Abstract
Purpose
Despite improved treatment options, recurrence rates remain high in ovarian cancer. Tumor microenvironment, particularly T cells, plays an important role in its natural history. The present study was conducted to study the kinetics of T cell subsets in homogenous population of advanced-stage ovarian cancer patients of serous histology with reference to treatment and outcome.
Methods
We assessed the frequencies of CD8+ T cells, CD4+ T cells, regulatory T cells (Tregs, CD4+ CD25intermediate to high FOXP3+ CD127low) and natural killer cells in peripheral blood at baseline (n = 41) and post-treatment (n = 27) in the patients and compared it with blood of healthy controls (n = 15). The ascitic fluid of 12 of these patients was compared with ascitic fluid of patients with cirrhotic liver disease (n = 5).
Results
Higher numbers of Tregs, CD8+ T cells and higher Treg/CD4 ratio and reduced numbers of CD4+ T cells and reduced CD4/CD8 ratio were observed in patients as compared to controls. An increase in total number of T cells was observed with treatment, while no change in frequency of any lymphocyte subset was evident.
Conclusions
These results support previous studies exhibiting an increase in Tregs in ovarian cancer patients. Unchanged levels of T cell subsets subsequent to treatment suggest that they may be resistant to reset to healthy control homeostatic levels with standard chemotherapeutic treatments. Lower CD4 levels, higher CD8 levels, altered CD4/CD8 ratio and T cell subsets call for evaluation of role of immunomodulating agents or Treg-targeted immunotherapy in the treatment of advanced-stage serous carcinoma ovary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epithelial ovarian cancer (EOC) is the third most common gynecological cancer among women in world and accounts for 6.1/100,000 new cases per year, while in India, it is second most common gynecological cancer among women with age standardized rate of 4.9/100,000 [1]. EOC is the main subtype accounting for more than 90 % of all malignant ovarian tumors. Most patients of ovarian cancer are diagnosed in advanced stages [International Federation of Gynaecology and Obstetrics, (FIGO) stages III and IV] and prognosis, and thus, it is generally poor. Despite the significant advances in surgery and chemotherapy, relapse occurs in about 70 % of the patients [2].
The tumor microenvironment utilizes the physiological regulatory mechanisms to evade anti-tumor immunity and promote angiogenesis and growth. T regulatory cells (Tregs) play a pivotal role in peripheral immune tolerance, immune suppression and angiogenic programming of tumor microenvironment [3]. In addition, Tregs modulate the anti-tumor responses through secretion of immunosuppressive cytokines, such as IL-10 and TGF-β, and suppression of antigen-presenting dendritic cells preventing them from presenting tumor antigens to tumor-infiltrating cytotoxic T cells and other effecter cells [4]. It has been previously reported that the Tregs are increased in malignant ascites and peripheral blood of patients with ovarian carcinoma and are associated with poor outcome [5, 6]. However, there is paucity of information about post-treatment kinetics of Tregs and other T cell subsets in ovarian cancer.
In this prospective study, we assessed the frequency of Tregs and other T cell subsets in malignant ascites and peripheral blood from epithelial ovarian cancer patients collected at the time of diagnosis and at the end of the treatment. Peripheral blood from age- and sex-matched healthy subjects and ascites form patients of chronic liver disease were taken as control and compared with the patient samples.
Subjects and Methods
Subjects
Peripheral blood was collected from patients of EOC of stages III and IV with serous histology between age 18 and 70 years at the time of diagnosis (n = 41) and 4 weeks after completion of treatment (n = 27) in ethylenediaminetetraacetic acid (EDTA). Peripheral blood samples were also collected from 15 age- and sex-matched healthy subjects. Ascitic fluid was also collected from patients with EOC (n = 12) and cirrhotic liver disease (n = 5). Patients with auto immune diseases or with comorbidities which may affect the immune system were excluded. The study was approved by institute ethics committee, and written informed consent was obtained from all the participants as per the guidelines of the ethics committee.
Flow Cytometric Analysis of Regulatory T Cells
Mononuclear cells were separated from peripheral blood using density gradient media (Ficoll-Hypaque; Sigma 1077, Sigma-Aldrich Inc., USA) and stained with the following monoclonal antibodies: CD3 Perdinin Chlorophyll Cychrome 5 (PerCPCy5), CD8 Fluorescin isothiocyanate (FITC), CD16 (PerCPCy7), CD56 Phycoerythrin (PE), CD25 Allophycocyanin (APC), CD4 (PE-CF594), CD127 (PE) and FoxP3 Alexa 488 (BD Biosciences, USA). Briefly, 1 × 106 peripheral blood mononuclear cells were incubated with antibodies for surface staining followed by fixation and permeabilization for FoxP3 staining as per the manufacturer’s recommendations (BD Biosciences, USA). The cells were then washed and suspended in phosphate-buffered saline and kept at 4 °C till acquisition. A total of 105 events were acquired on a flow cytometer (FC500, Beckman Coulter, USA), and analysis was done using FCS express software version 4.0 (Denovo software). Using sequential gating strategy, the lymphocytes were gated by the forward and side scatter properties, T cells were identified by expression of CD3, NK cells by CD16 and CD56, cytotoxic T cells by CD8+ CD3+, helper T cells by CD3+ CD4+ and Tregs as cells with CD4+ CD25intermediate to high FOXP3+ CD127low.
