Abstract
Purpose
Interval cytoreduction (IC) after a course of neoadjuvant chemotherapy is the preferentially followed management for advanced epithelial ovarian cancers. When planning an interval cytoreduction, it is important to assess the probability of achieving optimal surgery so as to avoid futile laparotomies. Several assessment techniques like CECT of the abdomen and pelvis, tumour marker-based response assessment and diagnostic laparoscopy are being used for this purpose with varying results. In our institute, we evaluated the utility of CA-125 value to predict the possibility of optimal surgery in advanced carcinoma ovary after neoadjuvant chemotherapy (NACT).
Methods
The data of treated advanced epithelial ovarian cancer were collected from the hospital records for the study period between February 2015 and February 2018 (n = 83). The cut-off value and percentage reduction of CA 125 in predicting optimal cytoreduction were analysed by receiver operator curve (ROC). These values were validated prospectively from March 2018 to September 2019 in 60 cases treated during this period. Statistical analysis was performed using SPSS 25.
Results
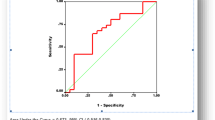
ROC analysis revealed CA-125 value of 88.5 U/ml (i.e. twice the upper limit of normal value) had sensitivity of 100% and specificity of 94% (AUC = 0.98; P value < 0.001) to predict optimal cytoreduction. Similarly, 90.46% reduction in CA-125 value had a sensitivity of 72% and specificity of 71% (AUC = 0.72; P value = 0.047) to predict optimal IC. In the prospective validation, among those who had post-NACT CA-125 value of less than twice the upper limit of normal value, 98.14% (53 out of 54) had optimal surgery with a P value of 0.0001. Among those with more than 90% reduction in CA 125 value after neoadjuvant chemotherapy 94.8% (55 out of 58) had optimal cytoreduction (P value—0.012).
Conclusion
The post-chemo CA 125 value and percentage reduction of CA 125 after neoadjuvant chemotherapy have high sensitivity and positive predictive value in predicting optimal interval cytoreduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial ovarian cancer is the most lethal gynaecological malignancy, majority being advanced at presentation [1]. Though no survival differences were proven between primary and interval cytoreduction (IC), the latter is preferred in advanced disease as this increases the possibility of optimal surgery and has less perioperative morbidity and mortality. When planning an interval cytoreduction, it is important to assess the probability of achieving optimal surgery so as to avoid futile laparotomies. Several assessment techniques like CECT of the abdomen and pelvis, tumour marker-based response assessment and diagnostic laparoscopy are being used for this purpose with varying results [2]. Diagnostic laparoscopy is considered ideal, but it requires hospital admission, general anaesthesia with associated costs. In our institute, we evaluated the utility of CA-125 value to predict the possibility of optimal surgery in advanced carcinoma ovary after neoadjuvant chemotherapy (NACT).
Materials and Methods
The data were collected from the hospital records for the study period between February 2015 and February 2018 (n = 83). All cases of biopsy proven advanced ovarian epithelial cancer patients who underwent interval cytoreduction after NACT were included in the study. The regimen used was Paclitaxol 175 mg/m2 IV over 3 h followed by Carboplatin AUC 5-6 over 1 h as 3 weekly cycle. The stage of the disease, imaging findings, preneoadjuvant CA 125 values, number of cycles of neoadjuvant chemotherapy, post-neoadjuvant CA 125 value and completeness of cytoreduction were recorded. The staging was done by means of clinical examination, CECT abdomen and pelvis, chest X-ray. The post-neoadjuvant CA 125 was done after 2 weeks of chemotherapy. The surgery was considered optimal, if no visible macroscopic disease was left behind. All the surgeries were performed by qualified surgical oncologists. The median preneoadjuvant CA 125 value, post-neoadjuvant CA 125 value in the optimal and suboptimal group was calculated. The cut-off value and percentage reduction of post-neoadjuvant CA 125 in predicting optimal cytoreduction were analysed by receiver operator curve (ROC). These values were validated prospectively from March 2018 to September 2019 in 60 cases treated during this period.
Statistics
Statistical analysis was performed using SPSS version 25. By univariate analysis, odds ratio (OR) and the P value were calculated. Mann–Whitney U test was used as the values were non-normally distributed. Receiver operating curve (ROC) calculated the area under curve and the best cut-off value to predict the likelihood of optimal cytoreduction. By using ROC curve, the sensitivity and specificity of achieving optimal cytoreduction were analysed for each value of post-neoadjuvant CA125. Similarly, ROC analysis was done to assess the sensitivity and specificity of percentage reduction of post-neoadjuvant CA 125. The utility of CA 125 value in predicting optimal IC was prospectively validated in the validation cohort using the Chi-square test.
