Abstract
Application of fertilizers over agricultural fields, domestic sewage, and livestock are major point and non-point sources of nutrients such as phosphate and nitrate in stormwater runoff. These nutrients, specifically phosphate, is the major pollutant triggering eutrophication in freshwater bodies, thus damaging both- the aquatic ecosystem and the environment. The current study investigates the removal of phosphate using biochar-based treatment units, which can be an efficient and cost-effective removal method. The biochar used in this study was produced by slow pyrolysis of bamboo and it was further chemically modified with 1%, 5%, and 10% FeCl3 solutions. The iron modification transformed the biochar surface to be electro-positive. Characterization of both biochar and modified biochars was carried out using proximate analysis, elemental analysis, Fourier transform infrared spectroscopy, and scanning electron microscopy. Furthermore, a series of batch experiments were conducted to evaluate the phosphate and nitrate removal efficiencies from the synthetic stormwater runoff. The removal efficiencies were found to be 29%, 49%, and 61%, with an initial phosphate concentration of 5 mg/L, using 1%, 5% and 10% FeCl3 treated biochar respectively, while the nitrate removal efficiencies (with an initial concentration of 50 mg/L) were found to be 12.4%, 14.6%, and 17.0% respectively. The phosphate removal kinetics by 10% FeCl3 modified biochar indicated that the phosphate removal followed pseudo-second-order kinetics. Isotherm modelling indicated both Langmuir and Freundlich isotherms as best fit for phosphate adsorption using 10% FeCl3 modified biochar thus representing mono-layer and multi-layer adsorption, respectively. Langmuir isotherm reported the maximum phosphate adsorption capacity of 11.57 mg/g by 10% FeCl3 modified biochar. Additional experiments were performed using real urban stormwater runoff from various regions of Delhi, which revealed similar phosphate removal efficiencies. The treated stormwater runoff can be used for water capacity building and non-potable applications in water-scarce urban areas.
Highlights
Pyrolysis of bamboo has 40% yield.
Phosphates and nitrates in stormwater runoff can be effectively removed by biochar.
Biochar requires its treatment with electro-negative chemical species for effective removal of nutrients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The scarcity of water is becoming a challenge throughout the world leading to the crisis of potable water (He et al. 2021). India being the seventh largest country in the world and the most populous country, is rapidly advancing in terms of agricultural growth to feed its citizens (Ministry of Agriculture & Farmers Welfare 2022). Agricultural advancement also leads to the exploited application of chemical fertilizers in agricultural fields. These fertilizers consist of nutrients such as phosphate (PO43−) and nitrate (NO3−) which when mixed with stormwater runoff degrades the quality of the receiving waterbody. In addition, phosphate is a critical pollutant causing eutrophication of receiving freshwater bodies (Haritash et al. 2017). The runoff from agricultural fields is a non-point source of nutrient pollution and causes excess nutrient loading leading to eutrophication, harmful algal blooms, and fish kills in receiving water bodies such as lakes and ponds (Sinha et al. 2017; Smith et al. 2020).
In addition, during dry seasons when no rainfall is being received in urban areas, pollutants such as pet wastes and biogenic wastes such as leaf litter and grass clippings get deposited on roads, streets, house roofs, trees, etc. During excess rainfall, the nutrients from the unutilized fertilizers, excess urban waste, and overflowing and leaking sanitary sewers get mixed with the stormwater runoff (Yang and Lusk 2018). The runoff traverses its flow path depending on the hydrology and imperviousness of the surface in that area (Kleinman 2006).
Nutrients in stormwater runoff are usually treated using several techniques such as biological treatment, ion exchange, ultrafiltration, membrane separation, and chemical precipitation. Employing some of these chemical techniques removes the pollutants through redox and ion exchange reactions, however, results in the release of harmful chemical species (Romero-Gueiza et al. 2015). Wetlands are also used to remove the nutrients from the receiving water, however, this method requires a large area and higher retention time moreover, the technique is not viable and efficient during the winter season (Haritash et al. 2015; Nandakumar et al. 2019; Pipil et al. 2021, 2023; Jain et al. 2024). Adsorption, on the other hand, is an effective technique that can be employed as a low-cost-efficient method to remove nutrients (Huang et al. 2020).