Outcome Measures and Statistical Analysis
Complete remission, partial response and disease progression were defined as described in Response Evaluation Criteria in Solid Tumors (RECIST) criteria 1.1 guidelines [7]. Optimal debulking was defined by ≤1 cm diameter of residual tumor post-surgery. Suboptimal debulking was defined by any residual tumor of >1 cm diameter. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of disease progression or death, whichever occurred first; the data for patients who were alive without disease progression were censored as of the date of their last assessment. Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause; data for patients still alive were censored at the date the patient was last known to be alive. Two-sample t test and paired t test were used to find the differences between two groups and between paired samples before and after therapy, respectively. Statistical analysis was carried out using STATA 11 software.
Results
Patient Characteristics and Treatment
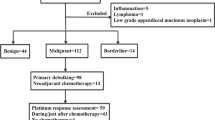
Of the 41 newly diagnosed patients of advanced-stage (stage III and IV) EOC with serous histology enrolled in the study, follow-up samples were available for evaluation in 27 patients. Of the remaining 14 patients, five patients died (one due to myocardial infarction before initiation of chemotherapy, one due to post operative complications and three due to chemo-toxicity); five patients were lost to follow-up before completion of chemotherapy; and four patients refused for repeat sampling. Twenty-six (65 %) patients received neoadjuvant chemotherapy [paclitaxel (175 mg/m2) and carboplatin (AUC 6) on day 1 q 3 weekly, NACT] followed by interval debulking (IDS). Fifteen patients (35 %) underwent primary surgery followed by adjuvant chemotherapy [paclitaxel (175 mg/m2) and carboplatin (AUC 6) on day 1 q 3 weekly × 6 cycles]. Other patient characteristics and treatment outcomes are listed in supplementary Table 1.
Frequency of T Cell Subsets in the Peripheral Blood and Ascitic Fluid of Patients and Controls
Peripheral Blood
Of the various T lymphocyte subsets, the frequency of helper T cells was lower (50.96 ± 14 vs. 61.8 ± 13.78 %, p = 0.005), while cytotoxic T cells (39 ± 12 vs. 31 ± 11.2 %, p = 0.01) and Tregs (9.3 ± 2.8 vs. 7.1 ± 2.08 %, p = 0.004) were significantly higher in patient samples (supplementary figure 1). There was no statistically significant difference in the frequency of NK cells with respect to controls (8.36 ± 6.86 vs. 6.06 ± 3.7 %, p = 0.36). The CD4/CD8 ratio was significantly lower (1.55 ± 0.93 vs. 2.36 ± 1.22, p = 0.006), and Treg/CD4 ratio was higher in patients (0.2 ± 0.1 vs. 0.1 ± 0.03, p = 0.004). There was no statistically significant difference in the Treg/CD8 ratio with respect to controls (0.26 ± 0.13 vs. 0.26 ± 0.12, p = 0.95) (Table 1).
When we compared the absolute values of T lymphocyte subsets, helper T cells were significantly lower and Tregs showed a trend toward lower numbers in patients (612 ± 323.5 vs. 1207 ± 861.3, p = 0.007; 9.8 ± 41.4 vs. 78.2 ± 40.8, p = 0.097). Cytotoxic T cells (519 ± 351 vs. 1066 ± 2342, p = 0.88) and natural killer cells (231.3 ± 282 vs. 209.5 ± 157, p = 0.57) were not statistically different with respect to controls. Post-treatment there was increase in T lymphocytes (50.04 ± 15.4 vs. 58.4 ± 13.5, p = 0.04), but no significant changes were observed in any other lymphocyte subset (Table 2).
Ascitic Fluid
Mean frequencies of T lymphocytes (45.9 ± 19.5 vs. 76.9 ± 7.4, p = 0.02) and CD4+ cells (48.1 ± 14.1 vs. 57.4 ± 12.2, p = 0.01) were higher in ascitic fluid as compared to the paired peripheral blood sample in the patients. However, no statistically significant difference was noted in the frequency of T cell subsets between the peripheral blood and ascitic fluid of the patients (Table 3). When the ascitic fluid of patients and controls were compared, T cells were significantly higher and NK cells lower in the patients (76.96 ± 7.4 vs. 44.56 ± 14.98, p = 0.01) (4.25 ± 1.9 vs. 21 ± 10.4, p = 0.002, supplementary figure 2) (Table 3).
