Abstract
Freshwater quality is declining globally due to changes in natural and human-driven land use. Lake Tana, Ethiopia’s largest lake, exemplifies this change. Human activities within its watershed have pushed it toward a trophic state, evident in the expansion of water hyacinths since 2011. This research aimed to evaluate the degree of pollution and trophic state of Lake Tana and identify the most important factors for effective management strategies. Eleven water quality parameters were collected from 20 sampling stations in Lake Tana from July 2018 to June 2019. Spatial distribution maps for water quality, trophic state and pollution index were generated using ArcMap. Water quality indicators were explored through factor analysis (FA) and principal component analysis (PCA). Lake Tana’s trophic level index ranges from 37.1 (oligotrophic) to 87.9 (hypereutrophic) annually. The pollution index classification indicated that the lake fell within categories three and four, changing from slightly polluted to polluted. According to the PCA and FA methods, oxygen depletion, nutrients, eutrophication, and suspended solids are identified as major factors influencing deteriorating water quality. Shores exhibit higher eutrophication and pollution levels than pelagic zones. Thus, all stakeholders must actively participate in management strategies prioritizing coastal areas and targeting the identified pollution factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The decline in lake water quality globally, especially in developing countries, poses significant risks to health, economic stability, and societal well-being (Akhtar et al. 2021). A combination of natural and human elements causes this decline. Human-related factors contributing to lake water quality deterioration include industrial wastewater, agricultural runoff, and domestic sewage. The increase in population and the expansion of industries have led to lake pollution, resulting in issues such as salinization, eutrophication, and the degradation of wetlands (Mishra 2023). These factors disrupt ecosystem functioning and compromise drinking water safety. Harmful infestations of planktonic algae, known as algal blooms, significantly interfere with lake water and aquatic life (Kumar et al. 2023; Monteiro et al. 2021). Also, algal blooms have become more prevalent in lakes worldwide (Ho et al. 2019; Huang et al. 2020).
Nutrient and light availability play crucial roles in supporting the growth of plants and animals in lake ecosystems. Plankton community growth can be affected by light conditions, while high chlorophyll-A concentrations contribute to eutrophication and potentially reduce dissolved oxygen (DO) levels in lakes (Chao et al. 2022). Phytoplankton growth is influenced by nutrient flux, chlorophyll levels, and physical and chemical factors (Girdner et al. 2020; Ishikawa et al. 2022; Tasnim et al. 2021).
Freshwater ecosystems are facing major threats from floods and the use of agrochemicals. Excessive phosphorus and nitrogen inputs trigger the transition from an oligotrophic to a hypertrophic state, leading to the harmful overgrowth of cyanobacteria (Dubey and Dutta, 2020). This includes the resuspension of sediments, significant internal nutrient loads from the bottom residue to the upper water, and lake residence time (Yang and Yin, 2022).
Lake Tana, Ethiopia’s largest freshwater body, is experiencing rapid deterioration in water quality (Moges et al. 2017; Tibebe et al. 2019; Wondim 2016). Increased agricultural activity in upstream areas and sediment deposition contribute to nutrient-rich inflows. Various studies have been conducted on Lake Tana. For example, water hyacinth (Anteneh et al. 2015; Dersseh et al. 2019; Tewabe 2015), water balance (Alemu et al. 2020; Dessie et al. 2015), sediment available nutrient characteristics (Kebedew et al. 2020a), which reassesses the accuracy of lake level data obtained through radar altimetry (Kaba 2007), suspended sediment flux budgeting (Zimale et al. 2018), and water quality evaluation using Landsat 7 ETM + images (Moges et al. 2017). Abate et al. (2017) have also reported on escalating nutrient-rich inflows and sediment deposition. Despite these previous research efforts, comprehensive assessments of lake-wide water quality, eutrophication patterns, and pollution status are still lacking. This has resulted in inadequate information about the trophic state and water pollution level of the Lake. Moreover, none of the prior studies employed PCA or FA methodologies to investigate the water quality. As a result, there is a limited understanding of the major factors contributing to the deterioration of water quality in Lake Tana. The objective of this study is to use statistical techniques such as PCA and FA to uncover the underlying relationships among various water quality parameters in Lake Tana. This will provide valuable insights into the factors contributing to water quality degradation. The information is essential for developing effective management strategies to mitigate water pollution, control eutrophication, and preserve ecological integrity. Additionally, the findings of this study can be used as a basis for future research and policymaking initiatives related to freshwater resource management and environmental conservation in the region.
Materials and methods
Study area
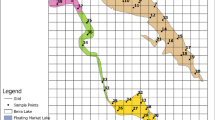
Lake Tana is located northwest of the Ethiopian highlands and has coordinates between 11030’ N, 3700’ E, and 12020’ N, 37040’ E. It received recognition from UNESCO as a biosphere reserve (Kalmbach 2017). Satellite images and the bathymetric survey show that the lake is nearly cylindrical, with an axis of 80 km in the north-south direction and 65 km east-west. At the average lake elevation of 1786.5 m a.s.l, the area of the lake was 3046 km2, with a volume of 29.6 km3 and a shore length of 431 km. The average depth is 9.7 m, the median depth is 10.5 m and the maximum depth is 14.8 m. Since the length and the width are much larger than the depth, Lake Tana is classified as a shallow and well-mixed lake (Vijverberg et al. 2009; Alemu et al. 2020). Consequently, since the calculated wave base depth extends up to Lake Tana’s maximum depth of 14.8 m, there is a strong relationship between the dissolved and available phosphorus (Kebedew et al. 2020b, 2023). Four large rivers and more than 40 small, intermittent rivers feed the lake. The four major rivers, Rib, Megech, Gilgel Abay, and Gumara, contribute 90% of the lake’s water and most of the materials that are transported to the lake (Alemu et al. 2020). Dessie et al. (2015) found that Gilgel Abay contributes 58% of the inflow water, Rib and Gumara from the east contribute 30%, Megech from the north contributes 10%, and numerous small streams in the west contribute 2%. Zimale et al. (2018) also estimated that the sediment budgets from the Megech, Rib, Gumara, and Gilgel Abay Rivers were 0.9 Mt. yr-1, 3.9 Mt. yr-1, 8.2 Mt. yr-1, and 14.3 Mt. yr-1, respectively.