Among various adsorbents available, biochar, a product obtained from pyrolysis or gasification of biomass in the absence of oxygen, can be an efficient low-cost adsorbent owing to several favourable properties such as high specific surface and internal porosity (Weber and Quicker 2018). The adsorption capacity of biochar depends on several factors like the functional group, the surface charge, and the specific surface area. Few studies were conducted in the past to explore the potential removal of nutrients from wastewater using biochar (Zhang et al. 2020; Reddy et al. 2014). The surface of biochar is negatively charged which makes it unsuitable for the effective removal of anionic chemical species such as phosphate and nitrate, and thus, requires treatment with cationic chemical species like Fe(III) to effectively remove these nutrients from runoff (Mukherjee et al. 2011). FeCl3 can effectively remove phosphate from the aqueous medium. Fe-P coprecipitate shows high stability under high pH fluctuation (3.0 < pH < 11.0). Moreover, FeCl3 acts as an alternate terminal electron acceptor oxidising the other chemical species present in aqueous medium (Li et al. 2019).
The characterisation of stormwater runoff is necessary to estimate the suspended solids, pollutants, and nutrient loading in the runoff so that the requisite and necessary treatment can be provided for it. In previously published studies, the stormwater runoff samples were collected from various land uses based on associated activity in Delhi, India, which includes commercial, industrial, institutional, residential, and road/highways (Pipil et al. 2022a). Study shows phosphate concentration ranges between 0.04 and 17.4 mg/L while nitrate concentrations range between 1.6 and 478.8 mg/L. The higher values of phosphates and nitrate can be attributed to the addition from organic matter, mulch, applied compost, etc. in lawns and parks, littering of food waste, and the excretory waste of stray animals and pets. Water-sensitive urban design (WSUD) such as rain gardens and wetlands can remove pollutants such as suspended solids, nutrients, and heavy metals when amended with an adsorbent such as biochar (Pipil et al. 2022b).
In the present study, biochar was produced by pyrolysis using bamboo and was subsequently impregnated with Fe(III). A detailed characterization of the biochar and Fe(III)-modified biochar was performed. A series of batch adsorption experiments were conducted using bamboo-biochar and Fe(III)-modified biochar to determine their phosphate removal kinetics and maximum adsorption capacity. Synthetically prepared stormwater consisting of phosphate and nitrate was subjected to treatment process using biochar. Furthermore real stormwater runoff consisting of phosphate and multiple co-existing chemicals was also treated using the same. The experimental results helped to assess the extent of phosphate removal and the mechanisms responsible for the removal.
Materials and methods
Preparation of biochar
Old refused bamboo was collected from the university campus which was cleaned thoroughly with Type-I water. The pieces were cut out of it to prepare biochar in bulk quantity to have homogeneous characteristics. The bamboo samples were then dried in a hot air oven for 24 h at 105 oC. The dried samples were then placed inside a muffle furnace at 500 oC for 30 min for pyrolysis in an air-tight chamber to ensure the presence of zero oxygen. The temperature was raised in the muffle at a rate of 16.67 oC/minute for 30 min to attain a final pyrolysis temperature of 500 oC. The biochar so formed was allowed to cool freely and naturally inside the muffle. The produced biochar was crushed and passed through IS 4.75 mm sieve but retained on the IS 2.36 mm sieve, which was the chosen grain size range for treatment. A solution of FeCl3 of strength 1%, 5%, and 10% was prepared in which an equal quantity of prepared biochar was added. It was subjected to shaking for 24 h in a bench-top shaker at a RPM of 150 and the temperature was set at 27 oC. This biochar impregnated with Fe(III) was then washed thrice thoroughly with Type-I water. It was then dried in a hot air oven at 105oC for 24 h. In addition, one set of experiments was conducted with untreated biochar. The untreated biochar is indicated with B0%, while biochar having 1%, 5%, and 10% FeCl3 was named as B1%, B5%, and B10%, respectively. The percentage yield and the cost associated with the preparation of biochar were also calculated.
Characteristics of biochar
Proximate analysis
To estimate the moisture, volatile, fixed carbon, and ash content of both untreated biochar and raw bamboo, proximate analysis was conducted following ASTM (ASTM E871-82, ASTM E1755-01, ASTM E872-82) standard methods using ELTRA - Thermogravimetric Analyzer (manufactured in Germany). The temperature for moisture, volatile, fixed carbon, and ash content was fixed at 105 oC, 915 oC, 750 oC, and 750 oC, respectively. The thermogravimetric analysis was conducted using 1100 ± 10 mg of samples in the crucibles.