T Cells Subsets with Clinical Parameters, Response to Therapy and Survival
There was no correlation of various T cell subsets with age (≤60 vs. >60 years), menopausal status, disease stage (III vs. IV) and baseline median CA 125 levels (≤623 vs. >623 U/ml). There was no significant difference in any of the T cell subsets as well as their ratios with respect to the response to chemotherapy. NK cell frequency was significantly higher in patients who had undergone NACT followed by IDS as compared to primary surgery followed by chemotherapy (−1.3 ± 4.5 vs. 4.3 ± 6.4, p = 0.01). There was no effect of residual disease, i.e., optimal (n = 9) versus suboptimal surgery (n = 18), on various T cell subsets.
Median follow-up was 13.6 months (range 1–24.13 months). Eleven (26.8 %) patients had progressed, and five (12.2 %) patients had died by the date of data censoring (31/7/14). None of the baseline T cell subsets frequencies as well as their ratios correlated with PFS or OS (supplementary table 2).
Discussion
The regulatory T cells have the potential to suppress several lymphocyte subsets. It has been suggested that they suppress anti-tumor immunity and help the cancer cells to survive. The up-regulation of Tregs in cancer has been demonstrated in previous studies [3, 8]. In addition to studies of Tregs in tumor tissues, increased Tregs have been demonstrated in peripheral blood, ascitic fluid and pleural fluid as well [6, 9, 10]. Similar to previous studies, frequency of Tregs in peripheral blood at baseline was significantly higher in patients than the controls (9.3 ± 2.8 vs. 7.1 ± 2.08, p = 0.004) in our cohort [11, 12]. We also found significantly lower CD4+ T cells in patients as compared to the healthy controls (50.96 ± 14 vs. 61.8 ± 13.78, p = 0.005), and as a result, there was no significant difference in absolute numbers of Tregs in patients and control groups.
CD8+ T cells are cytotoxic to tumor cells and generally considered as good prognostic marker for survival and were higher in patients (39 ± 12 vs. 31 ± 11.2, p = 0.01). However, no statistically significant difference was found in the levels of CD8+ cells post-treatment in the present study. Wu et al. [11] demonstrated that the CD8+ T cells were lower in patient group, while Milne et al. [12] did not find any significant difference between the patient and control group. This gives a compelling reason to look for subsets of CD8+ cells which may play a role in tumor induced immunosuppression. Karagoz et al. [14] have reported an increase in CD8+ Tregs in patients with carcinoma lung with increase in CD8+ CD28− cells and ratio of CD8+ CD28−/CD8+ CD28+ cells. Similarly, higher frequency of CD8+ CD28− cells has been reported in other malignancies like cutaneous T cell lymphomas [15], hepatocellular carcinoma [16] and prostate cancer [17]. In the present study, however, although an increase in CD8+ T cells was noted, further subset evaluation was not done.
The ascitic fluid represents local milieu of ovarian cancer, and therefore, ascitic fluid may be a better indicator of tumor microenvironment than PB. Considering this, the T cell subsets were evaluated in ascitic fluid, but apart from an increase in total number of T cells, we did not find any significant differences in either of the T cell subsets when compared with paired PB samples. This, therefore, suggests that PB is as good an indicator of tumor microenvironment with respect to T cell subsets as the ascitic fluid. Two recent studies have reported relatively higher numbers of CD4+ CD25+ Tregs in ascitic fluid compared to peripheral blood in ovarian cancer patients [6, 20]. This difference can be attributed to the use of FoxP3 expression along with CD4 and CD25 to enumerate Tregs in our study.
We evaluated the changes in T cell subsets with treatment. There was no significant change in relative percentages of CD4+ T, CD8+ T cells, Treg cells and NK cells post-treatment which is similar to the results reported by Park et al. [13]. In an earlier study, Napoletano et al. [18] observed decrease in Tregs post-treatment. A recent study by Wu et al. [11] also indicated that levels of IFN-γ produced by peripheral blood CD4 and CD8 effector T cells may temporarily increase, while CD4+ CD25+ Treg levels may decrease at the start of chemotherapy, suggesting an increase in effector function after the first round of chemotherapy. The possible explanation of this temporary effect can be that the standard chemotherapeutic agents do not affect the tumor immunology as such. Antibodies to Treg cells such as ONTAK [19], which have been used to deplete Tregs from peripheral blood of patients with melanoma to enhance immune response, may be useful in treatment of other solid tumors including ovarian cancer. In addition, immune modulators like lenalidomide and thalidomide which are known to correct the altered CD4/CD8 ratio in diseases like multiple myeloma and chronic lymphocytic leukemia may be effective in improving treatment efficacy by modulating T cell microenvironment in EOC patients as altered CD4/CD8 ratio was also observed in EOC patients in the current study.
