Abstract
Introduction
Endometrial stromal sarcomas (ESS) are difficult to diagnose, both due to the rarity of the tumor and because of the close resemblance of the tumor to normal stromal tissue. The most common extra-uterine site of ESS is the ovary. We report a case of, huge primary low-grade endometrial stromal sarcoma in the ovary, infiltrating bladder and right ureter.
Case Report
A 40-year-old lady presented with lower abdominal pain and fullness. On radiological examination, a huge abdomino-pelvic mass, with solid and cystic areas, with right hydroureteronephrosis was found.
Discussion
ESS pose a diagnostic challenge at extra-uterine sites. Malignant mixed mullerian tumor and sex cord stromal tumor are the close differential diagnoses. The other issue is deciding whether the tumor is primary or secondary in origin, especially in a previously hysterectomised patients. Various treatment options such as hormonal therapy, chemotherapy and radiotherapy exist but are not beneficial owing to the slow-growing nature of the tumors.
Conclusion
Primary ovarian ESS is a possibility after excluding possible metastatic spread from uterine ESS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial stromal sarcomas (ESS) are uncommon mesenchymal tumors of the uterus, and they are even rare in ovaries as a primary tumor [1]. Previously, ESS were classified into low-grade (LGESS) and high-grade. Currently, the World Health Organization (WHO) classifies ESS as LGESS and undifferentiated endometrial sarcoma. High-grade ESS are termed as undifferentiated ESS because of their pleomorphic nature and lack of endometrial stromal differentiation, histologically. Extra-uterine sites of involvement are extremely rare in ESS, and only around 100 cases have been reported in the literature with more than 50 % of the cases being associated with pre-existing endometriosis [2]. The primary site in extra-uterine ESS is ovary in 76 % of cases.
Case Report
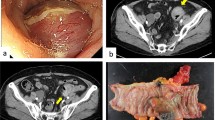
A 40-year-old lady with no comorbids presented, with history of abdomen distension and mild pain for last 1 month. She underwent hysterectomy 2 years back for dysfunctional uterine bleeding. On examination, she had an abdomino-pelvic mass, of variable consistency, reaching just up to umbilicus. Tumor markers for epithelial and germ cell tumors of ovary were within normal limits. Contrast-enhanced CT scan showed a huge heterogeneous mass about 23 cm × 18 cm with solid and cystic areas and was occupying entire pelvis, with loss of fat planes between bladder and rectum, with right-sided moderate hydroureteronephrosis (Fig. 1). No evidence of ascites or lymphadenopathy. Biopsy done elsewhere was reported as spindle cell neoplasm, favor leiomyosarcoma (IHC included: SMA positive, inhibin/calretinin/EMA/C-kit/desmin-negative, Ki67-positive 10–15 %). Patient underwent exploratory laparotomy and the findings demonstrated, huge solid cystic mass occupying entire pelvis and lower abdomen adherent to rectum and pelvic side walls (Fig. 2). A separate 5 cm cystic mass was infiltrating right lower ureter and posterior wall of bladder. Gross residual disease was left behind after resection of the abdominopelvic mass in order to avoid cystectomy as consent not obtained. The excision specimen revealed predominantly solid mass with a multiloculated cyst containing hemorrhagic fluid. Solid areas were homogenous, fleshy, tan white with areas of hemorrhage. Adequate sampling was done. Tumor was composed of diffuse sheets of stromal cells with interspersed prominent spiral arterioles and arborizing vascular pattern. The tumor cells were small with uniform round to oval nuclei and scanty cytoplasm. Areas of hemorrhage, tumor infarction, and hyalinization were noted along with hypocellular edematous areas. Mitotic count is 2–3/10 hpf. Sections from cyst wall reveal fibrous stroma with congested vessels, hemorrhage and hemosiderin laden macrophages. Compressed ovarian parenchyma was noted at periphery. Endometriosis was not identified in the sections studied. Patient was started on tablet letrozole 2.5 mg single dose daily and advised 3 monthly follow-up (Figs. 3, 4, 5).
Discussion
This case depicts primary ovarian sarcomas comprise only 1–3 % of all ovarian neoplasm [1]. ESS are also rare tumors, contributing for 0.2 % of all uterine malignancies and 7–15 % of all uterine sarcomas. ESS can occur in patients with endometriosis and in patients with a prolonged exposure to oestrogens. There are two hypotheses regarding the pathogenesis of extra-uterine ESS. The first one is that it arises from foci of pre-existing endometriosis and the second one is that it arises from sub-mesothelial pluripotential cells [3]. LGESS of ovary, in a previously hysterectomised patient, could also be a recurrence of the tumor in ovary, following an unidentified sarcoma in uterus. Recurrences of LGESS of uterus occur in 36–56 % of patients, most frequently in the pelvic cavity or the lungs. Metastatic spread to the cerebrum and bones and invasion in the great vessels and abdomen cavity have also been described [4]. Disease-free survival and prognosis are substantially better for low-grade versus undifferentiated sarcomas [5]. Our patient, a previously hysterectomised case, presented with LGESS in the ovary, which is the most common extra-uterine site. But, due to non-availability of slides of previous surgery, the histopathology examination could not be reviewed for the presence of any suspicious sarcomatous elements in uterus. Our patient never had any previous history suggestive of endometriosis or any history of prolonged hormonal pills intake.