Elemental analysis
Elemental analysis was performed for the determination of carbon, hydrogen, nitrogen, and sulphur (CHNS) using Eurovector - EuroEA3000 Series elemental analyser at 950 oC. The calibration was performed using L-Cystine as the reference standard material in tin capsules. CHNS analysis was conducted in duplicates using ≈ 2.5 mg samples in tin capsules.
Fourier transform infrared (FTIR) spectroscopy
A non-destructive method was used to identify the various functional groups present in biochar. The FTIR was conducted for untreated biochar and Fe(III) modified biochar before and after the experiments, to characterize the functional groups present. A Thermo Fisher Nicolet spectrometer enabled with a KBr beam splitter was used in the mid-infrared region. All the samples were ground in a mortar pestle which was cleaned thoroughly with an organic solvent. Sample pellets were prepared in the ratio of 1:10 with KBr. Spectra of KBr were run initially to cancel out its effect and its spectrum in the final results. The spectra were collected in the range of 400 cm− 1 and 4000 cm− 1 with a resolution of 2 cm− 1.
Scanning electron microscopy (SEM)
A non-destructive method for Scanning Electron Microscopy was performed using Zeiss EVO 18 special (Zeiss, Germany). The biochar samples were used to analyse their structure before and after the adsorption in the experiment. The samples were dried in a hot air oven for 24 h and then it was visualised on the copper-coated grid after gold coating between 750x and 1500x magnification.
Experimental setup
Three combinations of concentration for both phosphate (PO43−-P) and nitrate (NO3−-N) were selected while preparing the mixed synthetically prepared stormwater runoff. Phosphate and nitrate were present in the mixed synthetically prepared stormwater having the concentration of 2.0 mg/L PO43−-P, 25.0 mg/L NO3−-N; 5.0 mg/L PO43−-P, 50.0 mg/L NO3−-N; and 10.0 mg/L PO43−-P, 75.0 mg/L NO3−-N. Initially, a dose of 1.0 g/L of biochar was introduced for the treatment which was later increased to 5.0 g/L. The experiments were conducted in triplicates in a conical flask with 200 mL of synthetically prepared stormwater runoff and the respective doses of biochar. The sample with biochar was subjected to shaking at 150 RPM at 27 oC. An aliquot of 1.0 mL was extracted at every 1-hour time interval. pH, electrical conductivity, and total dissolved solids (TDS) were noted using a Labman make (LMMP 30 model) bench-top multiparameter before and after the experiment. The residual concentrations of phosphate and nitrate were analysed using single beam spectrophotometer (Labtronics make LT-290 Model) at λmax = 690 nm for phosphate, and at λmax = 220 nm for nitrate.
Removal of nutrient
The removal efficiency of nutrients from synthetically prepared and real stormwater runoff were calculated using the Eq. (1) given below:
Where Ci is the initial concentration of nutrients in stormwater runoff in mg/L; Ce is the concentration of nutrients at equilibrium after treatment in mg/L. The removal efficiency was calculated based on the extraction of samples at an interval of 1 h which was extracted for 4 h.
Results and discussion
Characterisation of biochar
Proximate analysis
Based on thermogravimetric analysis, raw bamboo has more moisture and volatile content while its biochar has more carbon and ash content as compared to raw bamboo (Table 1). Raw bamboo has moisture, fixed carbon, volatile, and ash content as 7.1%, 16.8%, 76.1%, and 5.1%, respectively. On the other hand, bamboo biochar has moisture, fixed carbon, volatile, and ash content of order 3.5%, 59.6%, 17.4%, and 16.8%, respectively. During pyrolysis, the temperature was raised from room temperature to a peak 500 oC, resulting in the loss of moisture and water of hydration in the form of vapours, while the sample could have under decomposition which released complex organic compounds including CO2, CO, H2, CH4 and other hydrocarbons in the form of syngas. This might have reduced the volatile content in biochar significantly. For the same reason of loss of moisture and water of hydration, the moisture content also reduced to half. The substantial increase in the fixed carbon content of bamboo biochar compared to raw bamboo is a result of the loss of other volatile organics during the pyrolysis (Sun et al. 2014).
