Abstract
Introduction
Endometrial cancer usually has a good prognosis. The recurrence and survival in endometrial cancer are based on multiple prognostic factors like patient age, histological grade, myometrial invasion, and lymphovascular space invasion. We investigated various clinicopathological features determining tumor recurrence in stage I endometrial cancer with endometrioid histology.
Methods
We retrospectively reviewed stage I endometrial cancer patients who underwent surgery at the Basavatarakam Indo American Cancer Hospital between 2010 and 2015. Patients who had tumor recurrence were documented. Various risk factors like size, grade, depth, lymphovascular involvement, etc., were studied, their relation with recurrence was noted, and statistical analysis was done.
Results
Twenty-three patients exhibited tumor recurrence in stage I EEC (13.3%). When considering the depth of myometrial invasion, the 5-year RFS of stage IA EEC is 90.4% in comparison with 66.6% when the depth of invasion is more than half of myometrial invasion. The 5-year RFS of the patients with stage I EEC is 100% in tumors with size less than 2 cms, 92.15% in tumor size 2–4 cms, and 70.45% when the tumor size is greater than 4 cms. The 5-year RFS of the patients is 94.7% in grade 1, 87.3% in grade 2, and 54.2% in grade 3.
Conclusion
Depth of myometrial invasion, grade, and size of the primary tumor are shown to affect recurrence. LUS involvement, intracervical glandular involvement, and the lymphovascular space invasion did not affect recurrence in endometrioid endometrial cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In India, the total number of estimated new cases of endometrial cancer in 2018 was 13,328 with an estimated 5010 deaths. The age-standardized incidence rate (ASIR) of endometrial cancer in India is 2.1/100,000 women [1]. Surgery is the primary treatment modality for endometrial cancer. Surgery alone is curative in most of the cases. Chemotherapy and radiation are used as adjuvant treatment in selected cases to decrease the recurrence and improve survival. Patients with endometrial cancer are usually having a good prognosis. The high mortality is mainly related to patients presenting with advanced stage and with high-risk histology (serous carcinoma). Fortunately, most of the endometrial cancers present in the early stage with endometrioid histology being the most common sub-type. Stage I comprises about 70–80% of cases of all endometrial cancers. Recurrence and survival in endometrial cancer are determined by various clinical and pathological prognostic factors. These factors are important in planning adjuvant therapy. A patient with two or more poor prognostic factors usually requires adjuvant treatment. Over the past few decades, several studies have demonstrated the prognostic importance of different parameters like age, size of tumor, lymph node status, histological type, grade, depth of myometrial invasion, lymphovascular space involvement, and cervical involvement. Analysis of SEER data suggests that survival is increased in patients who are younger, have early-stage disease, and have lower-grade disease [2]. In addition to grade and depth of myometrial invasion, other risk factors associated with poor prognosis include age, lymph node status, tumor size, lymphovascular space invasion (LVSI), and tumor involvement of the lower uterine segment [3, 4].
Early-stage endometrial cancer is typically divided into low-, intermediate-, and high-risk groups. Risk groups were classified using many factors that affect recurrence and survival [5]. There is a noticeable disparity in the criteria used for allocating patients into three risk groups among various studies. Each study has applied its own criteria for risk group classification [6]. However, there is limited evidence of the number and weight of these adverse risk factors to be taken into account in deciding adjuvant treatment [7]. To improve outcome in patients with endometrial cancer, physicians need to identify high-risk patients and to tailor adjuvant treatment appropriately to provide the best long-term survival.
We investigated the prognostic factors determining tumor recurrence in stage I endometrial cancer with endometrioid histology (EEC). We have excluded histology other than endometrioid histology and higher stage (stage II and above) in our paper. Our paper studies various clinicopathological features determining recurrence in endometrial cancer. This may help in planning adjuvant treatment.
Methods
Patients
We retrospectively reviewed the medical records of patients diagnosed with endometrial cancer at the Basavatarakam Indo American Cancer Hospital between 2010 and 2015. Stage I endometrial cancer patients with endometrioid histology who underwent upfront surgery were included in the study. Women who had fatal co-morbidity to affect survival, took hormone therapy for fertility-sparing, were in the stage greater than stage I, or were diagnosed with atypical histology were excluded. Grade, myometrial invasion, size of the primary tumor, LUS involvement, intracervical glandular involvement, and lymphovascular space invasion were noted from the final histopathology report and documented. We have a strict protocol for calling patients every 3 months for routine follow-up. All patients underwent routine pelvic examination and imaging [ultrasound or CT] on follow-up. Tumor recurrence suspected on clinical pelvic examination and imaging were confirmed on biopsy. Recurrence that occurred in the pelvic lymph node, vagina, or pelvic peritoneum was defined as loco-regional recurrence, whereas distant metastasis included inguinal lymph nodes, para-aortic lymph nodes, extra-pelvic peritoneum, lung, liver, and bone. Survival was documented, and an exact cause of death was noted whether it’s due to disease recurrence or non-oncological cause.