The lake and its surrounding wetlands support the livelihoods of more than half a million people (Vijverberg et al. 2009). Lake Tana and its surrounding areas were designated as growth corridors by both regional and federal governments, leading to the implementation of various irrigation systems. The increasing trend in agricultural land use in lake watersheds significantly impacts sediment yields (Andualem and Gebremariam 2015). Recent growth of water hyacinths has occurred (Anteneh et al. 2015; Tewabe 2015; Dersseh et al. 2019).
Location map of the study area: (a) location map of Ethiopia in Africa (https://datacatalog.worldbank.org/dataset/world-bank-official-boundaries); (b) map of Ethiopia and Ethiopia’s lakes; and (c) map of Lake Tana, which indicates the sampling stations, including the major inlets and outlet rivers
Sampling design
The water samples were collected from 20 sampling locations in Lake Tana, as shown in Fig. 1. The sampling stations were identified by considering the proximity and accessibility of the locations, the level of anthropogenic activity in the areas, the potential for agricultural runoff loading, and the major river outlets. A 10 km by 10 km grid was used to assess eleven water quality indicators: temperature (T), turbidity, Secchi depth (SD), pH, dissolved oxygen (DO), electrical conductivity (EC), total dissolved solids (TDS), nitrate, chlorophyll-A (Chl-a), phosphate, and ammonia. The water samples were collected quarterly throughout a single research year (2018–2019), representing the distinct seasonal changes. A Van Dorn water sampler (Fig. 2a) was used to collect the water samples at the designated sampling locations within the grid system.
Data collection
Water quality parameters were measured both in situ and in the laboratory. For parameters analyzed in the laboratory, water sampling, sample preservation, transportation, and analysis adhered to standard protocols (Bartram and Balance 1996). In situ measurements of dissolved oxygen (DO), temperature (T), pH, Total Dissolved Solids (TDS), and electrical conductivity (EC) were conducted using a portable field kit instrument (Aqua Probe-7000) (Fig. 2c). Secchi disk (SD) was also measured in-situ using a 20-cm diameter black and white marked circular disk (Fig. 2b) to measure the visible depth. A marked rope was used to submerge the disk, and the depths at which the disk was no longer visible from the surface were recorded. Turbidity and nitrate, phosphate, and ammonia levels were determined using an automated wavelength photometer system at the Water Treatment and Hydrology Lab of Bahir Dar Institute of Technology, Bahir Dar University.
Optical density measurement and chlorophyll-A (Chl-a) calculations
The chlorophyll-A (Chl-a) analysis involved an acetone extraction process, where the water samples were filtered through a pre-washed 0.47-µm-diameter membrane filter. The optical density of the filtered samples was measured at wavelengths of 750 nm, 663 nm, 645 nm, and 630 nm using a Jenway UV-6405 spectrophotometer. The Chl-a concentration was calculated using the methodology described in Han et al. (1994).
Ve is the acetone volume used for extraction (ml), Vf is the volume of water in the sample water filtered (ml), and L represents the length of the cell path (cm).
Interpolation
Spatial interpolation was performed to generate maps depicting patterns of water quality, trophic level index (TLI), and pollution index (PI) using ArcGIS software. The maps were created based on the average values of the four seasonal’ measurements utilizing the kriging interpolation tool in ArcGIS.
Trophic level index (TLI)
A comprehensive TLI was conducted following the methods described by Huo et al. (2013). The concentrations of Chl-a, SD, total phosphorus (TP), and total nitrogen (TN) were input parameters to calculate the TLI. The overall TLI (∑) represents the combined TLI, where Wj is the correlative weightiness of parameter j, and TLIj is the trophic level index of parameter j.
Chl-a is the standard parameter, and \(\:{r}_{j}\) represents the correlation coefficient between parameter j and Chl-a. SD, TP, and TN were used as quantitative trophic status indicators.
Pollution index (PI)
The pollution status of Lake Tana was assessed using the pollution index (PI) method, as described by Yan et al. (2015). This approach involved calculating the concentration of pollutants (Ci) and comparing them to the tolerable limits (Si) for freshwater quality. The resulting PI value was then used to categorize the overall pollution level of the lake water.
where m represents the number of pollutants.
Statistical analysis
Correlation analysis
All the statistical analyses were performed using Microsoft Excel 2019 and statistical software IBM SPSS 24.0. Pearson correlation analysis was employed to assess the relationships among the various water quality indicators in Lake Tana.
