Abstract
Background and Purpose
The availability of more advanced technology like hypo-fractionation has the potential of being the new standard of care in breast cancer. This study evaluates whether 3DCRT with a field-in-field technique (FIF) and a simultaneous integrated boost (SIB) could provide a dosimetrically comparable plan delivered to VMAT or IMRT.
Materials and Methods
3DCRT-FIF-SIB, VMAT and IMRT-SIB plans were generated for 20 patients. The plans were compared for planning target volume coverage (PTV 95), homogeneity and conformity, dose delivered to lungs, heart and C/L breast.
Results
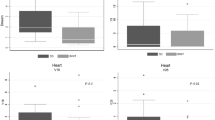
3DCRT FIF provided better sparing of C/L breast V1 and V5, whole lung V5, p = 0.000. The VMAT plans spared heart V30: (0.1 ± 0.46 vs. 11.5 ± 18.3) p = 0.000 and I/L lung V20: (19.3 ± 5 vs. 32.2 ± 11.1) p = 0.000. It provided a better coverage V95: (97 ± 0.8 vs. 95 ± 2.9) p = 0.002 and sparing of the heart V30: (0.1 ± 0.5 vs. 8.6 ± 11.5) p < 0.002 and lungs I/L V20: (19.3 ± 5.0 vs. 30.7 ± 6.1) p = 0.000. The treatment was faster with less exposure in terms of MU: (529 ± 57.8 vs. 1024 ± 298) p = 0.000.
Conclusions
3DCRT provides a dosimetrically acceptable alternative to more advanced technologies. VMAT and IMRT provide better sparing of heart and lungs. VMAT has a slight benefit of conformity, reduced exposure and shorter treatment time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The past few decades have seen radical changes in the concepts of breast cancer radiotherapy following conservative surgery. Conventional fractionation spanning 6–7 weeks of treatment has been followed for nearly half a century due to concerns regarding limiting long-term side effects of late responding tissue. But now with level 1 evidence to suggest that fraction sizes in the range of 2.7–3.3 Gy are both tolerable and feasible, the focus on breast-conserving radiotherapy has shifted to optimizing the potential shorter schedules [1]. The current era of advanced technology has seen a shift from 2D-based tangential to various forms of intensity modulation. Forward IMRT with field-in-field (FIF), three-dimensional radiotherapy (3DCRT), volumetric modulated arc therapy (VMAT) and intensity-modulated radiotherapy are radiation techniques that provide better sparing of the heart and lungs.
Most consensus guidelines and cooperative groups now accept hypo-fractionated schedules with shorter overall treatment time as the new standard of care [2]. However, the optimal technique and sequence of delivering the tumour bed boost remain a grey area. The method of integrating the boost with the primary whole breast radiotherapy, i.e. SIB, is gaining popularity. Several studies support the non-inferior cosmoses with promising dosimetric sparing to the heart and lungs when delivering SIB with VMAT and IMRT [3,4,5,6]. There are also a few studies to support the feasibility of doing the same with 3D FIF treatment [7, 8]. In a country like India, IMRT facilities are available in only a minority of treatment centres [9]. In addition to poor access, VMAT and IMRT techniques are expensive, labour-intensive and require experienced personal and dedicated quality checks.
We undertook this study to evaluate the dosimetric feasibility of optimizing a shorter treatment schedule by integrating SIB into FIF 3DCRT for primary whole breast irradiation. Our study compares the 3DCRT (FIF) with SIB with VMAT and IMRT plans generated from the same data set. The aims were to evaluate the non-inferiority and feasibility of this technique. As a secondary objective, we also compared the dosimetric difference/efficacy of SIB technique using VMAT versus IMRT.
Materials and Methods
In this study, we have used image data sets of 20 patients who had undergone breast conservative radiotherapy during the period of 2015–2017. The laterality was evenly distributed to nine patients having left-sided lesions and the remaining 11 right-sided breast cancers.
All 20 patients had identical treatment immobilization in supine position with 3-mm slice CT image data sets. The breast CTV, PTV and organs at risk were contoured based on the standard RTOG guidelines [10].
