Abstract
Purpose
Programmed death-ligand 1 (PD-L1) was expressed in various gynecology tumors. High-grade ovarian cancers could be a potential target for immune anti-PD-L1 modulate therapy. Antibodies targeting PD-L1 molecules are emerging cancer therapeutics. This study was designed to evaluate the expression of PD-L1 marker in the high-grade ovarian cancer types and evaluate its prognostic potential.
Methods
The study included 18 patients with ovarian high-grade serous cancer (HGSC) and 11 patients with clear cell cancer (CCC) histology type, both in the International Federation of Gynecology and Obstetrics (FIGO) stage I. The expression of the PD-L1 marker was measured by tissue microarray-based immunohistochemistry. Expression levels of PD-L1 were correlated with the presence of tumor-infiltrating lymphocyte (TIL) and other histopathology parameters.
Results
HGSC ovarian cancers predominantly had low PD-L1 expression, while CCC ovarian cancers had high PD-L1 expression (p < 0.001). PD-L1 expression did not show significant differences considering analyzed parameters other than histology type (localization, size, FIGO stage, lymphovascular invasion, tumor necrosis, and presence of TIL) among all ovarian cancers. There was no statistically significant difference in any of the tumor characteristics within histologic types of ovarian cancers.
Conclusion
PD-L1 expression was significantly higher in clear cell histology type than in high-grade serous ovarian cancers in FIGO I stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer (OC) is the eight most common cancer in female population [1]. Ovarian cancer research works focused primarily on high-grade serous cancer (HGSC), the most common ovarian cancer histology type, accounting for 70% of all ovarian cancer cases [2]. Ovarian clear cell cancer (CCC) is a rare histology type, approximately 10% of all ovarian cancers [3]. Both CCC and HGSC composed high-grade ovarian cancers and represent aggressive forms of ovarian cancer often harbor resistance to therapy and have an overall poor prognosis [2]. Ovarian cancers were diagnosed usually in advanced stages [4]. On the contrary to HGSC, CCC was diagnosed usually in early stage disease, but also with bad prognosis [3].
Programmed cell death-ligand 1 (PD-L1) is a molecule expressed on tumor cells. Also, it could be expressed on cells in tumor microenvironment such as dendritic cells, fibroblast, T-lymphocytes. PD-L1 protein on tumor cells interacts with programmed cell death-1 (PD-1) on T-lymphocytes and makes them inactive without effector functions [5,6,7]. Considering immune-cancer cycle, cancer cells produce tumor antigens which were phagocitosed by macrophages and presented to effector cytotoxic T-lymphocites. Such activated T-lymphocytes gain ability to destroy cancers cells. Ovarian cancer cells have ability to express PD-L1 on their surface and escape autitumor immune mechanism [5].
Regardless chemotherapy and relatively new thepary modalites as vascular endothelial growth factor (VEGF) and poly(ADP-ribose) polymerase (PARP) inhibitors, recurrences are still very often [8, 9]. There is a need to find new therapeutic options that would improve the patient outcomes. PD-L1 inhibitors should be promising strategies in ovarian cancer treatment. Assesement of patients who could be appropriate for anti-PD-L1 target immune therapy might be done by immunohistochemistry scoring of PD-L1 expression on cancer cells. Simultaneous analysis of the presence of tumor-infiltrating lymphocytes (TIL) could contribute to selection [10]. Previous studies report diversion results about immune target therapy by PD-L1 inhibitors. Cancer microenvironment shows significant effects on therapy outcomes [8].
The aim of the study was to analyze the expression of PD-L1 marker in the high-grade ovarian cancer types and evaluate its prognostic potential.
Materials and Methods
Patient Selection
The study included 18 patients with HGSC and 11 patients with CCC histology type, both in International Federation of Gynecology and Obstetrics (FIGO) stage I. Inclusion criteria were age 18 and over, tumors with pure clear cell or high-grade serous histology type and other types of ovarian tumors, any mixed component or lack of adequate available tissue for staining merited exclusion. Recorded parameters were patient’s age, menopausal status, tumor size and localization, presence of necrosis, presence of tumor lymphovascular invasion and degree of TIL. The ethical approval was obtained from the Ethics Committee of the University Clinical Center of Serbia, and all study participants gave their informed consent. A total of 29 patients met the above criteria.
