Abstract
Backgrounds
Matrix metalloproteinases (MMP) and fascin-1 play roles in epithelial–mesenchymal transition and tumour invasion.
Aims
This study was performed to investigate the relationships of fascin-1 and MMP-9 expression with prognostic parameters in endometrioid-type endometrial carcinoma (EEC).
Methods
A total of 100 cases of EEC were included in the study. Tissues were stained with antibodies against fascin-1 and MMP-9. The relationships between the immunohistochemical findings and clinicopathological prognostic parameters of EEC were examined.
Results
Tumour diameter was significantly related to lymphovascular invasion, FIGO stage and FIGO grade (p < 0.05). A tumour size > 2 cm was associated with a poor prognosis. The staining score for fascin-1 was ≤ 5 in 81 cases and > 5 in 19 cases (p > 0.05), and that for MMP-9 was ≤ 5 in 39 cases and > 5 in 61 cases (p > 0.05). Neither fascin-1 nor MMP-9 expression was significantly related to any of the clinicopathological parameters examined.
Conclusion
There were no significant relationships of fascin-1 and MMP-9 expression with the clinicopathological parameters of EEC. The results of this study suggested that these molecules do not contribute to the clinical behaviour of EECs. A tumour size > 2 cm is associated with a poor prognosis in EEC patients. To verify these results, more studies are needed with larger patient groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introductıon
Endometrial carcinoma (EC) is the fifth most common cancer in women worldwide and ranks second in incidence among gynaecological cancers after cervical uterine cancer [1]. EC is the fourth most prevalent cancer and the first most prevalent gynaecologic cancer among women in Turkey according to the 2014 cancer statistics [2].
ECs are divided into two groups according to their clinical, endocrine and morphological features. The first group develops due to exposure to unopposed oestrogen, and the precursor lesion is endometrial hyperplasia. The prototype of this group is endometrioid-type EC (EEC). The other group is associated with p53 mutation, and its prototype is serous carcinoma.
The endometrioid type accounts for 80% of endometrial cancers. Most are well to moderately differentiated and have a good prognosis. The 5-year survival rate of patients with International Federation of Gynecology and Obstetrics (FIGO) stage I EEC is 87% [3].
The mechanism of cancer invasion is an area of active research. Determining the aggressiveness of cancer in early-stage disease is very important in terms of predicting the clinical course of the disease and developing targeted therapies.
Epithelial cells in carcinoma tissue invade stromal tissues by gaining mesenchymal features via epithelial–mesenchymal transition (EMT) [4, 5]. Studies that examined the mechanism of invasion have identified complex pathways involving many mediators, including the gelatinase subtype of matrix metalloproteinases (MMP-2 to MMP-9), involved in local invasion [6], and fascin-1, which was demonstrated in vitro to play an important role in EMT [7].
Understanding the invasive potential of cancer is very important because of the high prevalence rate and poor prognosis of some types of EEC. This study was performed to evaluate the relationships of fascin-1 and MMP-9 expression with prognostic parameters, as well as invasive potential, in patients with EEC.
Materıals and Methods
The archive of İnönü University Turgut Özal Medical Center Medical Pathology Laboratory was scanned, and 106 cases diagnosed with EEC between 2011 and 2017 were retrospectively included in this study. All patients underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy. Bilateral pelvic and para-aortic lymph node dissection was also performed in 88 of these cases. Six cases without myometrial invasion were excluded from the study.
The clinicopathological prognostic parameters, FIGO grade and stage, myometrial invasion depth, lymphovascular invasion, cervical invasion, lymph node involvement and positive peritoneal cytology were evaluated. In addition, data regarding age, tumour size and distant organ metastases were obtained from the medical files of the patients (Table 1).
Immunohistochemical staining using antibodies against fascin-1 and MMP-9 was performed using a fully automated immunohistochemical staining device (BenchMark ULTRA; Ventana Medical Systems, Tucson, AZ, USA). Table 2 lists information such as the clone numbers, dilutions and production site of the antibodies used in the study.
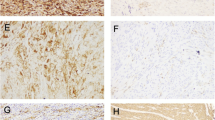
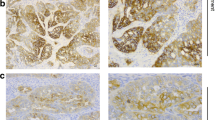
The prevalence and level of fascin-1 and MMP-9 cytoplasmic staining were evaluated. The average percentage of cells showing positive staining for both fascin-1 and MMP-9 in five areas at 100 × magnification was calculated. Staining was scored semi-quantitatively: 0, negative; 1, focal positivity (< 25%); 2, moderate positivity (26–50%); 3, diffuse positivity (> 50%).