Principal component analysis (PCA)
PCA is used to identify the key water quality parameters (factors) that contribute the most to the observed variability. It can determine the principal components that account for most water quality data variance. These principal components represent the dominant factors influencing water quality. The main objective of PCA was to maintain the original variability in the data (Cho et al. 2023). Primarily, the covariance matrix was determined. If X represents the original dataset in a matrix setup with m rows (representing different measurements of a particular characteristic) and n columns (the attributes), the covariance matrix, \(\:{C}_{x}\), is estimated as follows:
Next, the eigenvalues (λ) and eigenvectors (𝑣) are computed as follows:
PCA uses XT as the transpose matrix of X and I as the identity matrix of the same dimension as \(\:{C}_{x}\). The number of components is equal to the number of datasets. Most of the information is found in the first component, followed by the second, third, and so on. The number of components is typically determined by eigenvalues greater than 1 (Cho et al. 2023).
Factor analysis (FA)
The factor analysis (FA) was employed to examine the deviations in each water quality variable and identify the dominant pollution factors influencing Lake Tana. The FA model interpreted the principal factors using composite variables and calculated the percentage of variation by the factor loadings. Eigenvalues for each factor were subsequently computed using the principal component analysis (PCA) extraction method, as presented in Table 1.
Results
Spatiotemporal results of the water quality indicators
The analysis of the water quality parameters revealed variations in space and time. Turbidity levels were significant (p < 0.05), ranging from 11.5 to 273 NTU, with an average of 22 to 231 TU during the dry and wet seasons. High turbidity concentrations, predominantly exceeding 50 NTU, were observed at the major river entrance sites (Rib, Gumara, Megech, and Gilgel Abay), as illustrated in Fig. 3a.
The water temperature of Lake Tana ranged from 18 °C during the wet season to 29 °C during the dry season. Parts of the lake have been observed to exceed 25 °C, which is the maximum limit for freshwater ecosystems. The pH of Lake Tana was observed to range from 5.9 to 8.4, with an average annual range of 6.8 to 7.2. Spatially, station S15 exhibited nearly acidic values in August 2018, while station S19 exhibited an alkaline pH in April 2019, just before the rainy season. Higher pH values were documented in the northeastern direction (stations S10 and S17) and at the entrance of the Megech River, as illustrated in Fig. 4a.
Lake Tana’s electrical conductivity (EC) and total dissolved solids (TDS) ranged from 101to 169 µS/cm and 67 109 mg/L, respectively. The Minimum EC and TDS values were recorded during the wet season, while maximum values occurred throughout the dry season. The dissolved oxygen (DO) concentrations in Lake Tana ranged between 3.1 and 5.9 mg/L, with average values varying from 3.45 to 4.93 mg/L. Additionally, the ammonia concentrations ranged from 0.006 to 0.07 mg/L, with an average value ranging from 0.014 to 0.033 mg/L.
Trophic state variables
The Secchi depth (SD) in Lake Tana ranged from 15 to 107 cm, and the average depth was between 25 and 86 cm. The minimum and maximum values of SD were observed during the wet and before the rainy seasons. The phosphate concentration in the lake was between 0.03 and 0.61 mg/L (Fig. 4e). The mean phosphate concentrations were 0.58, 0.51, 0.45, and 0.50 mg/L in the rainy, rainy, dry, and before rainy seasons. The nitrate concentrations ranged from 7.3 to 17.6 mg/L, and the mean values ranged between 8.9 and 15.3 mg/L (Fig. 4d). The spatial variability in the nitrate concentration was significant (p < 0.05). The nitrate concentrations in the western, eastern, and northern parts of the lake were greater than those in the open water and southern (outflow) areas (Fig. 4d). Seasonally, nitrate concentrations tend to increase from the dry season to the rainy season.
Chl-a concentrations varied between 31 and 98 g/L, with average levels falling between 35 and 89 µg/L (Fig. 3e). Compared to previous studies, the Chl-a concentration observed in our study (Fig. 3d) notably exceeded those found elsewhere, particularly in the southeast and east of the lake. Recent investigations showed that the Rib and Gumara outlets east of the lake have greater nutrient influxes and amplify Chl-a levels.
Eutrophication and pollution status in Lake Tana
The findings of a single TLI for Chl-a concentration, phosphate concentration, nitrate concentration, and SD at twenty sampling stations across four seasons are presented in Table S5 of the supplementary material. The seasonal distributions of TLI for every sampling station are plotted in Fig. 5. The average TLI values varied from 37 to 87 in the dry and rainy seasons, respectively (i.e., higher oligotrophic to hypereutrophic). Comprehensive PI values are depicted in Fig. 6. The annual PI results were between 0.68 and 1.86.
Statistical analysis
Correlation analysis
Table 2 shows the results of a Pearson correlation analysis among turbidity, temperature, SD, pH, EC, TDS, DO, ammonia, phosphate, nitrate, and Chl-a. The significance test for all water quality variables in the correlation matrix was at the 95% confidence level (p = 0.05). The strong correlation exists between sediment-related parameters such as turbidity, and Secchi disk was expected. Phosphate concentration and turbidity were also related because the lake is constantly mixed, and the phosphate is in equilibrium with the suspended sediment (Kebedew et al. 2020b) The correlation of other parameters, with the exception of nitrate, is poor because they are not affected by the suspended sediment.
Principal component analysis
The principal factors extracted from the eleven water quality variables are shown in Table 1.
Factor analysis
Using the PCA analysis, three principal factors were extracted (Table 3). The first factor (F1) had a strong negative correlation with DO concentration (-0.82) and was strongly positively correlated with turbidity (0.91). The second factor (F2) was strongly negatively linked to EC and TDS and positively related to Chl-a. The third factor (F3) had a strong positive relationship with temperature. In the second section of Table 3, the rotated component matrix shows the cumulative deviation percentage by extracted components.