Elemental analysis
Based on elemental analysis, as presented in Table 1, it was observed that pyrolysis has significantly changed the elemental composition of biochar. The carbon content of bamboo biochar increased to 73.2% from the 43.0% as compared to raw bamboo. However, the hydrogen and oxygen content in the biochar was reduced from 7.3 to 3.9% and 48.8–22.4%, respectively (Table 1). Nitrogen and sulphur content remained < 1.0% for both raw bamboo and bamboo biochar. O/C and H/C ratios reduced from 1.13 to 0.31 and 0.17 to 0.05, respectively, on the conversion of bamboo biomass to biochar. The lower ratio of O/C and H/C impart greater stability and degree of aromaticity within the biochar (Kumar et al. 2013).
Fourier transform infrared (FTIR) spectroscopy
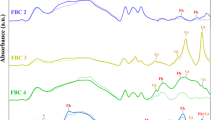
As a result of pyrolysis, the increase in temperature changes the functional groups in the biochar. Non-destructive FTIR spectroscopy shows the peak intensity of the various organic groups commonly observed in biochar (Table 2). Between 3550 cm− 1 to 3200 cm− 1, organic O-H stretching is a result of contribution from water retained in the biochar. C-H stretching between 3000 cm− 1 to 2840 cm− 1 corresponds to the presence of an alkane group. The peak between 2000 cm− 1 to 1650 cm− 1 is C = O stretching which consists of aromatic compounds, ketones, and amides for all the types of biochar. FTIR peak from 1400 cm− 1 to 1000 cm− 1 corresponds to C-H bending and C-O stretching that corresponds to the aldehyde and alcohol functional groups, respectively. 1000 cm− 1 to 650 cm− 1 corresponds to C = C stretching and C-H bending that relates to the alkene functional group (Fig. 1).
Scanning electron microscopy (SEM)
SEM images of biochar samples show the surface morphology of the biochar before and after treatment with FeCl3. In both the biochar, complex surface morphology can be seen along with macro to micropores present over the surface which are produced as a result of a vascular bundle of raw bamboo biomass (Hernandez-Mena et al. 2014). However, in biochar treated with FeCl3, agglomeration of this electropositive chemical can be seen (Fig. 2).
Removal of nutrients
Removal from synthetic stormwater runoff
For the phosphate strength of 5 mg/L, different types of biochars have shown different removal efficiencies. B0% biochar without any treatment was not able to remove the phosphate from synthetically prepared stormwater runoff in a period of 4 h. This could be attributed to the non-availability of adsorption sites on the surface of untreated and negatively charged biochar. Negatively charged sites cannot remove anionic species due electrostatic force of repulsion. However, biochar which was pre-treated with FeCl3 was able to remove phosphate. B1%, B5%, and B10% biochars removed 29%, 49%, and 61%, respectively, from synthetically prepared stormwater runoff when initial phosphate strength was 5.0 mg/L (Fig. 3). B10% treated biochar removed phosphate almost twice the B1% treated biochar. This could be attributed to the availability of sites having Fe(III) present on the porous surface of biochar that can easily remove phosphate from the sample. The increase in phosphate removal efficiency is almost 60 times for B10% treated biochar to that of the untreated B0% biochar in the present study. B10% biochar was further used to study its nutrient removal efficiency for different phosphate strengths of 2 mg/L, 5 mg/L, and 10 mg/L, and the results are presented in Fig. 4. It was observed that biochar can remove almost 63% of phosphate for the initial phosphate strength of 2 mg/L at a dose of 5.0 g/L absorbent and a contact period of 4 h. At the same time, the removal efficiency of phosphates reduced with the increase in the initial strength of phosphate (Fig. 4). For the same dose and contact period, only 59% and 49% of phosphate were removed from synthetically prepared stormwater runoff of strength 5 mg/L and 10 mg/L, respectively. On the other hand, for the initial strength of nitrate as 50 mg/L, biochar without any given treatment was able to remove approximately 9%. Increasing Fe concentration in biochar did not show significant removal efficiency towards nitrate. The removal efficiency of nitrate using B1%, B5%, and B10% was of order 12.4%, 14.6%, and 17.0%, respectively. The lower adsorption of nitrate compared to phosphate can be due to the preferential adsorption of phosphate over nitrate by Fe(III) modified biochar.