Tumor-Infiltrating Lymphocytes (TIL)
The evaluation for the presence of tumor-infiltrating lymphocyte was done on HE tissue sections before the construction of tissue microarray on microscopic magnification × 50. TIL signed as positive considered at least one detected lymphocyte in tumor tissue. Cancers without lymphocytes or other mononuclear inflammatory cells were designated as TIL negative [11].
Tissue Microarray (TMA)
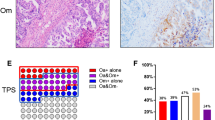
From each donor tissue block, one cylinder was taken by 3 mm puncture needle using the tissue microarray (TMA) method (Fig. 1a). All cylinders were moved to a recipient paraffin block [12]. Standard optimization protocol for PD-L1 is considered using placental tissue as a positive internal control for immunohistochemical analysis [13] (Fig. 1b). In the first row of each block, a placental tissue was placed to serve for orientation.
Immunohistochemical Analysis
Paraffin embedded formalin fixed tissue from both ovarian cancers types was stained for PD-L1 marker on the Autostainer Link 48, Agilent, Denmark. Epitope unmasking for PD-L1 antibody was done in EnVision FLEX epitope unmasking solution pH 6.1 (K8005, Agilent). Visualization system EnVision FLEX (Agilent) was used for immunohistochemical analysis. It was used as primary monoclonal anti-human PD-L1 antibody (clone 22C3, M3653, Agilent) in dilution of 1:30. Expression was analyzed by counting positive tumor cells on the × 400 power field and noticing their percentage from the total number of tumor cells. Only the membranous PD-L1 staining was considered positive. The following score was used to describe the expression levels: negative (0) without positive cells or with a single positive cell (< 1%); low (1 +) expression with less than 10% positive cells; moderate (2 +) expression with 10–50% positive cells and strong (3 +) expression with more than 50% positive cells. Associated moderate and strong positivity was considered as a high expression which is in positive correlation with therapy response [14,15,16].
Statistical Analysis
Categorical variables were presented as absolute and relative numbers. Numerical variables were expressed as arithmetic mean with standard deviation or median with range, depending on the data distribution. Normal distribution was evaluated by mathematical and graphical methods. Two independent study groups were compared by categorical variables using Chi-square or Fisher’s exact test if criteria for the previous one were not met, while they were compared by numerical variables using Mann–Whitney U test. All statistical methods were considered significant if p ≤ 0.05. Statistical analysis was performed in IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.
Results
Patient Characteristics
The mean age of 29 patients was 56.90 ± 11.61 years. Almost all patients were in menopause (79%). Eighteen (62%) had HGSC, and 11 (38%) had CCC. The distribution of unilateral and bilateral localized cancers was almost equal (55% vs. 45%). According to FIGO classification, the IC2 stage was the most common (62%), while IA, IC1, and IC3 stages were much rarer (7%, 17%, and 14%, respectively). Lymphovascular invasion was present in almost all cancers (83%), and almost 60% of them had tumor necrosis. TIL was present in 25 (86%) cancer cases.
PD-L1 Expression in Relation to Analyzed Parameters
Low and high PD-L1 expressions were equally distributed (55% vs. 45%). All analyzed parameters concerning PD-L1 expression are presented in Table 1. HGSC ovarian cancers predominantly had low PD-L1 expression, while CCC ovarian cancers had high PD-L1 expression (p < 0.001). PD-L1 expression did not differ according to other characteristics (localization, size, FIGO stage, lymphovascular invasion, tumor necrosis, and presence of TIL) among all ovarian cancers.
Immunohistochemical Analysis of PD-L1 Expression in High-Grade Serous Ovarian Cancer
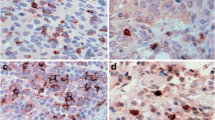
The PD-L1 expression in HGSC ovarian cancers is presented in Fig. 2. A low level of PD-L1 expression was most common (44%), and PD-L1 expression was absent in almost 40% of all HGSC ovarian cancers.
The most common pattern of PD-L1 expression in analyzed HGSC is presented in Fig. 3.
Immunohistochemical Analysis of PD-L1 in Clear Cell Ovarian Cancer
The PD-L1 expression in CCC ovarian cancers is presented in Fig. 4. The majority of all CCC ovarian cancers had a moderate level of PD-L1 expression, while a low level of PD-L1 expression was present in only 9% of CCC ovarian cancers.
The most common pattern of PD-L1 expression in analyzed CCC is presented in Fig. 5.