Discussion
Physical and chemical water quality indicators
Turbidity, temperature and dissolved oxygen (DO)
Lake Tana (Fig. 3a) has high turbidity concentrations, especially near the outlet of the four major rivers (Rib, Gumara, Megech, and Gilgel Abay) that transport. Sediment in these rivers originates mainly from active gullies upstream (Zegeye et al. 2016). Similarly, Wondim (2016) observed a high turbidity concentration (5–989 NTU) in Lake Tana. Sediment clogs fish gills, impairing their ability to find food (Newport et al. 2021), hindering visual attack feeders and benefiting large-body zooplankton survival. The western and northern shorelines of Lake Tana experience increasing temperatures (Fig. 3b), which can boost biochemical reactions and impact microbial development in water. The variation between the lowest and highest temperatures during this research period was 12 °C. This variability could significantly impact biochemical reactions and the lake’s ecosystem balance. Lake Tana has nearly comparable temperature ranges to the lakes in Annex 2.
The low dissolved oxygen concentrations in Lake Tana, ranging from 3.1 to 5.9 mg/L, pose a significant limitation for fish. The littoral areas had lower DO concentrations than the pelagic parts (Fig. 4b). The annual average DO concentration did not exceed 4 mg/L, suggesting that DO is a major limiting factor for fish in Lake Tana. The primary cause of this low DO concentration is attributed to the high turbidity in Lake Tana. Turbidity can reduce oxygen availability by limiting light penetration and hindering plant growth in the water. Other factors, such as temperature, turbulence, rainfall events, and plant productivity, could reduce the lake’s DO. The lowest DO levels were at locations S11, S12, S16, and S17 (Fig. 1) in the northeast of Lake Tana, which is the shallowest and has the greatest dissolved P concentrations (Kebedew et al. 2023). DO levels increase slightly during the wet season due to plant growth, lower temperatures, higher rainfall intensity, and freshwater mixing. Lake Tana’s DO level is in the lower range of other tropical lakes (Annex 2), which is consistent with the findings of Tibebe et al. (2019).
Electrical conductivity, total dissolved solids, pH and ammonia
Lake Tana exhibited the highest EC values during the rainy season, after which the values decreased. The maximum EC value of 169 µS/cm is much lower than Lakes Hawassa, Abaya, Chagan, Chamo, and Bellunder but higher than the lake’s EC in Arenguade and Shalla (Annex 2). This is positive news for aquatic health, biodiversity, and other impacts in Lake Tana. However, continuous monitoring is necessary to maintain low salinity levels. The TDS concentrations consistently remained below 100 mg/L. The current findings indicate no chronic effects on aquatic or human health. Nonetheless, slight fluctuations in TDS suggest the introduction of anthropogenic and naturally occurring inorganic salts and organic chemicals into the lake. Figure 3e & f demonstrate relatively higher EC and TDS levels in the northern regions of the lake, attributed to agricultural runoff, saline chemicals, and solid materials.
Aquatic organisms thrive within pH ranges of 5.0–9.0 (Cotruvo 2017). In our study, the annual average pH ranged from 5.9 to 8.4, consistent with previously published values of 7.0–9.9 (Wondim 2016) and 6.5–9.2 (Tibebe et al. 2019). Similarly, other studies have reported pH values within the range of 8.9–9.0 for Lake Chamo (Ghebremedhin and Gupta, 2023) and 7.1–9.3 for Lake Hawassa (Menberu et al. 2021) in Ethiopia. The pH level observed in Lake Tana falls within the recommended limits for aquatic health and human health. The pH level plays a critical role in predicting the toxicity of ammonia in aquatic animals.
The ammonia levels in Fig. 4c are elevated, especially near the eastern shore, threatening aquatic fauna, particularly fish. Fish are extremely sensitive to concentrations of ammonia (Wang et al. 2017). When the pH rises, even small amounts of ammonia can become toxic to fish, decreasing the capacity of the blood to carry oxygen. Depending on the pH and temperature conditions, this can result in suffocation (Wang et al. 2017). Therefore, fish in Lake Tana may be at risk of ammonia toxicity in areas with elevated pH and temperature. Ammonia likely originates from the urea used by farmers as fertilizer. Reducing its input to the lake demands immediate attention to protect aquatic life.
Trophic state parameters
The Secchi Disk (SD) is widely used to measure water transparency. It involves using a circular disc with white and black sections to determine the visible depth of the water. According to Duggan and Kotalik (2019), an annual average SD value should not be less than 10% of the lake’s natural mean depth. Lake Tana, with an average depth of 9.7 m (Kebedew et al. 2020b), is taken as a reference. Our study revealed that the average SD value at each station and season fell below the threshold, indicating the presence of algae and suspended sediments. SD values decrease from the lake’s coastal regions toward its pelagic zones (Fig. 3c). The dry season exhibits higher SD values than the wet season. Turbidity and algal blooms, particularly during rainfall, are the primary factors affecting water clarity in coastal regions of lakes.
In our study, the highest phosphate concentration (0.61 mg/L) occurred during the wet season, while the lowest value (0.02 mg/L) was recorded during the dry season. The phosphate levels in Lake Navaishia (Kenya) are similar and range from 0.021 to 0.83 mg/L (Ndungu et al. 2013). Phosphorus accelerates the growth of algae and aquatic plants and reduces oxygen levels when in excess (Fig. 4e). During the wet season, phosphate concentrations are highest near the outlets of the Gilgel Abay, Rib, Gumara, and Megech Rivers. In Lake Tana, nitrate is limiting, and phosphorus concentrations are not directly linked with eutrophication levels (Dersseh et al. 2022).
