Abstract
Aim
To assess the immunohistochemical patterns of p53 in malignant surface epithelial tumours of ovary and correlate it with clinico-pathological parameters.
Methods
Forty cases of histopathologically proven surface epithelial carcinomas of the ovary were studied. Immunohistochemistry was done and expression of p53 was analysed and these findings were correlated with clinico-pathological parameters.
Results
p53 expression was found in 55% of malignant surface epithelial tumours of ovary. There was significant association of p53 expression with histological grade (p = 0.034) and omental deposits (p = 0.005).
Conclusion
p53 expression was associated with higher grade and omental deposits. Routine analysis of this gene could have profound implications on prognosis and could open new avenues for management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Of all the gynaecologic malignancies, surface epithelial ovarian carcinomas have the highest case fatality rate, as greater than two-thirds of patients have advanced disease during the time of diagnosis. Ovarian carcinoma is the fourth most frequent cause of death from cancer in women. It poses a significant surgical challenge needing meticulous and frequent complex therapies and is extremely challenging to the patient's physical and psychological equilibrium [1].
Ovarian cancer is a group of genetically, biochemically and morphologically distinct diseases. Patient survival depends on tumour type, tumour grade and International Federation of Gynaecology and Obstetrics (FIGO) stage. However, none of these prognostic factors offer a target for therapy [2]. Any mutant, overexpressed or abnormally expressed protein in cancer cells can be a target for cancer vaccines and/or CAR T cell therapy [3].
p53 is a tumour suppressor gene which is located on chromosome 17p13. It encodes a nuclear phosphoprotein which has a critical role as a negative regulator of the cell cycle and in initiating carcinogenesis. p53 mutation is one of the most frequent mutations among the heterogeneous group of ovarian malignancies which have a poorly defined etiopathogenesis [4].
Epithelial ovarian tumours showing p53 overexpression are significantly less sensitive to chemotherapy and are more aggressive than those with functional p53 [5].
This study was taken up to correlate p53 expression with histological subtype, grading, staging of malignant surface epithelial tumours and to investigate the value of p53 as a marker for prognostic purposes.
Patients and Methods
In the present exploratory study, forty malignant surface epithelial ovarian tumours were studied. Patients who had received neo-adjuvant chemotherapy prior to surgery were excluded. Ethical clearance from the institutional ethical committee was obtained. The patients were not directly involved in the study. The patient’s clinical history and investigations were retrieved from the archives. Data were collected as per case report forms. A detailed gross examination was performed to record the tumour size, cut section, consistency, secondary changes and capsular breech.
The specimens were fixed in 10% neutral buffered formalin followed by paraffin embedding and staining with haematoxylin and eosin (H&E). Sections were studied to evaluate histologic type, histologic grade and architectural pattern.
Immunohistochemistry for p53 was done on 4-μm-thick paraffin-embedded wax sections on poly-l lysine-coated slides. Antigen retrieval was done in tri sodium citrate buffer at pH 6. Monoclonal antibody FLEX Monoclonal Mouse Anti-Human p53 Protein Clone DO-7 Ready to Use, Code IS616 was used for p53 antigen detection by one step horseradish peroxidase (HRP) polymer method. The sections were washed with wash buffer and counterstained with haematoxylin and again rinsed in water for five minutes. Sections from colorectal carcinomas were taken as positive control.
Brown granular nuclear reactivity was taken as positive. Tumours with nuclear immunoreactivity of more than 10% were considered positive [6]. The entire section was screened to determine the region with the maximum proportion of stained nuclei. p53 over expression was recorded as intensity of staining and score based on the percentage of the cells staining positively.
Staining intensity was scored on a 4-point scale, negative (no staining): 0, weak: 1+, moderate: 2+ and strong: 3+.
The intensity score was based on the proportion of tumour cells positively stained as < 10%: 0, 11–25%: 1+, 26–50%: 2+ and 51–75%: 3+ and ≥ 76%: 4+.
The relationship of clinicopathological parameters with p53 over expression was analysed separately for staining intensity and intensity score.