The intensity of staining was scored as follows: 1, mild; 2, medium; 3, strong. The two staining scores were multiplied, and the total staining score was between 0 and 9. In the statistical analysis, cases were divided into two groups according to a total staining score ≤ 5 or > 5. This grouping was preferred in order to include the cases with the least score 2 in the staining extent and intensity scores to be in the highly stained group.
Statistical analyses were performed using SPSS for Windows (SPSS Inc., Chicago, IL, USA). Quantitative variables are presented as the arithmetic mean ± standard deviation, and qualitative variables are presented as numbers (n) and percentages (%). The Shapiro–Wilk normality test showed that the quantitative data did not have a normal distribution (p < 0.05). Quantitative variables were analysed using the unpaired t test, Mann–Whitney U test and Kruskal–Wallis variance analysis. Qualitative variables were analysed using Pearson’s Chi-square test, Monte Carlo simulation of Pearson’s Chi-square test and Fisher’s exact test. In all analyses, p < 0.05 was taken to indicate statistical significance.
Results
The 100 patients included in the study had a mean age of 59.81 years (range 37–87 years). The mean tumour diameter was 3.80 cm (range 0.5–11.5 cm).
According to the FIGO grading system, 34 cases were classified as grade 1, 56 as grade 2 and 10 as grade 3; 67 were classified as stage IA, 18 as stage IB, 3 as stage II, 10 as stage III, and two as stage IV. There were statistically significant relationships of the FIGO grade with both FIGO stage and tumour diameter (p < 0.05) (Table 3).
A statistically significant relationship was found between lymphovascular invasion and tumour size (p < 0.05). However, tumours were larger in patients with myometrial invasion, cervical invasion, adnexal involvement, pelvic and/or para-aortic lymph node metastasis, distant metastasis and positive peritoneal cytology, but the relationships were not statistically significant (p > 0.05) (Table 4).
Distant organ metastasis was detected in 2 of 100 patients (2%). The lung hilar region lymph nodes were involved in both cases.
There were no significant correlations of the fascin-1 and MMP-9 staining scores with FIGO grade or stage (p > 0.05) (Table 5) (Figs. 1, 2).
There were no statistically significant relationships of fascin-1 and MMP-9 expression scores with myometrial invasion, cervical invasion, adnexal involvement, lymphovascular invasion, regional lymph node involvement, distant metastasis or positive peritoneal cytology (p > 0.05) (Table 6).
Dıscussıon
The incidence of EC is second among gynaecological cancers worldwide and the first in developed countries [1]. According to the 2014 cancer statistics, the incidence of EC in Turkey was 9.8/100,000 [2].
EEC generally has a better prognosis than other uterine neoplasms. Some subgroups, however, have a rather poor prognosis. The 5-year survival rate of patients with FIGO stage I disease is 87%. However, high FIGO stage, deep myometrial invasion and lymph node metastasis are associated with a poor prognosis and survival.
Distant metastasis is an important cause of death in EEC patients. Understanding the pathophysiology of the mechanism of metastasis will be useful in guiding treatment and determining prognosis.
Metastatic cancer cells are characterised by increased invasion and migration. Cell invasion in tumour progression is largely related to EMT. In the EMT process, epithelial cells lose their basal apical polarity, adopt the form of spindle cells and gain mobility. In addition, transformed cells acquire new markers, such as smooth muscle actin, fibronectin and vimentin and show increased activities of MMPs (MMP-2, MMP-3 and MMP-9) [8].
MMP-9 is a zinc-dependent endopeptidase that regulates extracellular matrix protein metabolism. Tumour invasion is associated with angiogenesis and metastasis, and MMP-9 may be associated with invasion and tumour progression in many organ cancers [9]. Expression of MMPs in carcinomatous tumour cells may be an indicator of tumour aggressiveness [8].
Planaguma et al. [10] reported that MMP-9 is expressed on the invasive surface of endometrial and ovarian cancers and plays an important role in the progression of carcinogenesis.
The role of MMP-9 in the benign-to-malignant differentiation of endometrial lesions has been evaluated, and MMP-9 expression has been detected in almost all EECs but at varying frequencies [17]. MMP-9 expression was positive in all cases in the present study, which was consistent with the high MMP-9 expression in EEC reported previously [10].
Some previous immunohistochemical studies of EECs using anti-MMP-9 antibodies indicated statistically significant relationships between some clinicopathological prognostic parameters and MMP-9 expression [11,12,13,14,15] (Table 7). However, other studies did not detect significant relationships between MMP-9 expression and clinicopathological prognostic parameters [11,12,13,14,15,16,17] (Table 7). In the present study, MMP-9 expression showed no statistically significant relationships with any clinicopathological parameter examined (p > 0.05).