The boost volume (CTV-B) was identified from operative clips, seroma and preoperative imaging when available. A 5-mm expansion was generated to provide a PTV margin and create the PTV-boost volume (PTV-B). The organs at risk contoured were heart, ipsilateral, contralateral and combined lung volumes as well as contralateral breast.
Treatment Planning
A radiotherapy treatment plan was individually created using 3DCRT (FIF) SIB, VMAT-SIB and IMRT-SIB for each data set.
Plan A: FIF 3DCRT with 45 Gy/25fr to PTV-T with SIB 15 Gy to PTV-B
Plan B: VMAT plan with SIB 60 Gy/25fr delivered to PTV-B and 45 Gy/25fr to PTV-T
Plan C: IMRT plan with SIB 60 Gy/25fr delivered to PTV-B and 45 Gy/25fr to PTV-T.
The FIF 3DCRT treatment plans used multi-leaf collimation and non-co-planar beams with gantry angles adjusted to optimal sparing of the heart and lungs. The PTV-boost was generated through manual optimization of beam weightage and MLC settings so as to encompass the 95% isodose. The SIB treatment plan was created by copying the whole breast plan and the boost plan both independently planned with FIF technique. The same isocentre and dose normalization points were used. The whole breast component plan received a daily dose of 1.8 Gy, and the boost plan delivered a dose of 0.6 Gy. This allowed for the boost volume alone to have the advantage of hypo-fractionation. A similar planning technique has been elaborated by Van Der Laan et al. [11].
Plan B used dynamic-field IMRT technique with the same set of treatment goals. We used the eclipse treatment planning system version 13.7 (Varian medical systems, USA). The treatment fields were designed with gantry angles ranging from 330° to 150° for left-sided tumours and from 50° to 200° for right-sided tumours.
Plan C was generated with Monaco TPS version 5.11(VMAT technique); gantry incremental angle was kept at 10°. Monte Carlo dose calculation algorithm one co-planar semi-arc with gantry angles between 290° to 160° in clockwise rotation was used for left-sided lesions and 200° to 80° in right-sided disease. The optimization objectives are given in Table 1.
Plan Evaluation and Statistical Analysis
Attempts were made for attaining similar and comparable coverage, homogeneity and optimal dose sparing of OAR.
Dose volume histograms were used for evaluation and dosimetric comparison of target volume coverage and organ at risk parameters.
A subset analysis was done to consider patient-specific parameters that can define the superiority or necessity of inverse modulated techniques. The parameters used were laterality of disease, size of the boost volume more or less than 100 cc and overlap of the heart within breast PTV > or < 1.2 cm.
Statistical Analysis
Paired sample statistics and students t test were used to evaluate planning and goals and dosimetric differences between generated DVH data. The reported p value was two tailed, and p < 0.05 was considered statistically significant.
Results
Plan A Versus Plan B
Dosimetric comparison for target coverage and organs at risk sparing between FIF 3DCRT (SIB) with IMRT-SIB. The comparative data sets are given in Table 2.
The FIF-3DCRT (SIB) was found to be non-inferior to IMRT (SIB) with several significant dosimetric advantages.
The FIF-3DCRT (SIB) plans show less dose scatter to contralateral lung and breast.
The IMRT plans had a contralateral lung dose V5 (volume of organ receiving 5 Gy) that was significantly higher (10.5 ± 18.23) versus (1.13 ± 4.24) p = 0.039 versus Vmean (2.12 ± 2.18) versus (0.595 ± 0.89) p = 0.008
The contralateral breast also received a higher dose V1: (43.49 ± 23.64) versus (20.725 ± 10.12) p < 0.001 and V5: (17.7 ± 8.78) versus (10.69 ± 8.76) p = 0.016.
The VMAT IMRT technique has an advantage of full optimization of clinical goals with reduced treatment and delivery time when compared to standard dynamic arc IMRT. In centres where all three modalities are available, as in the current institute an advantage would significantly impact by providing for a faster treatment and better allocation of resources. With this in mind, the third arm of the study plan C is a comparison of the VMAT technique with dynamic IMRT to evaluate any dosimetric equivalence or advantage. Table 3 compares plan efficacy between Plan B IMRT-SIB versus VMAT-SIB in terms of PTV primary and boost and OAR parameters.