Removal from field stormwater runoff
Based on the observations obtained for nutrient removal from synthetically prepared stormwater, real (field) stormwater runoff was treated using B10% biochar, to verify its adsorption capacity in chemically complex real stormwater runoff. The phosphate removal efficiency of biochar for stormwater runoff collected from commercial, institutional, industrial, and road/highway areas of the Delhi region was of order 40.5%, 57.1%, 40.3%, and 59.1%, respectively (Fig. 5). It shows that the removal efficiency observed is relatively similar to that of the synthetically prepared stormwater runoff experiments. Also, up to 67.3% of nitrate was removed from institutional area runoff. The removal efficiency depends upon the initial concentrations of the pollutants. The equilibrium of the reaction shifts towards another side that reverses and releases pollutants from the adsorbed surface of biochar. The shift of equilibrium depends upon the release of pollutants from the solid phase (biochar) to an aqueous phase.
Adsorption capacity
The adsorption capacity qe (mg/g) of the biochar was calculated using the Eq. (2)
Where qe is the adsorption capacity of biochar in mg/g; Ci is the initial concentration of nutrients in stormwater runoff in mg/L; Ce is the concentration of nutrients at equilibrium after treatment in stormwater runoff in mg/L; V is the volume of sample used for analysis in litre; and W is the quantity of absorbent in g. The adsorption capacity of biochar for a dose of 5.0 g/L was calculated in batch experiments for varying strength of phosphate in synthetic and real stormwater runoff which varied from 2.0 mg/L to 10 mg/L, and 0.4 mg/L to 3.2 mg/L, respectively. The experiment was conducted at 7.0 pH for synthetically prepared stormwater runoff at 27 oC temperature while the natural pH of real stormwater runoff is considered for this study. The dose of biochar was kept fixed at 5.0 g/L for Fe(III) treated B10% biochar for experimentation. The adsorption capacity of biochar increased with the increase in the concentration of phosphate which may be attributed to the availability of vacant sites for its adsorption which is represented by a strong correlation value (R2 > 0.99) as shown in Fig. 6.
Mechanism of removal
Removal of chemical species by biochar depends upon the presence of adsorption sites over the surface which is correlated with the specific surface area of the biochar produced. The increase in temperature for biochar production by pyrolysis, the specific surface area of biochar increases that enhances the removal of phosphates (Zeng et al. 2013). However, it was reported in earlier studies that biochar has an electro-negative charge over its surface, and it cannot remove anions (PO43− and NO3−) by adsorption (Wang et al. 2015; Zeng et al. 2013). The pyrolysis of biomass produces biochar having functional groups such as -OH and -COOH as shown by the FTIR spectrum peak at 3400 cm− 1 and 1600 cm− 1, respectively (Fig. 1), which may result in a negative surface charge. The net negative surface charge may lead to poor electrostatic attraction of PO43− and NO3− with biochar and thus, require treatment with electro-positive chemical species like FeCl3. The impregnation of FeCl3 over the biochar surface has played a role in nutrient removal. The SEM images before and after the adsorption (Fig. 2) revealed that the surface was smooth before adsorption while, after adsorption, the surface was occupied with adsorbed particles. This change in the surface morphology of biochar suggests that nutrients were removed by the action of electrostatic attraction between positively charged Fe(III) ions and negatively charged phosphate ions. FTIR spectrum peak at 1000 cm− 1 after adsorption suggests the interaction of Fe-OH with P ions (Zhang et al. 2009).
Kinetics of nutrient removal
To find the kinetics of nutrient removal, pseudo-first-order and pseudo-second-order models were applied. Pseudo-first-order kinetic model is given by Eq. (3)
Where qe is the adsorption capacity of biochar in mg/g; qt is the adsorption capacity of biochar in mg/g at any time t; K1 is pseudo-first order rate constant (min− 1).