The maximum desirable nitrate levels should be maintained at 50 mg/L for aquatic life and 45 mg/L for drinking water usage (Cotruvo 2017; Duggan and Kotalik, 2019). High nitrate concentrations were recorded in the shoreline regions of Lake Tana (Fig. 4d), most likely due to agrochemical release and fish farming activities. Ewnetu et al. (2014) observed comparable nitrate concentrations (2.7–12.1 mg/L). However, the current Lake Tana exceeded those of most tropical lakes (Annex 2). Nitrate levels (1.8–8.0 mg/L) reported by Gezie et al. (2018) were slightly lower than those in the present study. The increasing trend of nitrate is associated with intensifying agriculture and the increase in the use of fertilizers on farmland in the catchment.
The current Chl-a concentrations (35 to 89 g/L) showed an increasing trend compared to previous findings (27–50 µg/L) by Tibebe et al. (2019). This indicates nutrient availability, promoting algal bloom growth. This study identified factors influencing Chl-a levels in Lake Tana, polluted with various pollutants. Phosphorus and nitrogen significantly impact algae growth, reflecting the poor health of the lake ecosystem. The water hyacinth dispersion in Lake Tana could be linked to lake circulation that deposited P-rich sediment in the Northeastern part of the Lake (Kebedew et al., 2022a). The lowest Chl-a concentration occurred in the rainy season due to reduced algal blooms from increased wind waves, rainfall, diminished light, and greater dilution caused by runoff. The highest levels of Chl-a were found in the post-rainy and dry seasons and were attributed to stagnant water, ample light, enhanced phytoplankton growth, and biochemical changes.
Eutrophication and pollution indices
A comparison of the mean eutrophication values with the criteria of Duggan and Kotalik (2019) confirmed a middle-eutrophic to hypereutrophic state, potentially due to nutrient flux. Nutrient intrusions from sewage, agriculture, and cattle enrich the lake, expanding water hyacinths and algal blooms. Lake Tana’s eutrophication status affects its ecological network, requiring the monitoring of nutrient loads. Figure 7a also demonstrated eutrophic conditions across all sites in Lake Tana. Higher TLI values are observed in the northeastern (Megech and Rib Rivers joining), southeastern (near the Gumara River), and southwestern (near the entrance of the Gilgel Abay River) areas. Eutrophication levels increase from the central to the shoreline parts of the lake. The eutrophication status in Fig. 5 shows important seasonal variability. The maximum TLI (92) was observed during the rainy season. Most of Lake Tana is in middle-eutrophic to higher-eutrophic conditions, covering 30% and 40% of the lake area, respectively. Approximately 10% of the lake is in a hypereutrophic state. Blue-green algal blooms, dead fish, and unpleasant odors were observed during the field assessments, particularly near the shorelines and islands. This is associated with hypereutrophic conditions. Approximately 20% of the lake remains oligotrophic, mesotrophic, and moderately clear but susceptible to eutrophication. Previous studies reported lower average TLIs for Lake Tana (Tibebe et al. 2019). The present study confirmed an increasing trend in TLI associated with intensifying agriculture and a lack of conservation practices limiting the influx of sediment and nutrients.
The seasonal variations in PI in Fig. 6 showed that Lake Tana’s water exceeded the initial PI level of 0.4 set by Yan et al. (2015). Based on the determined comprehensive PI, the pollution level in Lake Tana was predominantly in classes III (slightly polluted) and IV (polluted), according to Annex 1. Multiple sources contribute to pollution, including domestic waste, agriculture, fishing, industrial activities, and swimming. The production of water hyacinths in lakes is an example of increasing pollution levels. Key locations that influence water quality are near Bahir Dar town and the mouths of the Megech, Rib, Gumara, and Gilgel Abay Rivers (Fig. 7b). These areas experience significant deterioration in water quality due to wastewater inflow from nearby towns and high sediment loads, particularly during the rainy season.
Correlation, principal component and factor analysis
Turbidity and temperature were strongly positively correlated with ammonia, phosphate, nitrate, and Chl-a (Table 2). These findings suggest that organic pollutants, such as agrochemicals, may be potential sources of pollution. The correlation matrix showed that the concentration of Chl-a (p < 0.05) increased with nutrient intrusion and turbidity but reversed with DO. Temperature exhibited a strong negative correlation with DO and a weak negative correlation with EC and total TDS. pH (p > 0.05) exhibited a weak positive relationship with turbidity, ammonia, nitrate, and Chl-a, while it was moderately correlated with temperature, EC, TDS, DO, and phosphate. Although pH is not a primary factor in Lake Tana’s water pollution, increased alkalinity can significantly impact fish due to the presence of ammonia. Notably, there was a strong negative correlation between turbidity and SD, indicating that sediment loads and topsoil inflows adversely affect the water clarity of the lake. The oxygen levels were negatively correlated with phosphate, indicating that nutrient levels were linked to oxygen depletion.
In PCA, the top three principal components were identified when eigenvalues larger than one were extracted. These components explain most of the variability in the initial eleven variables. The initial and extracted components are displayed in the first and second sections of Table 1. These three components explained a total of 91% of the initial variables, significantly reducing the complexity of the dataset with only a 9% loss of information. PCA summarized the aggregate percentage of variation described by the rotated components. Convergence was achieved after five iterations in SPSS.