Statistical Analysis
Clinical and pathological characteristics were analyzed with Student’s t test, Chi-square test, or Fisher’s exact test. We used univariate and multivariate Cox proportional hazard model and the Kaplan–Meier method with the log-rank test. The recurrence-free survival (RFS) indicates the time from the date of surgery to recurrence or the last follow-up date without recurrence. A P value less than 0.05 is considered statistically significant.
Results
During the study period, 342 patients underwent radical hysterectomy for endometrial cancer. Among 342 patients, 256 patients justified the inclusion criteria. Among 256 patients, 84 women were lost to follow-up, leaving 172 for the analysis. The median age of the patients with stage I EEC was 56.67 years (35–76 years). The known adverse risk factors between stages IA EEC and IB EEC are shown in Table 1.
Most patients in stage IB EEC had at least one of the adverse risk factors, such as high histological grade, large tumor size, positive LVSI, and positive lower uterine segment involvement or surface cervical glandular involvement than patients in stage IA EEC (81.48% vs. 42.06%; P = 0.0002).
Recurrence
The median follow-up period was 32.26 months (6–69 months), and 23 patients exhibited tumor recurrence in stage I EEC (13.3%). Of the recurrent cases, the median interval period between surgery and tumor recurrence was 23.4 months (6–53 months).
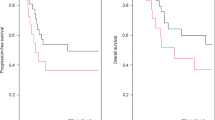
When considering the depth of myometrial invasion, the 5-year RFS of stage IA EEC is 90.4% in comparison with 66.6% when the depth of invasion is more than half of myometrial invasion (P value 0.001) (Fig. 1). If tumor size is greater than 4 cms, it was associated with an increased risk of recurrence (P < 0.0008). The 5-year RFS of the patients with stage I EEC was 100% in tumors with size less than 2 cms, 92.15% in tumor size 2–4 cms, and 70.45% when the tumor size is greater than 4 cms (P value 0.04) (Fig. 2). The tumors with grade 3 histology were associated with an increased risk of recurrence (P < 0.0001). The 5-year RFS of the patients was 94.7% in grade 1, 87.3% in grade 2, and 54.2% in grade 3 (P value 0.0001) (Fig. 3).
The rest of the risk factors, i.e., LUS involvement, intracervical glandular involvement, and lymphovascular space invasion, were not associated with an increased risk of recurrence (P = 0.7769, P = 0.5166 and P = 0.1837, respectively). The patients with stage IB exhibited a tendency for distant metastasis; however, the difference was not significant (P = .3998).
Discussion
The various prognostic factors that are postulated with the development of recurrent disease in endometrial cancer include age, histology, grade, histological type, cervical invasion, myometrial invasion, LVSI, peritoneal cytology, ovarian metastasis. Potential adverse risk factors according to the latest NCCN guidelines include the following: age, positive lymphovascular invasion, tumor size, and lower uterine segment or surface cervical glandular involvement. The risk factors like in any malignancy are important for explaining prognosis to the patient and deciding the adjuvant treatment.
Age at the presentation of cancer is considered an important prognostic factor in endometrial cancer. We have not studied age as a prognostic factor in this paper. Many studies have reported an inverse relationship between advancing age and poor outcome [8]. Younger patients have a good prognosis as compared to older patients. Some histological types like clear cell are associated with poor prognosis. In our study, we have excluded other histology and studied about the endometrioid type only which is considered as having a better prognosis and is the most common sub-type seen in endometrial cancer. As endometrioid histology is having a good prognosis, the effect of adverse risk factors on prognosis may not be the same when compared with other histologies. We wanted to study the effect of risk factors on endometrioid histology.
Our study like many other studies did show a grade of tumor to be a significant risk factor for disease recurrence. The PORTEC-1 study showed that tumor grade is important for tumor recurrence [7]. Five-year survival rates of 94% in patients with grade 1 tumors, 84% in those with grade 2 tumors and 72% in those with grade 3 tumors are reported. We have reported in our paper that the 5-year RFS of the patients is 94.7% in grade 1, 87.3% in grade 2, and 54.2% in grade 3 (P value 0.0001). Our results closely match the results of bigger studies. Like grade, depth of myometrial invasion is proven as an independent predictor of poor outcome. The PORTEC-1 study showed that myometrial invasion has an important role in tumor recurrence [7]. Recurrence developed in only 1% of patients with no myometrial invasion, and 7.7% developed recurrence with the invasion of the inner one-third of the myometrium. 14.5% developed recurrence with the invasion of the middle-third of the myometrium, and 15% developed recurrence with the invasion of the outer-third of the myometrium. Our paper showed the 5-year RFS of stage IA EEC is 90.4% in comparison with 66.6% when the depth of invasion is more than half of myometrial invasion. Depth of myometrial invasion is an indicator of disease recurrence and death. As the depth of invasion increases, tumor is exposed to more lymphatic and vascular channels leading to more dissemination of cancer cells leading to more recurrence and poor survival. Size of the tumor less than 2 cms, according to our study, had 100% 5-year survival, whereas the survival decreased with increasing tumor size 2–4 cms having RFS of 92%. The 5-year survival is just 70% when tumor size is > 4 cms; this is in accordance with other studies [9].