Pseudo-second-order kinetic model is given by Eq. (4)
Where qe is the adsorption capacity of biochar in mg/g; K2 is pseudo-second-order rate constant (g mg− 1 min− 1) and t is time in minutes. The plots of pseudo-first-order and pseudo-second-order models for the adsorption capacity of biochar are shown in Fig. 7a and b respectively. The various parameters obtained from these models are given in Table 3. Pseudo-first-order model fits relatively poor for the removal of phosphates in which the R2 value varied from 0.867 to 0.945. Also, the adsorption capacity calculated at equilibrium (qe(cal)) and adsorption capacity from the experiments (qe(exp)) varied significantly. Thus, it shows that adsorption kinetics does not follow the pseudo-first-order kinetic model. On the other hand, the R2 value varied between 0.999 and 0.996 for pseudo-second-order kinetics which shows that the plot fits very well. Also, adsorption capacity varied between 0.35 and 0.60 mg/g for phosphate. Thus, the adsorption of phosphate follows pseudo-second-order rather than pseudo-first-order kinetics as reported in another study (Zhang et al. 2012).
Adsorption Isotherm
To find the adsorption isotherm, both Langmuir and Freundlich isotherm models were applied. Langmuir isotherm in its linear form is represented by Eq. (5)
Where qe is the adsorption capacity of biochar in mg/g; qmax is the maximum adsorption capacity of biochar in mg/L; KL is Langmuir’s isotherm constant (L/mg).
The separation factor RL is given by Eq. (6)
Where, RL is Langmuir constant which indicates the adsorption possibility which may be either favourable (0 < RL < 1.0), unfavourable (RL > 1.0), linear (RL = 1.0) or irreversible (RL =0).
Fruendlich isotherm in its linear form is represented by Eq. (7):
Where qe is the adsorption capacity of biochar in mg/g; Kf is the Freundlich’s constant ((mg.g− 1) (L.mg− 1)1/n); Ce is the concentration of nutrient at equilibrium after treatment in stormwater runoff in mg/L. In general, adsorption isotherm distinguishes the surface properties and affinity to adsorb the adsorbate. Isotherms corresponding to B10% biochar were studied to understand the same. The best curve was obtained by the Langmuir isotherm model with R2 = 0.9992 as against the Freundlich isotherm model with R2 = 0.9933 (Fig. 8). The maximum adsorption capacity qmax was obtained as 11.57 mg/g for phosphate (Table 4). The calculated value of Langmuir constant RL was less than 1.0 which suggests that the adsorption possibility is favourable (Ayub et al. 2020). A brief comparison of studies done in the past for the removal of phosphate is summarized in Table 5.
Yield of biochar
The yield of biochar produced during the experiment was calculated as the ratio weight of carbonaceous biochar produced after pyrolysis to the weight of bamboo biomass before pyrolysis as given by the Eq. (8)
Using the formula mentioned above, approximately 40% biochar was produced from the initial bamboo biomass. The 60% of the initial mass loss can be attributed to the removal of moisture, the production of syngas, and the production of bio-oil during pyrolysis (Panwar and Pawar 2020). Biomass with higher water content will lead to a poor yield of biochar.
Cost of biochar production
Biochar was made through pyrolysis in a muffle furnace having a power rating of 2.0 KW. The muffle furnace was operated for an hour, initial 30 min for raising the temperature at a rate of 16.67 oC/minute, and the next 30 min for the actual process of pyrolysis at 500 oC. Thus, 2.0 KW muffle operated for 1 h implies that 2 units of electricity, i.e., 2.0 kWh was consumed in this process. The average price of electricity units in Delhi ₹6.0 per kWh. Thus, electricity cost accounts for ₹12.0 (Table 6). FeCl3 was also used to impregnate the biochar in various configurations. The cost of FeCl3 used during experimentation was ₹400 per 500 g packaging. Thus, the total cost of biochar includes the cost of electricity used during pyrolysis and the cost of chemicals consumed. Hence, the cost of biochar varies between ₹12.0 per kg for non-impregnated biochar to ₹244.0 per kg for 10% FeCl3 impregnated biochar. Considering the currency exchange rate as US$1 equivalent to ₹83.17, the cost of biochar production becomes equivalent to US$ 0.14 per kg to US$ 2.93 per kg for the present study (Fig. 9).