Factor analysis also offers valuable insights for interpreting each factor, particularly in water quality analysis. In Table 1, the factor analysis findings demonstrated the three extracted factors. F1, which accounts for 50% of the total variance, is characterized by high loadings of turbidity, ammonia, phosphate, and nitrate (Table 3). Therefore, F1 is defined as the suspended solids pollution factor (SSPF) and the nutrient pollution factor (NPF). A higher concentration of Chl-a also had a strong influence on F2. This explains 26% of the overall variance. This factor is referred to as the eutrophication pollution factor (EPF) and supports the trophic level findings from both this study and Tibebe et al. (2019). F3 captured 15% of the total variance, primarily driven by temperature and DO. Temperature catalyzes a wide range of biochemical reactions in water, which can directly impact DO levels and lead to depletion. Hence, F3 is defined as the oxygen depletion factor (ODF).
Implications for future water quality management in Lake Tana
The variations in water quality in Lake Tana are likely linked to the area’s climate and seasonal changes. This study aimed to understand how nutrient levels and other water quality measures have changed over time and location in Lake Tana. The increasing pollution from sources such as agricultural runoff, sewage discharge, and upstream erosion may be causing a significant decline in the lake’s water quality. To address this, it’s important to control the release of fertilizer runoff and soil erosion near major river mouths in the northern and northeastern areas, and to reduce aquaculture growth and fertilizer use. Additionally, more research is necessary to assess water quality in Lake Tana at various scales. Remote sensing methods can also be valuable for studying water quality changes over long periods and large areas.
Conclusions
In this research, a comprehensive evaluation of the water quality, eutrophication, and pollution levels in Lake Tana was conducted. The findings underscore substantial pollution and eutrophication, particularly in the northern and northeastern areas. There were noticeable spatial and seasonal discrepancies, with higher levels of turbidity, suspended solids, phosphate, nitrate, and TLI during the rainy seasons, attributed to increased rainfall, runoff, and nutrient enrichment. Conversely, lower levels of ammonia, dissolved oxygen, and chlorophyll-A were observed during dry seasons due to low flows in the rivers and intensified phytoplankton growth. The presence of high turbidity concentrations, low DO levels, and pollutants such as ammonia, phosphate, nitrate, and Chl-a suggested potential sources of pollution from agricultural runoff, sewage discharge, and other human activities.
The lake was identified to be in a middle-eutrophic to hypereutrophic state, with significant nutrient fluxes contributing to algal blooms and degraded water quality. Pollution levels, based on pollution indices, were mainly categorized as slightly to heavily polluted, especially in areas near major river mouths and coastal regions. Strong correlations were observed between various water quality parameters, highlighting the interconnectedness of physicochemical factors in Lake Tana. Principal Component Analysis (PCA) and Factor Analysis (FA) identified significant pollution factors, including suspended solids pollution factor (SSPF), nutrient pollution factor (NPF), eutrophication pollution factor (EPF), and oxygen depletion factor (ODF), providing insights into the type and extent of pollution in the lake.
Water quality degradation in Lake Tana’s coastal areas is worse than in the pelagic areas due to higher nutrient and pollutant inputs from agricultural activities and sediment intrusions. Effective management strategies are needed to address these challenges, emphasizing proactive actions and conservation measures to safeguard this vital freshwater resource. Control measures targeting nutrient and sediment inputs, such as soil erosion and agricultural runoff, are essential for preserving the lake’s ecological health. Continued monitoring and remote sensing practices are recommended to track water quality dynamics and inform management decisions over time.
Annex
Data availability
All relevant data are included in the paper or its supplementary section.
References
Abate M, Nyssen J, Moges MM, Enku T, Zimale FA, Tilahun SA, Steenhuis TS (2017) Long-term landscape changes in the Lake Tana Basin as evidenced by delta development and floodplain aggradation in Ethiopia. Land Degrad Dev 28(6):1820–1830
Akhtar N, Ishak S, Bhawani MI, S. A., Umar K (2021) Various natural and anthropogenic factors responsible for water quality degradation: a review. Water 13(19):2660
Alemu ML, Worqlul AW, Zimale FA, Tilahun SA, Steenhuis TS (2020) Water balance for a tropical lake in the volcanic highlands: Lake Tana, Ethiopia. Water 12(10):2737
Andualem TG, Gebremariam B (2015) Impact of land use land cover change on stream flow and sediment yield: a case study of Gilgel Abay watershed, Lake Tana Subbasin, Ethiopia. Arba Minch University
Anteneh W, Tewabe D, Assefa A, Zeleke A, Tenaw B, Wassie Y (2015) Water hyacinth coverage survey report on Lake Tana Biosphere Reserve. Techn Rep Series, 2