Fascin-1 is a 55-kDa actin-bundling protein that plays a key role in the stability and regulation of cell protrusions and other actin-based structures involved in cell motility, migration and invasion. Its expression has been reported to be associated with increased invasion and metastatic potential in mouse xenograft tumour models and increased migratory capacity in carcinoma cells in culture [6].
A relationship between increased fascin-1 expression and aggressive clinical course has been reported in many organ cancers. Fascin-1 may be an early marker of metastatic potential in aggressive carcinomas. Fascin-1 expression has been suggested to increase the risk of mortality by 2.5 fold in breast, colorectal and oesophageal carcinomas [18]. It has also been shown to be associated with an increased risk of metastasis in colorectal and gastric carcinomas [18].
Fascin-1 has been investigated most extensively in ovarian tumours among gynaecological cancers and has been reported to be associated with high tumour grade and stage, drug resistance in serous carcinoma and poor survival and prognosis [19]. On the other hand, there have been very few studies of fascin-1 in EEC.
Stewart et al. [20] found fascin-1 expression in all cases of EEC examined. Gün et al. [21] reported that fascin-1 expression in EEC was significantly related to both tumour grade and neural invasion (Table 8). However, no significant relationships of its expression with tumour size, myometrial depth of invasion, lymphovascular invasion and tumour stage have been reported. In the present study, there were no statistically significant relationships between fascin-1 expression and clinicopathological prognostic factors.
When the relationship between MMP9 expression and survival was investigated, Mihalj et al. [15] indicated these two parameters significantly related. The relationship between MMP9 expression and survival was not found to be significant in the study conducted by Yu et al. [13] and Graesslin et al. [17]. However, there is no survival research with fascin-1.
There have been many studies regarding MMP-9 and fascin-1 expression in EC [10,11,12,13,14,15,16,17,18,19,20,21]. Expression of MMP-9 and fascin-1 only in EEC cases was not examined before. Therefore, it would not be appropriate to compare these previous studies with the present study. We identified no relationship of MMP-9 or fascin-1 expression with prognostic factors of EEC.
The numbers of cases in studies reported in the literature range from 20 to 128 [10,11,12,13,14,15,16,17,18,19,20,21]. In most of those studies, the number of cases was insufficient to draw definitive conclusions regarding prognosis. The number of cases included in the present study was greater than those of most previous studies reported to date.
Öz et al. [22] did not find a statistically significant relationship between tumour diameter and lymph node metastasis. However, in the present study, statistically significant relationships were detected between tumour diameter and FIGO grade, FIGO stage and lymphovascular invasion (p < 0.05).
Therefore, MMP-9 and fascin-1 appear to play roles in cancer invasion. However, as tumour invasion is the result of complex interactions among many molecules, the expression of proteins such as MMP-9 or fascin-1 alone may not be sufficient to detect the invasive capacity of cancer cells.
The significant relationships between tumour diameter and many clinicopathological parameters detected in the present study suggest that patients with tumours > 2 cm in diameter have a poorer prognosis.
Conclusion
There were no significant relationships of fascin-1 and MMP-9 expression with the clinicopathological parameters of EEC. This study’s results suggest that MMP9 and fascin-1 do not contribute to the clinical behaviour of EECs. A tumour size > 2 cm is associated with a poor prognosis in EEC patients. Although the number of patients in this study is higher than the previous studies, these results should be verified by studies with invitro and invivo methods on larger patient groups.
References
Fact Sheets by Population [Internet]; Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed 25 Dec 2017
2014 Yılı Türkiye Kanser İstatistikleri [Internet]. Available from: https://hsgm.saglik.gov.tr/tr/kanser-istatistikleri/yillar/2014-yili-turkiye-kanser-istatistikleri.html
Kurman RJ, Hedrick EL, Ronnett BM, Blaustein A. Endometrial Carcinoma. In: Kurman RJ, Hedrick EL, Ronnett BM, editors. Blaustein’s pathology of the female genital tract. 6th ed. New York, Dodrecht, Heidelberg, London: Springer; 2011. p. 319–476.
Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118(19):4325–6.
Abouhashem NS, Ibrahim DA, Mohamed AM. Prognostic implications of epithelial to mesenchymal transition related proteins (E-cadherin, Snail) and hypoxia inducible factor 1α in endometrioid endometrial carcinoma. Ann Diagn Pathol. 2016;22:1–11.
Akter H, Park M, Kwon O-S, Song EJ, Park W-S, Kang M-J. Activation of matrix metalloproteinase-9 (MMP-9) by neurotensin promotes cell invasion and migration through ERK pathway in gastric cancer. Tumor Biol Agust. 2015;36(8):6053–62.