Conclusions
The current study aimed to evaluate the enhanced phosphate and nitrate removal efficiency by Fe(III) modified biochar from synthetically prepared stormwater runoff containing phosphate and nitrate, and from actual stormwater runoff collected from the field. The biochar used in this study was produced by slow pyrolysis of bamboo biomass at a yield rate of 40%. The bamboo-biochar was then treated with 1%, 5%, and 10% FeCl3 solutions to induce surface electro-positivity. Biochar and modified biochars were then characterized based on proximate analysis, elemental analysis, Fourier Transform InfraRed (FTIR) Spectroscopy, and Scanning Electron Microscopy (SEM). The characterization results indicated that the Fe(III) modified biochars possess favourable surface and chemical properties for phosphate removal. Phosphate removal from synthetic stormwater was found to be 29%, 49%, and 61% for an initial phosphate concentration of 5 mg/L using 1%, 5%, and 10% FeCl3 treated biochar, respectively. However, the nitrate removal efficiency was found to be 9%, 12.4%, 14.6%, and 17.0% for B0%, B1%, B5%, and B10% respectively, at an initial nitrate concentration of 50 mg/L. This is possibly due to the preferential adsorption of phosphate instead of nitrate by Fe modified biochar, and the high initial concentration of nitrate. The removal efficiencies were found to reduce with increasing phosphate concentration, while no such trends were observed in the case of nitrate. The phosphate removal efficiency of modified biochar (B10%) for stormwater runoff collected from commercial, institutional, industrial, and road/highway areas of the Delhi region was 40.5%, 57.1%, 40.3%, and 59.1%, respectively, with the variations effected by the initial phosphate loading. A study of the kinetics of phosphate adsorption by B10% biochar showed that the adsorption of phosphate removal follows pseudo-second-order rather than pseudo-first-order kinetics. Both Langmuir and Freundlich isotherm models, which indicate mono-layer and multi-layered heterogeneous adsorption respectively, were found to be well fit for the phosphate adsorption by B10% biochar. The maximum adsorption capacity, obtained from Langmuir isotherm, was 11.57 mg/g for phosphate removal by B10%. Lastly, an analysis of production and modification costs of bamboo-biochar indicated that modification of biochar is economically feasible.
Data availability
Necessary data has been provided along with this manuscript.
References
Ayub A, Raza ZA, Majeed MI, Tariq MR, Irfan A (2020) Development of sustainable magnetic chitosan biosorbent beads for kinetic remediation of arsenic contaminated water. Int J Biol Macromol 163:603–617. https://doi.org/10.1016/j.ijbiomac.2020.06.287
Deng Y, Li M, Zhang Z, Liu Q, Jiang K et al (2021) Comparative study on characteristics and mechanism of phosphate adsorption on Mg/Al modified biochar. J Environ Chem Eng 9(2):105079. https://doi.org/10.1016/j.jece.2021.105079
Haritash AK, Sharma A, Bahel K (2015) The potential of Canna lily for wastewater treatment under Indian conditions. Int J Phytoremediation 17(10):999–1004. https://doi.org/10.1080/15226514.2014.1003790
Haritash AK, Dutta S, Sharma A (2017) Phosphate uptake and translocation in a tropical Canna-based constructed wetland. Ecol Process 6:12. https://doi.org/10.1186/s13717-017-0079-3
He C, Liu Z, Wu J, Pan X, Fang Z, Li J, Bryan BA (2021) Future global urban water scarcity and potential solutions. Nat Commun 12(1):1–11. https://doi.org/10.1038/s41467-021-25026-3
Hernandez-Mena LE, Pécoraa AA, Beraldob AL (2014) Slow pyrolysis of bamboo biomass: analysis of biochar properties. Chem Eng 37:115–120. https://doi.org/10.3303/CET1437020
Huang Y, Lee X, Grattieri M, Yuan M, Cai R, Macazo FC, Minteer SD (2020) Modified biochar for phosphate adsorption in environmentally relevant conditions. Chem Eng J 380:122375. https://doi.org/10.1016/j.cej.2019.122375
Jain N, Yadav S, Taneja S, Ray S, Haritash AK, Pipil H (2024) Phosphate removal from urban stormwater runoff using Canna lily and Cyperus alternifolius-based bioretention system. Sustainable Water Resour Manage 10(2):1–10. https://doi.org/10.1007/s40899-024-01076-5