Bartram J, Ballance R (1996) Water quality monitoring: a practical guide to the design and implementation of freshwater quality studies and monitoring programmes. CRC
Chao C, Lv T, Wang L, Li Y, Han C, Yu W, Yan Z, Ma X, Zhao H, Zuo Z, Zhang C (2022) The spatiotemporal characteristics of water quality and phytoplankton community in a shallow eutrophic lake: implications for submerged vegetation restoration. Sci Total Environ 821:153460
Cho Y-C, Im J-K, Han J, Kim S-H, Kang T, Lee S (2023) Comprehensive Water Quality Assessment using Korean Water Quality indices and Multivariate Statistical Techniques for Sustainable Water Management of the Paldang Reservoir. South Korea Water 15(3):509
Cotruvo JA (2017) 2017 WHO guidelines for drinking water quality: first addendum to the fourth edition. Journal-American Water Works Association 109(7):44–51
Dersseh MG, Kibret AA, Tilahun SA, Worqlul AW, Moges MA, Dagnew DC, Melesse AM (2019) Potential of water hyacinth infestation on lake Tana, Ethiopia: a prediction using a GIS-based multicriteria technique. Water, 11(9), 1921
Dersseh MG, Steenhuis TS, Kibret AA, Eneyew BM, Kebedew MG, Zimale FA, Worqlul AW, Moges MA, Abebe WB, Mhiret DA, Melesse AM, Tilahun SA (2022) Water Quality Characteristics of a Water Hyacinth Infested Tropical Highland Lake: Lake Tana, Ethiopia. Front Water 4:774710. https://doi.org/10.3389/frwa.2022.774710
Dessie M, Verhoest NEC, Pauwels VRN, Adgo E, Deckers J, Poesen J, Nyssen J (2015) Water balance of a lake with floodplain buffering: Lake Tana, Blue Nile Basin, Ethiopia. J Hydrol 522:174–186
Dubey D, Dutta V (2020) Nutrient enrichment in lake ecosystem and its effects on algae and macrophytes. Environmental Concerns and Sustainable Development: Volume 2: Biodiversity, Soil and Waste Management, 81–126.Duggan, S. B., & Kotalik, C. J. (2019). Not the Latest Science: National Recommended Water Quality Criteria for Aquatic Life Under the Clean Water Act. Minn. JL Sci. & Tech., 21, 371
Enawgaw Y, Lemma B (2018) Water Quality and Productivity Assessment of Lake Tinishu Abaya for multiple designated water uses, Ethiopia. J Environ Anal Toxicol 8(570):525–2161
Ewnetu DA, Bitew BD, Chercos DH (2014) Determination of surface water quality status and identifying potential pollution sources of Lake Tana: particular emphasis on the lake boundary of Bahirdar City, Amhara region, north west Ethiopia, 2013. Environment and Earth Science
Gezie A, Assefa WW, Getnet B, Anteneh W, Dejen E, Mereta ST (2018) Potential impacts of water hyacinth invasion and management on water quality and human health in Lake Tana watershed, Northwest Ethiopia. Biol Invasions 20:2517–2534
Ghebremedhin SG, Gupta B, Sen (2023) Spatiotemporal water quality assessment and pollution source apportionment of Lake Chamo using water quality index and multivariate statistical techniques. Eur J Environ Earth Sci 4(1):11–19
Girdner S, Mack J, Buktenica M (2020) Impact of nutrients on photoacclimation of phytoplankton in an oligotrophic lake measured with long-term and high-frequency data: implications for chlorophyll as an estimate of phytoplankton biomass. Hydrobiologia 847(8):1817–1830
Han L, Rundquist DC, Liu LL, Fraser RN, Schalles JF (1994) The spectral responses of algal chlorophyll in water with varying levels of suspended sediment. Int J Remote Sens 15(18):3707–3718
Ho JC, Michalak AM, Pahlevan N (2019) Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 574(7780):667–670
Huang J, Zhang Y, Arhonditsis GB, Gao J, Chen Q, Peng J (2020) The magnitude and drivers of harmful algal blooms in China’s lakes and reservoirs: a national-scale characterization. Water Res 181:115902
Huo S, Ma C, Xi B, Su J, Zan F, Ji D, He Z (2013) Establishing eutrophication assessment standards for four lake regions, China. J Environ Sci 25(10):2014–2022
Ishikawa M, Gurski L, Bleninger T, Rohr H, Wolf N, Lorke A (2022) Hydrodynamic drivers of nutrient and Phytoplankton Dynamics in a Subtropical Reservoir. Water 14(10):1544
Kaba E (2007) Validation of altimetry lake level data and its application in water resources management. Master’s Thesis, ITC, Enschede, The Netherlands
Kalmbach E (2017) Establishment of the Lake Tana biosphere reserve within the UNESCO world network of biosphere reserves. Social and Ecological System Dynamics: Characteristics, Trends, and Integration in the Lake Tana Basin, Ethiopia, 523–544
Kebedew MG, Tilahun SA, Zimale FA, Steenhuis TS (2020a) Bottom sediment characteristics of a tropical lake: Lake Tana, Ethiopia. Hydrology 7(1):18
Kebedew MG, Kibret AA, Tilahun SA, Belete MA, Zimale FA, Steenhuis TS (2020b) The relationship of lake morphometry and phosphorus dynamics of a tropical Highland Lake: Lake Tana, Ethiopia. Water 12(8):2243
Kebedew MG, Tilahun SA, Zimale FA, Belete MA, Wosenie MD, Steenhuis TS (2023) Relating lake circulation patterns to sediment, nutrient, and Water Hyacinth distribution in a Shallow Tropical Highland Lake. Hydrology 10:181. https://doi.org/10.3390/hydrology10090181
Keyombe J, Waithaka E (2017) Analysis of some aspects of water quality of Lake Naivasha. Int J Chem Lifesciences 6(1):2001–2005
Kumar S, Bauddh K, Singh R, Kumar N, Kumar R (2023) Aquatic Macrophytes: Ecology, Functions and Services. Springer Nature.Liu, X., Zhang, G., Sun, G., Wu, Y., & Chen, Y. (2019). Assessment of Lake water quality and eutrophication risk in an agricultural irrigation area: A case study of the Chagan Lake in Northeast China. Water, 11(11), 2380