PD-L1 Expression in HGSC and CCC Considering Histopathological Parameters
Table 2 shows all analyzed histopathological parameters concerning PD-L1 expression in two histology types of ovarian cancers (HGSC and CCC). There was no statistically significant difference in any of the tumor characteristics within histologic types of ovarian cancers.
Discussion
The evaluation of PD-L1 expression in high-grade ovarian cancers could determine more effective treatment and increase the overall survival for these patients [17]. PD-L1 status in the clear cell histologic cancer type is a certain intriguing predictive marker for determining the possibility of immune checkpoint inhibitor therapy [18]. The more frequent ovarian cancers such as HGSC were far more often analyzed considering PD-L1 expression [9, 14]. The comparison between these high-grade OC could bring novel results, significant for better evaluation of PD-L1 expression to determine more effective therapy protocols for these patients.
Our study analyzed differences in PD-L1 expression in two histology types of high-grade ovarian cancers (HGSC and CCC). All cancers in our study were in stage FIGO I. Patients were usually in menopause, in FIGO IC2, with the presence of tumor lymphovascular invasion and necrosis. Almost all analyzed cancers showed TIL. We did not find significant differences in PD-L1 expression considering all analyzed parameters others than histology tumor type.
We found significantly higher PD-L1 expression in CCC histology type of ovarian cancer. The most frequent level of PD-L1 expression in CCC was moderate, which is part of a high expression score. PD-L1 expression in HGSC was usually low. The number of HGSC without PD-L1 expression was conspicuous. Those could be the reasons for weaker PD-L1 status in HGSC. Considering our results, using PD-L1 inhibitors could be more effective in CCC than in HGSC ovarian cancers. Similar results were reported in the study which described a good therapy response by immune inhibitors for CCC tumor type, especially whereas existing detection of microsatellite instability [3, 19].
Our study did not show a significant correlation between TIL and PD-L1 status in CCC or HGSC histology types. The presence of TIL is in association with the response to PD-L1 inhibitors in many cancers. Significant PD-L1 expression in OC is often in positive correlation with remarkable TIL. The majority of studies reported significant TIL in association with higher PD-L1 expression in HGSC, which could lead to promising immune therapy responses for these patients [20, 21]. There were not any differences between CCC and HGSC OC considering TILs and PD-L1 expression, except in CCC with microsatellite instability, whereas it described a significantly higher number of TILs [3, 19].
The reason for these differences could be a different patient selection, where we choose only OC in stage I. CCC is a very rare type of OC, mostly diagnosed in the first stage of disease, and for better correlation, we analyzed HGSC in the same FIGO stages. HGSC is the most frequent OC which is mostly diagnosed in later stages because many studies analyzed PD-L1 expression, in association with TIL and OS just at that point, where results are different from this study [22].
Significant problems for standardizing PD-L1 immunohistochemical analysis are methodological inconsistency, various cutoff values, spatial and temporal factors, different sensitivity and clone types of PD-L1 antibodies. Complex tumor microenvironment certainly affects the accuracy of PD-L1 immunohistochemistry staining [23]. There are four main PD-L1 clones (28–8, 22C3, SP142, SP263), but clone 22C3 was the most used, analyzed and also applied in this study. This clone was expressed as membranous PD-L1 staining which is in positive correlation with therapy outcomes [15, 16, 23].
For the non-Asian population, a significant positive correlation between higher PD-L1 expression and overall survival (OS) in patients with OC was reported [5]. In HGSC, the majority of studies reported a positive correlation between PD-L1 expression and decreased OS [22, 24]. One study showed that remarkable PD-L1 expression in CCC indicates an unfavorable prognosis for these patients [17]. The results suggest that the analysis of PD-L1 expression in CCC certainly has the prognostic potential [17]. Considering our findings, whereas PD-L1 expression was significantly higher in CCC than in HGSC, we expect more unfavorable OS in CCC than in HGSC histology type. Since the HGSC with significant PD-L1 expression already has a quite unfavorable prognosis [22], patients with CCC could have an additionally worse OS. More studies are necessary in the future for a better evaluation of the association between PD-L1 expression levels and the prognosis of these patients.
The limitations of this study could be a relatively small sample size or PD-L1 expression analysis by immunohistochemistry alone. Considering the rarity of clear cell histology type and HGSC in stage FIGO I, we found this research enough challenging. Analysis of more additional proteins which are involved in the PD-1/PD-L1 regulatory pathway certainly could increase a better understanding of this mechanism. Involving additional methods for the evaluation of PD-L1 status could improve the analysis. Anyway, this study could be a great inspiration for further research.