Hashimoto Y, Skacel M, Adams JC. Roles of fascin in human carcinoma motility and signaling: prospects for a novel biomarker? Int J Biochem Cell Biol. 2005;37(9):1787–804.
Makker A, Goel MM. Tumor progression, metastasis, and modulators of epithelial–mesenchymal transition in endometrioid endometrial carcinoma: an update. Endocr Relat Cancer. 2016;23(2):R85-111.
Zhou X, Zhang H, Han X. Role of epithelial to mesenchymal transition proteins in gynecological cancers: pathological and therapeutic perspectives. Tumor Biol. 2014;35(10):9523–30.
Planagumà J, Liljeström M, Alameda F, et al. Matrix metalloproteinase-2 and matrix metalloproteinase-9 codistribute with transcription factors RUNX1/AML1 and ETV5/ERM at the invasive front of endometrial and ovarian carcinoma. Hum Pathol. 2011;42(1):57–67.
Di Nezza LA, Misajon A, Zhang J, et al. Presence of active gelatinases in endometrial carcinoma and correlation of matrix metalloproteinase expression with increasing tumor grade and invasion: MMPs in Endometrial Carcinoma. Cancer. 2002;94(5):1466–75.
Aglund K, Rauvala M, Puistola U, et al. Gelatinases A and B (MMP-2 and MMP-9) in endometrial cancer—MMP-9 correlates to the grade and the stage. Gynecol Oncol. 2004;94(3):699–704.
Yu F, Jiang Q, Zhou Y, et al. Abnormal expression of matrix metalloproteinase-9 (MMP9) correlates with clinical course in Chinese patients with endometrial cancer. Dis Markers. 2012;32(5):321–7.
Srdelić Mihalj S, Kuzmić-Prusac I, Zekić-Tomaš S, Šamija-Projić I, Čapkun V. Lipocalin-2 and matrix metalloproteinase-9 expression in high-grade endometrial cancer and their prognostic value. Histopathol Agust. 2015;67(2):206–15.
Inoue Y, Abe K, Obata K, et al. Immunohistochemical studies on matrix metalloproteinase-9 (MMP-9) and type-IV collagen in endometrial carcinoma. J Obstet Gynaecol Res. 1997;23(2):139–45.
Grybos A, Bar J. The relationships between the immunoexpression of KAI1, MMP-2, MMP-9 and steroid receptors expression in endometrial cancer. Folia Histochem Cytobiol. 2014;52(3):187–94.
Graesslin O, Cortez A, Fauvet R, Lorenzato M, Birembaut P, Daraï E. Metalloproteinase-2, -7 and -9 and tissue inhibitor of metalloproteinase-1 and -2 expression in normal, hyperplastic and neoplastic endometrium: a clinical-pathological correlation study. Ann Oncol. 2006;17(4):637–45.
Tan VY, Lewis SJ, Adams JC, Martin RM. Association of fascin-1 with mortality, disease progression and metastasis in carcinomas: a systematic review and meta-analysis. BMC Med. 2013;11(1):1.
Lin C, Su H-Y, Tsai W-C, Sheu L-F, Jin J-S. Association of cortactin, fascin-1 and epidermal growth factor receptor (EGFR) expression in ovarian carcinomas: correlation with clinicopathological parameters. Dis Markers. 2008;25(1):17–26.
Stewart CJR, Crook ML, Manso L. Fascin expression in low-grade uterine endometrioid adenocarcinoma: correlation with microcystic, elongated and fragmented (MELF)-type alteration at the deep invasive margin: Fascin in endometrial carcinoma. Histopathology. 2011;59(1):73–80.
Gun BD, Bahadir B, Bektas S, et al. Clinicopathological significance of fascin and CD44v6 expression in endometrioid carcinoma. Diagn Pathol. 2012;7(1):1.
Oz M, Korkmaz V, Meydanli MM, Sari ME, Cuylan ZF, Gungor T. Is tumor size really important for prediction of lymphatic dissemination in grade1 endometrial carcinoma with superficial myometrial invasion? Int J Gynecol Cancer. 2017;27(7):1393–8.
Acknowledgements
This project was realised with the contribution of Inonu University Scientific Research Projects Coordination Unit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tecellioglu, F.S., Akpolat, N. & Sahin, N. Mmp-9 and Fascin-1 Expression in Endometrioid-Type Endometrial Carcinoma and Their Prognostic Value. Indian J Gynecol Oncolog 19, 24 (2021). https://doi.org/10.1007/s40944-020-00492-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-020-00492-7