Treatment
As there are only isolated case reports of ESS in ovary, treatment principles are extrapolated from ESS of uterus. Standard treatment for ESS is total abdominal hysterectomy with bilateral salpingo-oophorectomy, even at premenopausal ages. Studies on the effect of removal or preservation of ovaries, with respect to recurrence and overall survival in early-stage disease, in premenopausal girls have shown contradicting results [6]. Effect of lymph node dissection on overall survival is also controversial in early-stage disease [7].
Adjuvant therapy following surgery can include radiotherapy, chemotherapy and hormonal therapy. In a study performed by Ioffe et al., ER and PR were expressed in 76 % of their cases of LGESS and all undifferentiated endometrial sarcomas showed no expression. Patients with advanced and recurrent disease respond well to progestins or aromatase inhibitors such as letrozole or gonadotropin-releasing hormone (GnRH) analogues [8]. Tamoxifen and estrogens are contraindicated because of the possible stimulatory effect on disseminated endometrial stromal cells.
There are some studies to determine the effects of chemotherapy in the treatment of LGESS, but all show contradicting results. This may be partly due to inclusion of all type and stages of sarcomas in these studies owing to rarity of disease [9]. The effect of chemotherapy demonstrated in these studies cannot be extrapolated to LGESS patients, as they are very indolent tumors. The chemotherapy regimens vary, generally including platinum drugs, taxanes, ifosfamide and doxorubicin, with greater preference to doxorubicin. Post-operative treatment with chemotherapy might be an option in patients with hormone-unresponsive tumors and for inoperable tumors. Post-operative radiotherapy decreases the risk of local recurrence, especially in grade II–IV disease, without having a significant effect on overall survival. Radiotherapy can also be given as palliation. Some studies have shown a substantial reduction in local recurrence, but these studies include a variety of histological subtypes of sarcomas, making it difficult to interpret the results in the scenario of LGESS [10]. Hence according to Haberal et al., one can conclude that there appears to be no difference in recurrence rates or survival between those who receive and those who do not receive adjuvant therapy.
In our patient, gross residual disease left behind, over the posterior wall of bladder to avoid cystectomy. In view of strong hormone receptor positivity and absence of clear role of chemotherapy and radiotherapy in LGESS, we started patient on hormonal therapy (T. letrozole 2.5 mg OD). She was advised close follow-up three monthly and to consider cystectomy or other adjuvant therapies if she does not respond to the above management.
Conclusion
Due to paucity of cases, there are no clear guidelines for managing LGESS. At this juncture, effects of hormonal therapy in advanced and metastatic tumors with positive ER and PR are promising. The effects of chemotherapy and radiotherapy in adjuvant settings are still controversial.
References
Prat J, Endometrioid tumors. In: Pathology of the ovary. Philadelphia: Saunders; 2004. p. 145–151.
Mourad WA, Abdelgaffar B, Tulbah A, Tulbah M, Subhi J. Multifocal extrauterine endometrial stromal sarcoma: a very rare complication of endometriosis associated with elevated serum CA-125. Ann Saudi Med. 2003;23:181–3.
Zaloudek C, Hendrickson MR, Soslow RA. Mesenchymal tumors of the uterus. In: Kurman RJ, Ellenson LH, Ronnett BM, editors. Blausteins pathology of the female genital tract. 5th ed. New York: Springer; 2002. p. 586–93.
Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol. 1990;14:415–38.
Ihnen M, Mahner S, Jänicke F, Schwarz J. Current treatment options in uterine endometrial stromal sarcoma: report of a case review of the literature. Int J Gynecol Cancer. 2007;17:957–63.
Haberal A, Kayikçioğlu F, Boran N, et al. Endometrial stromal sarcoma of the uterus: analysis of 25 patients. Eur J Obstet Gynecol Reprod Biol. 2003;109:209–13.
Reich O, Winter R, Regauer S, et al. Should lymphadenectomy be performed in patients with endometrial stromal sarcomas. Gynecol Oncol. 2005;97:982.
Pink D, Lindner T, Mrozek A, et al. Harm or benefit of hormonal treatment in metastatic low-grade endometrial stromal sarcoma: single center experience with 10 cases and review of the literature. Gynecol Oncol. 2006;101:464–9.
Sutton G, Brunetto VL, Kilgore L, et al. A phase II trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: a Gynecologic Oncology Groups Study. Gynecol Oncol. 2000;79:147–53.
Weitmann HD, Knocke TH, Kucera H, et al. Radiation therapy in the treatment of endometrial stromal sarcoma. Int J Radiat Oncol Biol Phys. 2001;49:739–48.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to this report. There was no financial support for the conduct of the study and the writing of the manuscript.
Rights and permissions
About this article
Cite this article
Ravichander, S.K., Boralkar, A.K., Borde, N.D. et al. A Rare Case Report of Primary Low-Grade Endometrial Stromal Sarcoma (LGESS) of Ovary Infiltrating Bladder. Indian J Gynecol Oncolog 14, 39 (2016). https://doi.org/10.1007/s40944-016-0066-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-016-0066-0