The VMAT plan provided better coverage V95 (volume receiving 95% of the prescribed dose) 97 ± 0.8 versus 95 ± 2.9, (p = 0.002) in comparison with IMRT. It was also significantly more conformal CI 0.9 ± 0.0 versus 0.7 ± 0.3 to IMRT with better sparing of the heart V30 0.1 ± 0.5 versus 8.6 ± 1.5, (p = 0.002) and lungs; ipsilateral lung V20 19.3 ± 0.5 versus 30.7 ± 0.1 (p < 0.001). VMAT provided an advantage of faster treatment with less exposure MU 529 ± 57.8 versus 1024 ± 298, (p < 0.001). IMRT dose distribution was more homogenous in terms of less probability of a hot spot V107 0.09 ± 0.2 versus 1.4 ± 1.5, (p = 0.008) and less dose to the contralateral breast.
Discussion
The current era of radiotherapy has the advantage of emerging radiobiological insights that can be used for designing new protocols with a potential for improved results and favourable toxicity profiles.
Adjuvant radiotherapy following breast-conserving surgery is now focused on utilizing anticipated lower alpha/beta value of around 4 to incorporate hypo-fractionated schedules into routine practice [12,13,14]. Four prospective randomized clinical trials have provided sufficient evidence of equivalence to conventional treatment to allow for incorporation into international treatment guidelines. The added radiobiological advantage of such schedules would be a reduction of overall treatment time, improved local control and favourable patient logistics in terms of convenience and compliance to treatment [15].
The benefit of reduced overall treatment time can be optimally provided by using a hypo-fractionated treatment regimen and integrating the boost component. The applicability of such a protocol has been proven through several techniques, of which dynamic arc IMRT remains the most popular. Alternative techniques that have proven feasible include 3DCRT with electron boost and 3D CRT-FIF [16, 17]. These are especially important in developing countries where 3DCRT is more accessible and affordable.
Several trials have supporting evidence to suggest that IMRT can be effectively and safely used to achieve acceptable dose profile and normal tissue sparing when integrating the boost [18, 19]. However, there are limitations and dosimetric issues of concern that restrict its universal usage.
The dosimetric issues of concern include increased dose to contralateral breast and lung, which can be two to three times higher than when using 3D CRT [20, 21]. This is of particular concern in the Asian population where we see a larger subset of younger patients with aggressive tumours that require intense chemotherapy regimens. The risk of second malignancies and late pulmonary toxicities cannot be ignored. The availability of IMRT facilities as well as gating is limited and in most centres 30–50% more expensive than 3DCRT. It also requires trained personnel. For planning and delivery, 3DCRT-FIF has been shown to provide optimal target goals and comparable organ of risk sparing to IMRT in several studies, including the one conducted in our centre earlier. Although 3DCRT-FIF technique for whole breast irradiation can be considered the standard of care, the best method of delivering the boost remains a grey area. Integrating the boost with the first phase of whole breast treatment would have radiobiological advantage of reducing treatment time by 1–2 weeks with a potential of improved local control.
In the current trial, we have tried to evaluate the dosimetric feasibility and safety of such a protocol (3DCRT FIF-SIB) and its comparative efficacy to more established techniques IMRT and VMAT.
The results of our study suggest that this protocol is non-inferior in terms of PTV coverage and in fact was better significantly to IMRT in a few target parameters. The 3DCRT FIF-SIB plan had less hot spots in both the whole breast and boost volume (Dmax p < 0.001, V107 p = 0.004). There was better sparing of contralateral lung (p = 0.008) as well as of contralateral breast (p < 0.001). The exposure in terms of monitor units and treatment time was 70% less than with IMRT-SIB (281 ± 20.2 MU vs. 1024 ± 298.3 MU, p = < 0.001).