PD-L1 expression in ovarian CCC has not been well studied. Our study could promote the significance of further analysis to find more effective therapy protocols for these patients, especially if we know that CCC is generally resistant to standard platinum-based chemotherapy [19, 25]. A recent study showed remarkable clinical responses by immunotherapy in patients with high PD-L1 expressions in recurrent, therapy-resistant OC [26]. Considering other cancer types, different from gynecologic, PD-L1 inhibitors brought many promising insights. Clear cell morphology could be a significant, independent characteristic for the indication of immunotherapy [19].
Conclusion
PD-L1 expression was significantly higher in clear cell histology type than in high-grade serous ovarian cancers in FIGO I stage. Significant PD-L1 expression in CCC could have an important predictive value for the implementation of anti-PD-L1 target immune therapy for these patients. Our results suggest further investigation of PD-L1 marker in clear cell histology type of ovarian cancers because immunotherapy is still controversial for these rare gynecologic tumors.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. https://doi.org/10.3322/caac.21590.
van Zyl B, Tang D, Bowden NA. Biomarkers of platinum resistance in ovarian cancer: what can we use to improve treatment. Endocr Relat Cancer. 2018;25(5):R303–18. https://doi.org/10.1530/ERC-17-0336.
Howitt BE, Strickland KC, Sholl LM, Rodig S, Ritterhouse LL, Chowdhury D, et al. Clear cell ovarian cancers with microsatellite instability: a unique subset of ovarian cancers with increased tumor-infiltrating lymphocytes and PD-1/PD-L1 expression. Oncoimmunology. 2017;6(2):e1277308. https://doi.org/10.1080/2162402X.2016.1277308.
Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK collaborative trial of ovarian cancer screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945–56. https://doi.org/10.1016/S0140-6736(15)01224-6.
Piao J, Lim HJ, Lee M. Prognostic value of programmed cell death ligand-1 expression in ovarian cancer: an updated meta-analysis. Obstet Gynecol Sci. 2020;63(3):346–56. https://doi.org/10.5468/ogs.2020.63.3.346.
Pietzner K, Nasser S, Alavi S, Darb-Esfahani S, Passler M, Muallem MZ, et al. Checkpoint-inhibition in ovarian cancer: rising star or just a dream? J Gynecol Oncol. 2018;29(6):e93. https://doi.org/10.3802/jgo.2018.29.e93.
Robainas M, Otano R, Bueno S, Ait-Oudhia S. Understanding the role of PD-L1/PD1 pathway blockade and autophagy in cancer therapy. Onco Targets Ther. 2017;10:1803–7. https://doi.org/10.2147/OTT.S132508.
Pawłowska A, Kwiatkowska A, Suszczyk D, Chudzik A, Tarkowski R, Barczyński B, et al. Clinical and prognostic value of antigen-presenting cells with PD-L1/PD-L2 expression in ovarian cancer patients. Int J Mol Sc. 2021;22:11563. https://doi.org/10.3390/ijms222111563.
Battaglia A, Piermattei A, Buzzonetti A, Pasciuto T, Zampetti N, Fossati M, et al. PD-L1 expression on circulating tumour-derived microvesicles as a complementary tool for stratification of high-grade serous ovarian cancer patients. Cancers. 2021;13:5200. https://doi.org/10.3390/cancers13205200.
Bansal A, Srinivasan R, Rohilla M, Rai B, Rajwanshi A, Suri V, et al. Immunotyping in tubo-ovarian high-grade serous carcinoma by PD-L1 and CD8+ T-lymphocytes predicts disease-free survival. APMIS. 2021;129(5):254–64. https://doi.org/10.1111/apm.13116.
Clark CA, Gupta HB, Sareddy G, Pandeswara S, Lao S, Yuan B, et al. Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res. 2016;76(23):6964–74. https://doi.org/10.1158/0008-5472.CAN-16-0258.
Choi CH, Kim KH, Song JY, Choi SJ, Kim L, Park IS, et al. Construction of high-density tissue microarrays at low cost by using self-made manual microarray kits and recipient paraffin blocks. Korean J Pathol. 2012;46(6):562–8. https://doi.org/10.4132/KoreanJPathol.2012.46.6.562.
Oh SY, Roh CR. Autophagy in the placenta. Obstet Gynecol Sci. 2017;60(3):241–59. https://doi.org/10.5468/ogs.2017.60.3.241.