Conclusions
An attempt to investigate the kinetics of T cell subsets in peripheral blood and ascitic fluid in patients of advanced-stage carcinoma ovary with serous histology was conducted in this study. The absence of statistically significant differences in the frequency of Treg cells between PB and ascitic fluid suggests that peripheral blood is as good an indicator of tumor microenvironment as the ascitic fluid in ovarian cancer. As compared to controls, the patient group had significantly increased Tregs, CD8+ cells and altered CD4/CD8 ratio. A persistent increase in CD8+ T cells as observed in this study further suggests that perhaps a specific CD8+ T cell subset may be one of key players in tumor immunology, and thus, further subset analysis of CD8+ T cells may be attempted in future studies. Unchanged levels of T cell subsets subsequent to standard chemotherapy call for evaluation of role of immunotherapy and immune modulators in decreasing the numbers and effectiveness of Tregs, promoting dendritic cell–NK cell cross-talk and augmenting CD8+ T cell differentiation into memory cells in patients of advanced-stage serous carcinoma ovary to enhance the efficacy of standard chemotherapy.
References
Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol Off J Am Soc Clin Oncol. 2003;21:3194–200.
Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–71.
Chen M-L, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–24.
Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+) CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72.
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Oxf Engl 1990. 2009;45:228–47.
Peng D-J, Liu R, Zou W. Regulatory T cells in human ovarian cancer. J Oncol. 2012;2012:345164.
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol Baltim Md 1950. 1995;155:1151–64.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61.
Wu X, Feng Q-M, Wang Y, Shi J, Ge H-L, Di W. The immunologic aspects in advanced ovarian cancer patients treated with paclitaxel and carboplatin chemotherapy. Cancer Immunol Immunother CII. 2010;59:279–91.
Milne K, Alexander C, Webb JR, Sun W, Dillon K, Kalloger SE, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med. 2012;10:33.
Park A, Govindaraj C, Xiang SD, Halo J, Quinn M, Scalzo-Inguanti K, et al. Substantially modified ratios of effector to regulatory T cells during chemotherapy in ovarian cancer patients return to pre-treatment levels at completion: implications for immunotherapy. Cancers. 2012;4:581–600.
Karagöz B, Bilgi O, Gümüs M, Erikçi AA, Sayan O, Türken O, et al. CD8+ CD28− cells and CD4+ CD25+ regulatory T cells in the peripheral blood of advanced stage lung cancer patients. Med Oncol Northwood Lond Engl. 2010;27:29–33.
Urbaniak-Kujda D, Kapelko-Słowik K, Wołowiec D, Dybko J, Hałoń A, Jaźwiec B, et al. Increased percentage of CD8+ CD28− suppressor lymphocytes in peripheral blood and skin infiltrates correlates with advanced disease in patients with cutaneous T-cell lymphomas. Postȩpy Hig Med Dośw Online. 2009;63:355–9.
Qiu Y-R, Yang C-L, Chen L-B, Wang Q. [Analysis of CD8(+) and CD8(+) CD28(−) cell subsets in patients with hepatocellular carcinoma]. 1 Jun Yi Xue Xue Bao Acad J First Med Coll PLA. 2002;22:72–3.
Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13:6947–58.
Napoletano C, Bellati F, Landi R, Pauselli S, Marchetti C, Visconti V, et al. Ovarian cancer cytoreduction induces changes in T cell population subsets reducing immunosuppression. J Cell Mol Med. 2010;14:2748–59.
Mahnke K, Schönfeld K, Fondel S, Ring S, Karakhanova S, Wiedemeyer K, et al. Depletion of CD4+ CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer J Int Cancer. 2007;120:2723–33.
Landskron J, Helland Ø, Torgersen KM, Aandahl EM, Gjertsen BT, Bjørge L, et al. Activated regulatory and memory T-cells accumulate in malignant ascites from ovarian carcinoma patients. Cancer Immunol Immunother CII. 2015;64:337–47.
Acknowledgments
This work was supported by a research Grant (No. 5/13/108/2011/NCD-III) from Indian council of Medical Research, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baghmar, S., Kumar, L., Rani, L. et al. Prognostic Relevance of T Regulatory Cells in Patients with Advanced-Stage Serous Carcinoma Ovary. Indian J Gynecol Oncolog 14, 20 (2016). https://doi.org/10.1007/s40944-016-0050-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40944-016-0050-8