In the past decade, many more centres have acquired alternative IMRT technology like VMAT and tomotherapy. An important issue in which IMRT was inferior to the 3DCRT plan was longer treatment delivery and exposure. The VMAT technique of IMRT can be executed with less planning time as well as delivery time and exposure. In our centre, we have recently acquired this newer technology and it would be imperative to identify whether such an advantage could be dosimetrically justified. In this study, the comparison of plan B IMRT-SIB with plan C VMAT-SIB showed an advantage of VMAT over IMRT in terms of better coverage V95 (97 ± 0.8) versus (95 ± 2.9) p = 0.002. It was also significantly more conformal to IMRT with better sparing of the heart V30 (0.1 ± 0.5) versus (8.6 ± 11.5) p = 0.002 and lungs, ipsilateral lung V20 (14.2 ± 0.5) versus (30.7 ± 0.1) p < 0.001. The potential advantage of faster treatment with less exposure was confirmed in our study with VMAT being associated with nearly 50% less MU (524 ± 51.8) versus (1024 ± 298), p < 0.001.
The only advantage IMRT had was in terms of less of hot spots and dose to contralateral breast (p = 0.004).
An earlier study by Laan et al. [22].had analysed the possibility of patent predictive factors that may help to identify patients that are benefitted by higher techniques. They found OHB > 1.4 cm and boost volume > 125 cc as significant parameters. Our subset analysis did not show a difference. However, a number was too small to achieve statistical significance. The limitation of this study is the reduced number of patients and dosimetric nature of data. However, integration of simultaneous boost into the treatment protocol is the current focus of many ongoing trials [23, 24] and very few studies have evaluated all three techniques as we have.
Conclusions
3DCRT FIF with SIB provides a dosimetrically acceptable and technically feasible alternative to the same treatment delivered with more advanced technologies. It may have an advantage in younger parents where second malignancies may be of concern. VMAT and IMRT do provide some sparing of heart and lungs and may be opted when this is of concern. VMAT has a slight edge over IMRT in terms of conformity, reduced exposure and shorter treatment delivery time and may be preferred when both modalities are available
References
Shelley W, Brundage M, Hayter C, Paszat L, Zhou S, Mackillop W. A shorter fractionation schedule for post lumpectomy breast cancer patients. Int J Radiat Oncol Biol Phys. 2000;47(5):1219–28.
Kim KS, Shin KH, Choi N, Lee SW. Hypofractionated whole breast irradiation: new standard in early breast cancer after breast-conserving surgery. Radiat Oncol J. 2016;34(2):81.
Yarnold J, Ashton A, Bliss J, Homewood J, Harper C, Hanson J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long- term results of a randomised trial. Radiother Oncol. 2005;75:9–17.
Owen JR, Ashton A, Bliss JM, Homewood J, Harper C, Hanson J, et al. Effect of radiotherapy fraction size on tumour control in patients with early- stage breast cancer after local tumour excision: long- term results of a randomised trial. Lancet Oncol. 2006;7:467–71.
START Trialists’ Group, Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, et al. The UK standardisation of breast radiotherapy (START) trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331–41.
START Trialists’ Group, Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, et al. The UK standardisation of breast radiotherapy (START) trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098–107.
Joseph B, Farooq N, Kumar S, Vijay CR, Puthur KJ, Ramesh C, Lokesh V. Breast-conserving radiotherapy with simultaneous integrated boost; field-in-field three-dimensional conformal radiotherapy versus inverse intensity-modulated radiotherapy—a dosimetric comparison: Do we need intensity-modulated radiotherapy? South Asian J Cancer. 2018;7(3):163.
Moorthy S, Sakr H, Hasan S, Samuel J, Al-Janahi S, Murthy N. Dosimetric study of SIB-IMRT versus SIB-3DCRT for breast cancer with breath-hold gated technique. Int J Cancer Ther Oncol. 2013;1(1):010110.
Kannan V, Bajpai R. Conforming modern radiation oncology facilities to the irregular contours of the vast and varied nation of India. Int J Radiat Oncol Biol Phys. 2016;94(4):645–51.
Rtog.org. 2018. cited 13 October 2018. https://www.rtog.org/LinkClick.aspx?fileticket=vzJFhPaBipE%3d&tabid=236.
van der Laan HP, Dolsma WV, Maduro JH, Korevaar EW, Hollander M, Langendijk JA. Three-dimensional conformal simultaneously integrated Boost technique for breast-conserving radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68(4):1018–23.