Results
In the retrospective analysis, among 83 patients, 12 patients had stage IVA disease and the remaining had stage IIIC disease. Fifty-four patients were operated after 3 cycles of neoadjuvant chemotherapy, while the remaining 29 patients received 6 cycles of neoadjuvant chemotherapy prior to surgery. After 3 cycles of NACT, patients were assessed by means of clinical examination, CA 125 and CECT abdomen and pelvis. In those with high possibility of inoperability or suboptimal surgery, all 6 cycles of NACT are given, i.e. the 29 patients. Optimal interval cytoreduction was achieved in 91.6% (76 patients) and suboptimal surgery in 8.4% (7 patients). The median pre-chemotherapy CA 125 value in optimal and suboptimal group was 672.5U/ml (range 104–8340) and 986.0 U/ml (range 432–4532), respectively. Similarly, the median post-NACT CA 125 value in optimal and suboptimal group were 31.5U/ml (range 3.3–122) and 112.0U/ml (range 89–131), respectively. The median CA-125 values are shown in Table 1.
ROC analysis revealed post-neoadjuvant CA-125 value of 88.5U/mlhad sensitivity of 100% and specificity of 94% (AUC = 0.98; P value < 0.001) to predict optimal cytoreduction. Similarly, 90.46% reduction in CA-125 value had a sensitivity of 72% and specificity of 71% (AUC = 0.72; P value = 0.047) to predict optimal IC. The ROC curves are shown in Figs. 1 and 2.
In the prospective validation, among those who had post-NACT CA-125 value of less than 88.5U/ml, 98.14% (53 out of 54) had optimal surgery with a P value of 0.0001. Among those with more than 90% reduction in CA 125 value, 94.8% (55 out of 58) had optimal cytoreduction (P value—0.012). The results of validation are shown in Tables 2 and 3.
Discussion
More than two-thirds of the patients with epithelial ovarian cancer have advanced disease at presentation, and their 5-year survival rate is less than 28% [3]. NACT followed by surgery is preferentially performed in many centres, as it increases the possibility of optimal IC which is the single most important prognostic determinant [4].
Several approaches have been used to predict the possibility of achieving optimal IC and these includes clinical examination, CECT of the abdomen and pelvis, post-NACT CA-125 value, diagnostic laparoscopy or combination of the above.
Nelson criteria, Bristow and Ferrandina criteria and Sugarbaker criteria are some of the CT-based criteria. Nelson CT-based criteria predicted the optimal surgery in only 69% cases [5]. Bristow and Ferrandina et al. evaluated the combination of clinical examination and CT imaging and reported a specificity of 75% with a PPV of 50% [6]. However, these results have not been consistently reproducible in independent cohorts.
Extensive peritoneal carcinomatosis diffuse mesenteric involvement, diffuse long segment bowel loop involvement, diaphragmatic disease, and porta hepatis deposits are common causes for inoperable disease. Laparoscopy offers the advantage of directly look into the abdomen and decide on operability. However, the presence of any of the individual parameters as such does not necessarily indicate inoperability which is evident from performance of individual parameters. Laparoscopic scores combine multiple parameters and provide a better assessment of intra-abdominal disease extent, the chance of inoperability. The sensitivity of diagnostic laparoscopy was studied by Fagotti et al. and reported it to be effective in predicting optimal surgery and proposed its use to avoid unnecessary laparotomies [7]. LapOvCa trial has shown a significant benefit in reducing futile laparotomies (10% vs. 39%) by using diagnostic laparoscopy [8]. Though laparoscopy appears a promising tool, it needs technical expertise, hospital administration, general anaesthesia and associated costs.
Several studies have used various CA 125 values as cut-off and had diverse results. Monisha et al. analysed the CA 125 value and per cent reduction to predict optimal surgery. In a cohort of 406 cases of post-NACT, in those with CA 125 value of less than 100 U/ml, 70% had optimal surgery and in those with more than 95% reduction in CA 125, 88% had optimal surgery [9]. Another study by George et al. in 496 patients used a cut-off value of 500U/ml which had 78% sensitivity [10].