Statistical Analysis
Statistical analyses were performed using IBM Statistical Package for the Social sciences (SPSS) Statistics for Windows, version 24.0 (IBM Corp., Armonk, New York). Independent sample t test and one-way ANOVA were employed to study the correlation of p53 as a continuous variable with known prognostic factors (tumour type, tumour grade, CA125 levels, p53 overexpression), and correlation of p53 as a categorical variable was determined by Chi-square test and independent samples t test. Data was expressed as mean. A p value of ≤ 0.05 was taken to be statistically significant.
Results
The demographic data, clinical, and histopathological parameters are given in Table 1.
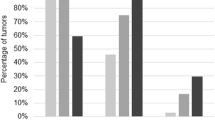
Out of 40 cases, 22 (55%) were p53 positive and 18 (45%) were p53 negative. p53 expression in different histological types of ovarian carcinomas and the intensity scores are given in Table 2, Figs. 1, 2, and 3. Out of the 22 positive cases, 20 (91%) belonged to the high-grade group and two (9%) belonged to the low-grade group. There was no statistically significant association between histological type of tumour and p53 intensity (p = 0.73). The staining pattern of the serous groups was found to be more intense as well as diffuse in comparison with the other types. There was a statistically significant association between p53 intensity and grade of the tumour with the p value being 0.04.
The expression of p53 was higher in high-grade serous group as compared to low-grade serous group. This difference was found to be statistically significant (p = 0.043). Among the serous carcinomas, a higher intensity score of 3+ and 4+ was seen in 12 out of the 16 positive cases (75%) and a much lesser intensity score of 1+ and 2+ in the rest four (25%) cases. Two out of the three p53 positive cases of mucinous carcinoma expressed an intensity of 3+, whereas one expressed 1+. Both cases of clear cell carcinoma were high grade and had a p53 intensity of 3+.
Omental deposits were documented in 22 cases, and all these 22 cases were positive for p53 with the p value being 0.005.
Twenty-six (65%) cases were categorised as FIGO stage III, seven (17.5%) as stage I, four (10%) as stage II and three as (7.5%) stage IV. Thus, 11 cases belonged to the early stage (FIGO stages I and II), whereas 29 cases belonged to the advanced stage (FIGO stages III and IV) (Table 3). In the present study, although 18/22 (81.8%) p53 positive cases belonged to the advanced stage, no statistically significant association was found between p53 positivity and FIGO stage at diagnosis (p value − 0.564). Among the eighteen p53 negative cases, 11 (61.1%) belonged to advanced stage, whereas seven (38.9%) cases belonged to the early stages.
The mean CA 125 level in malignant tumours was found to be 437.06 U/ml. Twenty (57%) cases with CA125 levels beyond the cut-off value were found to have a p53 intensity score of 3+, whereas only one (3%) case had an intensity score of 2+. Fourteen (40%) cases were negative for p53. A significant relationship between raised CA125 levels and p53 overexpression was found with the p value being 0.009.
Discussion
Ovarian cancer is the seventh most common cancer in women worldwide, and India has the second highest incidence of ovarian cancer globally [7]. Ovarian cancer has the least survival rate of all gynaecological cancers as it is characterised by absence of awareness of symptoms and diagnosis at a late stage [8].
Various genetic alterations have been identified in ovarian cancers, but their prognostic role is uncertain. The frequent occurrence of p53 mutation indicates a crucial role in ovarian carcinogenesis. Although available data indicate that p53 loss may have a prognostic significance in different types of human cancers, including lung and prostate, a similar outcome in ovarian cancers is unclear [5].
Unlike normal p53 protein which is rapidly removed from the nucleus, mutant forms have a prolonged half-life, which promotes intranuclear accumulation and can detected by immunohistochemistry [9].
Although various studies have found p53 overexpression in older age groups, statistically significant association has not been proven [8, 10]. The possible explanation for p53 overexpression with increasing age could be that there is accumulation of somatic mutation and accompanied by loss of heterozygosity on chromosome 17. Patients with Li-Fraumeni syndrome are found to have an earlier age at presentation. In the present study, no association was found between age and p53 expression (p value 0.407).