Herbert C, Nichol A, Olivotto I, Weir L, Woods R, Speers C, Truong P, Tyldesley S. The impact of hypofractionated whole breast radiotherapy on local relapse in patients with grade 3 early breast cancer: a population-based cohort study. Int J Radiat Oncol Biol Phys. 2012;82(5):2086–92.
Yarnold J, Haviland J. Pushing the limits of hypofractionation for adjuvant whole breast radiotherapy. Breast. 2010;19(3):176–9.
Deantonio L, Gambaro G, Beldì D, Masini L, Tunesi S, Magnani C, Krengli M. Hypofractionated radiotherapy after conservative surgery for breast cancer: analysis of acute and late toxicity. Radiat Oncol. 2010;5(1):112.
Jagsi R, Griffith KA, Boike TP, Walker E, Nurushev T, Grills IS, Moran JM, Feng M, Hayman J, Pierce LJ. Differences in the acute toxic effects of breast radiotherapy by fractionation schedule: comparative analysis of physician-assessed and patient-reported outcomes in a large multicenter cohort. JAMA Oncol. 2015;1(7):918–30.
Sioshansi S, Rivard MJ, Hiatt JR, Hurley AA, Lee Y, Wazer DE. Dose modeling of noninvasive image-guided breast brachytherapy in comparison to electron beam boost and three-dimensional conformal accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;80(2):410–6.
Baycan D, Karacetin D, Balkanay AY, Barut Y. Field-in-field IMRT versus 3D-CRT of the breast. Cardiac vessels, ipsilateral lung, and contralateral breast absorbed doses in patients with left-sided lumpectomy: a dosimetric comparison. Jpn J Radiol. 2012;30(10):819–23.
Singla R, King S, Albuquerque K, Creech S, Dogan N. Simultaneous-integrated boost intensity-modulated radiation therapy (SIB-IMRT) in the treatment of early-stage left-sided breast carcinoma. Med Dosim. 2006;31(3):190–6.
Guerrero M, Li XA, Earl MA, Sarfaraz M, Kiggundu E. Simultaneous integrated boost for breast cancer using IMRT: a radiobiological and treatment planning study. Int J Radiat Oncol Biol Phys. 2004;59(5):1513–22.
Michalski A, Atyeo J, Cox J, Rinks M, Morgia M, Lamoury G. A dosimetric comparison of 3D-CRT, IMRT, and static tomotherapy with an SIB for large and small breast volumes. Med Dosim. 2014;39(2):163–8.
Bucci MK, Bevan A, Roach M. Advances in radiation therapy: conventional to 3D, to IMRT to 4D, and beyond. CA Cancer J Clin. 2005;55(2):117–34.
van der Laan HP, Dolsma WV, Schilstra C, Korevaar EW, de Bock GH, Maduro JH, Langendijk JA. Limited benefit of inversely optimised intensity modulation in breast conserving radiotherapy with simultaneously integrated boost. Radiother Oncol. 2010;94(3):307–12.
Hurkmans CW, Meijer GJ, van Vliet-Vroegindeweij C, van der Sangen MJ, Cassee J. High-dose simultaneously integrated breast boost using intensity-modulated radiotherapy and inverse optimization. Int J Radiat Oncol Biol Phys. 2006;66(3):923–30.
Hijal T, Fournier-Bidoz N, Castro-Pena P, Kirova YM, Zefkili S, Bollet MA, Dendale R, Campana F, Fourquet A. Simultaneous integrated boost in breast conserving treatment of breast cancer: a dosimetric comparison of helical tomotherapy and three-dimensional conformal radiotherapy. Radiother Oncol. 2010;94(3):300–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joseph, B., Ikkurthi, V., Farooq, N. et al. Breast-Conserving Radiotherapy with Simultaneous Integrated Boost—A Dosimetric Comparison of 3DCRT, VMAT and IMRT: Do We Really have a Better Plan?. Indian J Gynecol Oncolog 17, 43 (2019). https://doi.org/10.1007/s40944-019-0289-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-019-0289-y