In our study, CA-125 value of more than twice the upper limit of normal value has sensitivity of 100% and specificity of 94% (AUC = 0.98; P value < 0.001) to predict optimal interval cytoreduction. Similarly, 90% reduction in post-neoadjuvant CA-125 value has sensitivity of 72% and specificity of 71% (AUC = 0.72; P value = 0.047) to predict operability. Both the CA 125 cut-off value and percentage reduction value on validation showed statistically significant. On comparing to the other studies, the results of our studies have shown that the post-neoadjuvant CA 125 value less than 88.5U/ml and 90% reduction in CA 125 value are more reliable cut-offs with a very good sensitivity and specificity to predict optimal IC which is strongly supported by the prospective validation.
This study, however, has several limitations. The sample size is small, laboratory errors could affect outcome, and as CA 125 fall is a continuum timing of post-NACT CA 125 test could alter result and clinical decision making.
Conclusion
The operability for advanced carcinoma ovary after neoadjuvant chemotherapy should be assessed accurately to avoid futile laparotomies. The post-chemo CA 125 value and percentage reduction of CA 125 after neoadjuvant chemotherapy has high sensitivity and positive predictive value in predicting optimal interval cytoreduction.
References
Rauh-Hain J et al. Ovarian cancer screening and early detection in the general population. PubMed - NCBI [Internet]. [cited 2019 Dec 6]. https://www.ncbi.nlm.nih.gov/pubmed/21629494.
Gómez-Hidalgo NR, Martinez-Cannon BA, Nick AM, Lu KH, Sood AK, Coleman RL, et al. Predictors of optimal cytoreduction in patients with newly diagnosed advanced-stage epithelial ovarian cancer: time to incorporate laparoscopic assessment into the standard of care. Gynecol Oncol. 2015;137:553.
Seigel R et al. Cancer Statistics, 2017. PubMed - NCBI [Internet]. [cited 2019 Dec 6]. https://www.ncbi.nlm.nih.gov/pubmed/28055103.
Chang L-C, Huang C-F, Lai M-S, Shen L-J, Wu F-LL, Cheng W-F. Prognostic factors in epithelial ovarian cancer: a population-based study. PLoS ONE. 2018;13(3):e0194993.
Nelson BE et al. Preoperative abdominopelvic computed tomographic prediction of optimal cytoreduction in epithelial ovarian carcinoma. PubMed - NCBI [Internet]. [cited 2020 Jan 21]. https://www.ncbi.nlm.nih.gov/pubmed/8418230.
Ferrandina G, Sallustio G, Fagotti A, Vizzielli G, Paglia A, Cucci E, et al. Role of CT scan-based and clinical evaluation in the preoperative prediction of optimal cytoreduction in advanced ovarian cancer: a prospective trial. Br J Cancer. 2009;101:1066–73.
Fagotti A et al. Introduction of staging laparoscopy in the management of advanced epithelial ovarian, tubal and peritoneal cancer: impact on prognosis in a single. PubMed - NCBI [Internet]. [cited 2020 Jan 21]. https://www.ncbi.nlm.nih.gov/pubmed/23938372.
Rutten MJ, van Meurs HS, van de Vrie R, Gaarenstroom KN, Naaktgeboren CA, van Gorp T, et al. Laparoscopy to predict the result of primary cytoreductive surgery in patients with advanced ovarian cancer: a randomized controlled trial. J Clin Oncol. 2017. https://doi.org/10.1200/jco.2016.69.2962.
Gupta M, Patel SM, Arora R, Tiwari R, Dave P, Desai A, et al. Does preoperative CA-125 cutoff value and percent reduction in CA-125 levels correlate with surgical and survival outcome after neoadjuvant chemotherapy in patients with advanced-stage ovarian cancer? Our experience from a tertiary cancer institute. South Asian J Cancer. 2020;9:30.
Can the preoperative Ca-125 level predict optimal cytoreduction in patients with advanced ovarian carcinoma? A single institution cohort study | Request PDF [Internet]. ResearchGate. [cited 2020 Jan 21]. https://www.researchgate.net/publication/23716664_Can_the_preoperative_Ca-125_level_predict_optimal_cytoreduction_in_patients_with_advanced_ovarian_carcinoma_A_single_institution_cohort_study.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, D.S., Noushad, S.N., Anandh, S.M. et al. CA-125 Levels Predict Optimal Surgery in Carcinoma Ovary: A Retrospective Analysis with Prospective Validation. Indian J Gynecol Oncolog 18, 51 (2020). https://doi.org/10.1007/s40944-020-00398-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-020-00398-4