The rest of the risk factors we studied like LUS involvement, intracervical glandular involvement, and lymphovascular space invasion were not associated with an increased risk of recurrence. This is in contrary to other studies where they have shown the above-mentioned factors are important in recurrence and prognosis. It is difficult to explain why results of our study don’t match with other studies when LUS involvement, intracervical glandular involvement, and lymphovascular space invasion are taken into account. Probably in endometrioid histology, these factors do not play a role in deciding recurrence and prognosis. We need larger studies to substantiate our result with respect to LUS involvement, intracervical glandular involvement, and lymphovascular space invasion as risk factors.
All these factors mentioned in this paper have been used to guide the decision regarding adjuvant therapy. Mariani et al. [10] and Milam et al. [11] have classified endometrial cancer into low risk and high risk based on these multiple risk factors. They propose endometrioid adenocarcinoma with grade 1 or 2, myometrial invasion < 50%, and tumor with the largest diameter ≤ 3 cm at low risk. Current management guidelines (NCCN/ESMO) have defined risk groups based on age, size, myometrial invasion, histological grade, LVSI, and positive lower uterine segment or surface cervical glandular involvement [12].
Although we investigated specific prognostic factors in stage I EEC, our study has some limitations. A retrospective design of the study might induce selection bias to include patients with stage I EEC. Secondly, the effect of adjuvant treatment is not considered to evaluate prognosis. In addition, there were no sufficient death events to analyze overall survival or cancer-specific survival. Prospective evaluation with long-term follow-up is needed to draw an accurate conclusion.
In the latest development, progesterone receptor expression may be useful in identifying prognosis in endometrial cancer. In the future, new molecular markers like LCAM and other factors like MSI, PI3 K-AKT, Wnt/β-catenin and P53 may be used for deciding the indication for adjuvant therapy [13]. Like in breast cancer, oncotype Dx is used for deciding adjuvant treatment; we will for sure develop some tool in low-risk endometrial cancer to decide patients with low risk of recurrence. In some low-risk patients, we can avoid adjuvant treatment. Our main aim in developing such a tool is to identify patients at low risk of recurrence and avoid morbidity in this low-risk group of patients.
Conclusion
Grade, myometrial invasion, and size of the primary tumor affect recurrence and prognosis in early-stage endometrioid histology. LUS involvement, intracervical glandular involvement, and lymphovascular space invasion were not associated with an increased risk of recurrence in endometrial cancer.
References
www.icmr.nic.in (Consensus document for the management of uterine cancer).
Chan JK, Sherman AE, Kapp DS, et al. Influence of gynecologic oncologists on the survival of patients with endometrial cancer. J Clin Oncol. 2011;29:832–8.
Benedetti Panici P, Basile S, Salerno MG, et al. Secondary analyses from a randomized clinical trial: age as the key prognostic factor in endometrial carcinoma. Am J Obstet Gynecol. 2014;210(363):e361–3.
Doll KM, Tseng J, Denslow SA, et al. High-grade endometrial cancer: revisiting the impact of tumor size and location on outcomes. Gynecol Oncol. 2014;132:44.
Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynecol Obstet. 2009;105:103–4.
Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094–108.
Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. Lancet. 2000;355(9213):1404–11.
Zusterzeel PL, Bekkers RL, Hendriks JC, et al. Prognostic factors for recurrence in patients with FIGO stage I and II, intermediate or high risk endometrial cancer. Acta Obstet Gynecol Scand. 2008;87(2):240–6.
Canlorbe G, Bendifallah S, Laas E, et al. Tumor size, an additional prognostic factor to include in low-risk endometrial cancer: results of a French multicenter study. Ann Surg Oncol. 2016;23:1717.
Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506–19.
Milam MR, Java J, Walker JL, Metzinger DS, Parker LP, Coleman RL, Gynecologic Oncology Group. Nodal metastasis risk in endometrioid endometrial cancer. Obstet Gynecol. 2012;119:286–92.
Colombo N, Creutzberg C, Amant F, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26:2–30.
Zeimet AG, Reimer D, Huszar M, et al. L1CAM in early-stage type I endometrial cancer: results of a large multicenter evaluation. J Natl Cancer Inst. 2013;105(15):1142–50.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goel, V., Ramani, K., Raju, K. et al. Various Clinicopathological Factors Impacting Recurrence in Stage I Endometrial Cancer: A Retrospective Study. Indian J Gynecol Oncolog 17, 56 (2019). https://doi.org/10.1007/s40944-019-0300-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-019-0300-7