Presently, based on histopathology, immunohistochemistry and molecular genetic analysis, the common types of surface epithelial tumors are: high-grade serous carcinomas (70%), endometrioid carcinomas (10%), clear cell carcinomas (10%), mucinous carcinomas (3%) and low-grade serous carcinomas (< 5%) [6]. These tumours are considered as intrinsically heterogeneous diseases, as indicated by differences in genetic and epidemiological risk actors, precursor lesions, pattern of spread, molecular changes in oncogenesis, response to chemotherapy and prognosis [11,12,13,14].
In the present study, the expression of p53 was higher in high-grade serous group as compared to low-grade serous group and was statistically significant. They appear to originate from fimbrial fallopian tube epithelium and require p53 dysfunction to develop their characteristic genomic instability [15]. However, many studies have observed a distinct p53 expression with various histological types but have failed to prove a statistically significant association with the same [7, 10, 16].
The significant association of p53 overexpression with higher tumour grades, as found in the present study and similar other studies [8, 17, 18], could probably be explained by the fact that the tumour biology alters with evolution of the disease process, thus giving aggressiveness to the tumour.
In a study on the prognostic importance of p53, bcl-2 and bax in early-stage epithelial ovarian carcinomas treated with adjuvant chemotherapy, positive p53 staining was seen most frequently in serous papillary carcinomas, and less frequently in clear cell carcinomas. Tumour grade (p = 0.014) and primary persistent disease (p = 0.016) were significantly associated with p53 status. A similar significant association was found between the p53 status and the cancer-specific and overall survival rate (p = 0.007; p = 0.020). The most favourable survival rate was seen in the subgroup of patients, whose tumours were negative for p53. Conversely, overexpression of p53 in the tumours was related to an increased possibility of mortality [19].
Tumour staging is an effort to stratify patients into prognostic groups based on the extent and volume of the disease at the time of diagnosis. Stage has been proven to be a strong predictor of outcome for all malignancies of the female gynaecological system with survival diminishing as stage at diagnosis increases [8]. The present study is in concordance with various studies, reiterating the fact that most ovarian cancers present at a later stage at the time of diagnosis and hence might account for the poor prognosis of these tumours [8, 20,21,22,23].
Studies by the Gynaecologic Oncology Group (GOG) and others have revealed that overexpression of p53 protein is linked to inferior survival in advanced ovarian cancers. p53 overexpression is significantly greater in advanced stage III/IV disease (40 to 60%) compared with stage I disease (10 to 20%). This endorses the fact that the greater frequency of p53 overexpression in advanced stage cancers as indicative of a late event in ovarian carcinogenesis [24]. According to our observation, p53 overexpression was not significantly associated with FIGO staging at diagnosis, as supported by many other studies [9, 10, 20, 23, 25, 26].
Intriguingly, a considerable association between p53 expression and FIGO stage was found, revealing that p53 protein levels have an increased negative impact on disease-specific survival (DSS) and progression-free survival (PFS) in FIGO stage I contrasted to FIGO stages II, III and IV. FIGO stage I patients who did not receive adjuvant chemotherapy after primary surgery and who showed tumour progression had varying p53 expression. In contrast, as many as 89% of the patients with no progression after primary surgery had low expression of the p53 protein, indicating that p53 expression may have clinical significance for low risk patients. Only ten patients (11%) who had low p53 expression experienced tumour progression, whereas 81 patients (89%) had no tumour progression. Therefore, it may be inferred that p53 could be helpful for stratifying FIGO stage I patients into a p53 high-risk group who may need additional treatment after primary surgery to avoid relapse, and a low-risk patient group (p53 ≤ 5%) who is most often treated without adjuvant chemotherapy. A careful follow-up of this low-risk patient group may help identify the section of patients who progress despite a favourable p53 status and initiate therapeutic treatment [18].
The present study has found a significant association between mean CA125 levels at the time of diagnosis and p53 overexpression with a p value of 0.009. Conflicting results have been found with other similar studies [18, 27]. However, further large-scale research is essential to establish a definitive relationship between CA125 levels, p53 expression and other prognostic markers.