Melese H, Debella HJ (2023) Comparative study on seasonal variations in physico-chemical characteristics of four soda lakes of Ethiopia (Arenguade, Beseka, Chitu and Shala). Heliyon, 9(5)
Menberu Z, Mogesse B, Reddythota D (2021) Evaluation of water quality and eutrophication status of Hawassa Lake based on different water quality indices. Appl Water Sci 11:1–10
Mishra RK (2023) Fresh water availability and its global challenge. Br J Multidisciplinary Adv Stud 4(3):1–78
Moges MA, Schmitter P, Tilahun SA, Ayana EK, Ketema AA, Nigussie TE, Steenhuis TS (2017) Water Quality Assessment by Measuring and using landsat 7 ETM + images for the current and previous Trend Perspective: Lake. 1564–1585. https://doi.org/10.4236/jwarp.2017.912099
Monteiro FM, de Moura GC, Severiano JdosS, Mendes CF, de Barbosa JE L (2021) Submerged macrophytes support cyanobacteria and microcystin production in a drawdown tropical semi-arid reservoir. Aquat Ecol 55(3):875–890
Ndungu J, Augustijn DCM, Hulscher SJMH, Kitaka N, Mathooko J (2013) Spatio-temporal variations in the trophic status of L ake N aivasha, Kenya. Lakes Reservoirs: Res Manage 18(4):317–328
Newport C, Padget O, de Perera TB (2021) High turbidity levels alter coral reef fish movement in a foraging task. Sci Rep 11(1):5976
Rashid A, Mehmood MA, Qadri H, Bhat RA, Dar GH (2018) Comparative water quality assessment of a tropical and a temperate lake of India. J Pharmacognosy Phytochemistry 7(4):2246–2249
Tasnim B, Fang X, Hayworth JS, Tian D (2021) Simulating nutrients and phytoplankton dynamics in lakes: Model development and applications. Water, 13(15), 2088
Tewabe D (2015) Preliminary survey of water hyacinth in Lake Tana, Ethiopia. Global J Allergy 1(1):13–18
Tibebe D, Kassa Y, Melaku A, Lakew S (2019) Investigation of spatiotemporal variations of selected water quality parameters and trophic status of Lake Tana for sustainable management, Ethiopia. Microchem J 148:374–384
Vijverberg J, Sibbing FA, Dejen E (2009) Lake Tana: source of the blue nile. The Nile: origin, environments, limnology and human use. Springer, pp 163–192
Wang H-J, Xiao X-C, Wang H-Z, Li Y, Yu Q, Liang X-M, Jungmann D (2017) Effects of high ammonia concentrations on three cyprinid fish: Acute and whole-ecosystem chronic tests. Sci Total Environ 598:900–909
Wondim YK (2016) Water quality status of Lake Tana, Ethiopia. J Civil Environ Res 8(9):39–41
Yan C-A, Zhang W, Zhang Z, Liu Y, Deng C, Nie N (2015) Assessment of water quality and identification of polluted risky regions based on field observations & GIS in the honghe river watershed, China. PLoS ONE, 10(3), e0119130
Yang C, Li J, Yin H (2022) Phosphorus internal loading and sediment diagenesis in a large eutrophic lake (Lake Chaohu, China). Environ Pollut 292:118471
Zegeye AD, Langendoen EJ, Stoof CR, Tilahun SA, Dagnew DC, Zimale FA, Guzman CD, Yitaferu B, Steenhuis TS (2016) Morphological dynamics of gully systems in the subhumid Ethiopian highlands: the Debre Mawi watershed, SOIL. 2:443–458. https://doi.org/10.5194/soil-2-443-2016
Zimale FA, Moges MA, Alemu ML, Ayana EK, Demissie SS, Tilahun SA, Steenhuis TS (2018) Budgeting suspended sediment fluxes in tropical monsoonal watersheds with limited data: the Lake Tana basin. J Hydrology Hydromechanics 66(1):65
Acknowledgements
We would like to acknowledge the dedication of the Bahir Dar University-Bahir Dar Institute of Technology for providing the laboratory equipment required during the data collection. We also thank Mr. Getahun Birara for his significant help during sample collection and his full commitment to facing risks during field work in Lake Tana.
Funding
This research was funded by the Bahir Dar Institute of Technology (BiT) of Bahir Dar University and the Blue Nile Water Institute, Ethiopia.
Author information
Authors and Affiliations
Contributions
M.D.N. contributed to setting the concepts, collecting the data, analyzing the collected data, writing the first draft article, and updating the draft based on the remarks of the coauthors. M.A.M. helped set the objectives, research approaches, task direction, appraisal, and supervision of the research work. M.G.K. contributed to the sampling design, shaped the structure of the paper, and provided comments. F.A.Z. and B.A.A. helped in reviewing and enhancing the paper for publication. B.W.A. also contributed in giving comments. T.S.S was instrumental in the revision process. He made significant contributions to reshaping the paper, providing thoughtful responses to the reviewers’ comments, and enhancing the paper’s overall grammar and readability. T.S.S was reviewed the paper thoroughly and provided significant feedbacks that enhanced the quality of the manuscript. His efforts were pivotal in preparing the paper for a successful resubmission. All the authors declare that they reviewed the manuscript and accepted the published paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they do not have a conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nerae, M.D., Kebedew, M.G., Abebe, B.A. et al. Assessment of pollution and trophic state of a water hyacinth infested tropical highland lake: Lake Tana in Ethiopia. Sustain. Water Resour. Manag. 10, 175 (2024). https://doi.org/10.1007/s40899-024-01152-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-024-01152-w