to endometrial stroma in the proliferative phase. Advances in the genetic knowledge of these tumors have revealed distinct genetic abnormalities in ESS, namely JAZF1-SUZ12 gene fusion in low-grade tumors and YWHAE-NUTM2A/B rearrangement in high-grade tumors [3, 4]. In this background, the two varieties of ESS, low grade and high grade, have been separately classified in 2014 WHO classification.
Primary extrauterine ESS (EESS) is a very rare phenomenon to occur; they are mostly seen in the premenopausal age, and the literature is limited to case series and reports. The extrauterine origin is largely thought to be due to endometriosis, where endometrial-like tissue is present outside the uterus. In majority of the cases, the endometriotic foci were demonstrated in the tumor vicinity. The largest reported series on EESS is by Masand et.al from M D Anderson Cancer Centre, which described 63 such cases [5]. The majority of extrauterine sites described in the literature are from other gyn (gynecological) organs, namely ovary (most common), fallopian tube, vagina, and adnexa. The second most common site of origin is GIT (gastrointestinal tract) and very rarely from the anterior abdominal wall, retroperitoneal tissue, lung and bladder are involved [2, 6, 7, 9]. Most of the patients are diagnosed between 40 and 50 years of age, and it is rare in younger patients.
Low grade is the most common subtype found at extrauterine sites; however, high-grade varieties have also been reported. For entailing a diagnosis of EESS, the uterus should be free of tumor and there should be foci of endometriosis in the site of origin. The presenting symptoms can depend on the sites of involvement and are not typical to the tumor per se. Approximately 25% of early-stage disease can be asymptomatic. It can even manifest several years after primary hysterectomy, and often, endometriosis would have been present in such cases [8]. This rare entity can be misdiagnosed for other tumors such as gastrointestinal stromal tumor, ovarian stromal neoplasm, leiomyosarcoma, granulosa cell tumor, cellular fibroma, adeno/synovial sarcoma, peripheral nerve sheath tumor and atypical stromal endometriosis[5].
For operable low-grade ESS, complete cytoreduction is the standard of care. Adjuvant endocrine therapy is indicated from stage II onward; however, the duration is still undefined. Adjuvant radiotherapy has shown to limit the local recurrence, but its survival advantage is very low. For advanced and inoperable disease, hormonal manipulation is recommended though the data are scarce. There are no specific staging or treatment guidelines for low-grade EESS and are most often managed as per the recommendations for low-grade ESS. Operable low-grade EESS is treated with complete cytoreductive surgery followed by adjuvant anti-estrogen therapy. The commonly used agents for endocrine manipulation are aromatase inhibitors, megestrol acetate, medroxyprogesterone acetate and GnRH analogues. There are very limited data on management of inoperable and metastatic low-grade EESS, but they often respond to endocrine therapy. The prognosis of low-grade ESS is relatively good with 5-yr survival of > 85% for stage 1 and 10% for advanced stages. Survival data of EESS are not reported so far.
Recent systematic review on EESS showed that prognosis depends on tumor size, site of origin and adjuvant endocrine therapy. Those with tumor size less than 5 cm, Gyn origin tumors (ovary) and those who have received adjuvant endocrine therapy had superior overall survival and progression-free survival [9]. Those with generalized involvement of the abdomen where the site of origin is unknown have poor prognosis compared to Gyn and GIT in origin EESS.
To conclude, this case highlights the need and importance of distinguishing EESS from the common epithelial ovarian cancer. The treatment is entirely different, and EESS is therapeutically amenable even in advanced stages of presentation.
References
Novetsky AP, Powell MA. Management of sarcomas of the uterus. Curr Opin Oncol. 2013;25(5):546–52.
Efared B, Sidibé IS, Erregad F, Hammas N, Chbani L, El Fatemi H. Extra-uterine low grade endometrioid stromal sarcoma arising from ovarian endometriosis: a case report and review of the literature. Gynecol Oncol Res Pract. 2019;6:2.
Huang HY, Ladanyi M, Soslow RA. Molecular detection of JAZF1-JJAZ1 gene fusion in endometrial stromal neoplasms with classic and variant histology: evidence for genetic heterogeneity. Am J Surg Pathol. 2004;28(2):224–32.
Lee CH, Mariño-Enriquez A, Ou W, Zhu M, Ali RH, Chiang S, et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012;36(5):641–53.
Masand R, Euscher E, Deavers M, Malpica A. Extrauterine endometrial stromal sarcoma: a pathologic study of 63 cases with clinical correlation. Am J Surg Pathol. 2013;37:1635–47.
Clair K, Wolford J, Veran-Taguibao S, Kim G, Eskander RN. Primary low-grade endometrial stromal sarcoma of the omentum. Gynecol Oncol Rep. 2017;21:119–21.
Liu Z, Ding J, Li X, Yu K. Endometrial stromal sarcoma arising in vagina. Int J Clin Exp Pathol. 2013;6:2997–3002.
Ayuso A, Fadare O, Khabele D 2013 A Case of Extrauterine Endometrial Stromal Sarcoma in the Colon Diagnosed Three Decades after Hysterectomy for Benign Disease. Hindawi Publishing Corporation. Case Reports in Obstetrics and Gynecology. 2013, Article ID 202458, 3 pages
Deb PQ, Heller DS. Extrauterine endometrial stromal sarcoma: a systematic review and outcome analysis. Ann Diagn Pathol. 2022;59: 151966.
Funding
There are no sources of funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no competing interests among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saikumar, S., Haridas, L. & Renu, S. Extrauterine Endometrial Stromal Sarcoma: A Case Report. Indian J Gynecol Oncolog 21, 44 (2023). https://doi.org/10.1007/s40944-023-00721-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-023-00721-9