Jovanović L, Janković R, Ćirković A, Jović M, Janjić T, Djuričić S, et al. PD-L1 expression in different segments and histological types of ovarian cancer according to lymphocytic infiltrate. Medicina (Kaunas). 2021;57(12):1309. https://doi.org/10.3390/medicina57121309.
Dako. PD-L1 IHC 22C3 pharmDx interpretation manual – gastric or gastroesophageal junction adenocarcinoma. PD-L1 IHC 22C3 pharmDx is FDA-approved for in vitro diagnostic use (2019). https://www.agilent.com/cs/library/usermanuals/public/29219_pd-l1-ihc-22C3-pharmdx-gastric-interpretation-manual_us.pdf
Dako. PD-L1 IHC 22C3 pharmDx interpretation manual—NSCLC—for in vitro diagnostic use ( 2018). https://usermanual.wiki/m/36cf5deff6015d44ebe696b439deba5ec850657a9b00689e6c52d5bc11706076.pdf
Zhu J, Wen H, Bi R, Wu Y, Wu X. Prognostic value of programmed death-ligand 1 (PD-L1) expression in ovarian clear cell carcinoma. J Gynecol Oncol. 2017;28(6):e77. https://doi.org/10.3802/jgo.2017.28.e77.
Alldredge J, Serna-Gallegos T, Gallegos N, VanLeer JP, Chang Jenny, Ziogas A, Goreal W, Randall L. Evaluation of clear cell subtypes of ovarian and uterine malignancies with anti-PD-L1 and anti-PD1 immunohistochemical expression and their association with stage and survival. Gynecol Oncol. 2019;155(3):483–8. https://doi.org/10.1016/j.ygyno.2019.10.008.
Willis BC, Sloan EA, Atkins KA, Stoler MH, Mills AM. Mismatch repair status and PD-L1 expression in clear cell carcinomas of the ovary and endometrium. Mod Pathol. 2017;30(11):1622–32. https://doi.org/10.1038/modpathol.2017.67.
Wan C, Keany MP, Dong H, Al-Alem LF, Pandya UM, Lazo S, et al. Enhanced efficacy of simultaneous PD-1 and PD-L1 immune checkpoint blockade in high-grade serous ovarian cancer. Cancer Res. 2021;81:158–73. https://doi.org/10.1158/0008-5472.CAN-20-1674.
Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7:1486–99.
Farrag MS, Abdelwahab K, Farrag NS, Elrefaie WE, Emarah Z. Programmed death ligand-1 and CD8 tumor-infiltrating lymphocytes (TILs) as prognostic predictors in ovarian high-grade serous carcinoma (HGSC). J Egypt Natl Canc Inst. 2021;33:16. https://doi.org/10.1186/s43046-021-00073-5.
Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther. 2016;9:5023–39. https://doi.org/10.2147/OTT.S105862.
Bansal A, Srinivasan R, Rohilla M, Rai B, Rajwanshi A, Suri V, et al. Immunotyping in tubo-ovarian high-grade serous carcinoma by PD-L1 and CD8+ T-lymphocytes predicts disease-free survival. APMIS. 2021;129:254–64. https://doi.org/10.1111/apm.13116.
Mackay HJ, Brady MF, Oza AM, Reuss A, Pujade-Lauraine E, Swart AM, et al. Prognostic relevance of uncommon ovarian histology in women with stage III/IV epithelial ovarian cancer. Int J Gynecol Cancer. 2010;20(6):945–52. https://doi.org/10.1111/IGC.0b013e3181dd0110.
Hamanishi J, Mandai M, Konishi I. Immune checkpoint inhibition in ovarian cancer. Int Immunol. 2016;28(7):339–48. https://doi.org/10.1093/intimm/dxw020.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by LJ, AĆ, RJ and MJ. The first draft of the manuscript was written by LJ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interest to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Faculty of Medicine, University in Belgrade (Date 28/11/2019, No. 1550/XI-40) and Ethics Committee of University Clinical Center of Serbia (date 19/07/2018, No. 747/3).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Patients signed informed consent regarding publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jovanović, L., Ćirković, A., Jović, M. et al. PD-L1 Expression in High-Grade Serous and Clear Cell Ovarian Cancer. Indian J Gynecol Oncolog 20, 47 (2022). https://doi.org/10.1007/s40944-022-00658-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-022-00658-5