In the present study, all cases with omental deposits showed p53 expression. Adhesion of cancer cells to the mesothelial cells of the peritoneum is considered as an initial, critical step for the metastatic spread of ovarian cancer. The mutant p53 by stimulating the intracellular integrin signalling may confer a metastatic advantage to the ovarian cancer cells in the peritoneal cavity. Thus, targeted inhibition of the mutant p53 or β4-integrin signalling may offer novel therapeutic strategies in ovarian cancer [28].
A study on prognostic value of hormonal receptors, p53, ki67 and HER2/neu expression in epithelial ovarian carcinoma has found a shorter time to progression in patients with p53 expression; however, a statistically significant relation could not be demonstrated between p53 overexpression and PFS [29].
Conclusion
Surface epithelial ovarian carcinomas have a dismal prognosis among the malignant tumours of female genital tract. The management of epithelial ovarian cancer requires expertise in surgery, chemotherapy, imaging, histopathology and palliation. Specialist multidisciplinary teamwork is necessary to attain best possible results. The histopathology of epithelial ovarian tumours is varied, and each epithelial ovarian cancer subtype has genetic mutations that are being evaluated for their potential to predict the effectiveness of targeted treatments. Use of established prognostic markers and detection of newer markers could be beneficial in guiding the therapy and strengthening the follow-up of patients. These results could also provide improved awareness of the biological behaviour of ovarian tumours.
High grade and metastasis have been long regarded as poor prognostic indicators. In the present study, a statistically significant association was seen between p53 expression, grade of the tumour and omental deposits. It has also been shown that loss of functional p53 might bestow a chemoresistant phenotype as p53 plays a role in chemotherapy-induced apoptosis. p53 could hence be included in the routine panel of IHC markers which would steer the clinician better in customised chemotherapy and expect the probable outcome and prognosis of ovarian cancers. However, prospective studies are required to assess the prognostic value.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Carter JH, Deddens JA, Mueller G, et al. Transcription factors WT1 and p53 combined: a prognostic biomarker in ovarian cancer. Br J Cancer. 2018;119(4):462–70.
Cheever MA, Allison JP, Andrea Ferris AS, et al. The prioritization of cancer antigens: a National Cancer Institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–37.
Amanullah NAR, Poothiode U, Vilasiniamma L. Expression of p53 in epithelial ovarian tumors. Indian J Pathol Microbiol. 2020;63:235–40.
Buttitta F, Marchetti A, Gadducci A, et al. p53 alterations are predictive of chemoresistance and aggressiveness in ovarian carcinomas: molecular and immunohistochemical study. Br J Cancer. 1997;75(2):230–5.
Vaughan S, Coward JI, Bast RC Jr, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11(10):719–25.
Puri S, Chadha V, Pandey AK. Epidemiology of ovarian tumours in Northern India—a tertiary hospital-based study. Indian J Community Fam Med. 2018;4:37–41.
Sylvia MT, Kumar S, Dasari P. The expression of immunohistochemical markers oestrogen receptor, progesterone receptor, Her-2-neu, p53 and Ki-67 in epithelial ovarian tumours and its correlation with clinicopathologic variables. Indian J Pathol Microbiol. 2012;55(1):33–7.
Kmet LM, Cook LS, Magliocco AM. A review of p53 expression and mutation in human benign, low malignant potential, and invasive epithelial ovarian tumours. Cancer. 2003;97(2):389–404.
Hamdi EA, Saleem SH. P53 expression in ovarian tumours: an immunohistochemical study. Ann Coll Med Mosul. 2012;38(2):73–9.
Prat J. New insights into ovarian cancer pathology. Ann Oncol. 2012;23(Suppl 10):X111–7.
Ezzati M, Abdullah A, Shariftabrizi A, et al. Recent advancements in prognostic factors of epithelial ovarian carcinoma. Int Sch Res Not. 2014;2014:953509.
Engel J, Eckel R, Schubert-Fritschle G, et al. Moderate progress for ovarian cancer in the last 20 years: prolongation of survival, but no improvement in the cure rate. Eur J Cancer. 2002;38(18):2435–45.
Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460(3):237–49.
Kohn EC, Ivy SP. Whence high-grade serous ovarian cancer. Am Soc Clin Oncol Educ Book. 2017;37:443–8.
Kanthikar SN, Dravid NV, Deore PN, et al. Clinico-histopathological analysis of neoplastic and non-neoplastic lesions of the ovary: a 3-year prospective study in Dhule, North Maharashtra, India. J Clin Diagn Res. 2014;8(8):FC04–7. https://doi.org/10.7860/JCDR/2014/8911.4709.
Yemelyanova A, Vang R, Kshirsagar M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24(9):1248–53.
Rask L, Høgdall CK, Kjaer SK, et al. Association of CD31 and p53 with survival of ovarian cancer patients. Anticancer Res. 2019;39(2):567–76.
Skirnisdóttir I, Seidal T, Gerdin E, Sorbe B. The prognostic importance of p53, bcl-2 and bax in early stage epithelial ovarian carcinoma treated with adjuvant chemotherapy. Int J Gynecol Cancer. 2002;12(3):265–76.
Klemi PJ, Pylkkanen L, Kiilholma P, et al. p53 protein detected by immunohistochemistry as a prognostic factor in patients with epithelial ovarian carcinoma. Cancer. 1995;76(7):1201–8.
Aune G, Stunes AK, Tingulstad S, et al. The proliferation markers Ki-67/MIB-1, phosphohistone H3, and survivin may contribute in the identification of aggressive ovarian carcinomas. Int J Clin Exp Pathol. 2011;4(5):444–53.
Anttila MA, Kosma VM, Hongxiu J, et al. p21/WAF1 expression as related to p53, cell proliferation and prognosis in epithelial ovarian cancer. Br J Cancer. 1999;79(11–12):1870–8.
Korkolopoulou P, Vassilopoulos I, Konstantinidou AE, et al. The combined evaluation of p27Kip1 and Ki-67 expression provides independent information on overall survival of ovarian carcinoma patients. Gynecol Oncol. 2002;85(3):404–14.
Cruickshank DJ, Fullerton WT, Klopper A. The clinical significance of pre-operative serum CA 125 in ovarian cancer. Br J Obstet Gynaecol. 1987;94(7):692–5.
Werness BA, Freedman AN, Piver S, et al. Prognostic significance of p53 and p21 (waf1/cip1) immunoreactivity in epithelial cancers of the ovary. Gynecol Oncol. 1999;75(3):413–8.
Kupryjańczyk J, Bell DA, Yandell DW, et al. p53 expression in ovarian borderline tumours and stage I carcinomas. Am J Clin Pathol. 1994;102(5):671–6.
Anderson KS, Wong J, Vitonis A, et al. p53 autoantibodies as potential detection and prognostic biomarkers in serous ovarian cancer. Cancer Epidemiol Biomark Prev. 2010;19(3):859–68.
Lee J-G, Ahn J-H, Kim TJ, et al. Mutant p53 promotes ovarian cancer cell adhesion to mesothelial cells via integrin β4 and Akt signals. Sci Rep. 2015;5:12642.
García-Velasco A, Mendiola C, Sánchez-Muñoz A, Ballestín C, Colomer RH. Prognostic value of hormonal receptors, p53, ki67 and HER2/neu expression in epithelial ovarian carcinoma. Clin Transl Oncol. 2008;10(6):367–71.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
Dr. RVN contributed to literature search, data acquisition, data analysis, manuscript preparation and statistical analysis. Dr. SS contributed to concepts, design, definition of intellectual content, manuscript editing, manuscript review and guarantor.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Ethics Approval
The study was approved by the institutional ethics committee of JSS Medical College, Mysore.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nagaraj, R.V., Satish, S. A Study of Expression and Significance of p53 in Malignant Ovarian Surface Epithelial Tumours. Indian J Gynecol Oncolog 19, 32 (2021). https://doi.org/10.1007/s40944-021-00506-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-021-00506-y