Kleinman PJ, Srinivasan MS, Dell CJ, Schmidt JP, Sharpley AN, Bryant RB (2006) Role of rainfall intensity and hydrology in nutrient transport via surface runoff. J Environ Qual 35(4):1248–1259. https://doi.org/10.2134/jeq2006.0015
Kumar S, Masto RE, Ram LC, Sarkar P, George J, Selvi VA (2013) Biochar preparation from Parthenium hysterophorus and its potential use in soil application. Ecol Eng 55:67–72. https://doi.org/10.1016/j.ecoleng.2013.02.011
Li S, Jiang F, Lei T, Ren Z, Wang S, Yang X (2019) Phosphorus removal by in situ sprayed ferric chloride in Dianchi Lake: efficiency, stability, and mechanism. Process Saf Environ Prot 131:320–328. https://doi.org/10.1016/j.psep.2019.09.021
Ministry of Agriculture & Farmers Welfare (2022) Contribution of Agriculture Sector towards GDP. Agriculture has been the bright spot in the Economy despite COVID-19. https://www.pib.gov.in/PressReleasePage.aspx?PRID=1741942. Accessed on 14 February, 2022
Mukherjee A, Zimmerman AR, Harris W (2011) Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 163:247–255. https://doi.org/10.1016/j.geoderma.2011.04.021
Nandakumar S, Pipil H, Ray S, Haritash AK (2019) Removal of phosphorous and nitrogen from wastewater in Brachiaria-based constructed wetland. Chemosphere 233:216–222. https://doi.org/10.1016/j.chemosphere.2019.05.240
Panwar NL, Pawar A (2020) Influence of activation conditions on the physicochemical properties of activated biochar: a review. Biomass Convers Biorefinery 1–23. https://doi.org/10.1007/s13399-020-00870-3
Pipil H, Haritash AK, Reddy KR (2021) Seasonal variability and kinetics of phosphate removal in a Phragmites-based engineered wetland. Rend Lincei Scienze Fis E Naturali 32(4):729–735. https://doi.org/10.1007/s12210-021-01017-w
Pipil H, Haritash AK, Reddy KR (2022a) Spatio-temporal variations of quality of rainwater and stormwater and treatment of stormwater runoff using sand–gravel filters: case study of Delhi, India. Rend Lincei Scienze Fis E Naturali 33:135–142. https://doi.org/10.1007/s12210-021-01038-5
Pipil H, Yadav S, Taneja S, Chawla H, Haritash AK, Reddy KR (2022b) Water Sensitive Urban Design (WSUD) for Treatment of Storm Water Runoff. In: Kanwar, V.S., Sharma, S.K., Prakasam, C. (eds) Proceedings of International Conference on Innovative Technologies for Clean and Sustainable Development (ICITCSD – 2021). Springer, Cham. https://doi.org/10.1007/978-3-030-93936-6_5
Pipil H, Yadav S, Taneja S, Chawla H, Haritash AK, Reddy KR (2023) Removal of phosphate from Stormwater Runoff using bench scale constructed Wetland. In: Yukselen-Aksoy Y, Reddy KR, Agnihotri AK (eds) Sustainable Earth and Beyond. EGRWSE 2022. Lecture Notes in Civil Engineering, vol 370. Springer, Singapore. https://doi.org/10.1007/978-981-99-4041-7_39
Qiu B, Duan F (2019) Synthesis of industrial solid wastes/biochar composites and their use for adsorption of phosphate: from surface properties to sorption mechanism. Colloids Surf a 571:86–93. https://doi.org/10.1016/j.colsurfa.2019.03.041
Reddy KR, Xie T, Dastgheibi S (2014) Evaluation of biochar as a potential filter media for the removal of mixed contaminants from urban storm water runoff. J Environ Eng 140(12):04014043. https://doi.org/10.1061/(ASCE)EE.1943-7870.0000872
Romero-Gueiza MS, Tait S, Astals S, del Valle-Zermeno R, Martinez M, Mata- Alvarez J et al (2015) Reagent use efficiency with removal of nitrogen from pig slurry via struvite: a study on magnesium oxide and related by-products. Water Res 84:286–294. https://doi.org/10.1016/j.watres.2015.07.043
Sinha E, Michalak AM, Balaji V (2017) Eutrophication will increase during the 21st century as a result of precipitation changes. Science 357(6349):405–408. https://doi.org/10.1126/science.aan2409
Smith JS, Winston RJ, Tirpak RA, Wituszynski DM, Boening KM, Martin JF (2020) The seasonality of nutrients and sediment in residential stormwater runoff: implications for nutrient-sensitive waters. J Environ Manage 276:111248. https://doi.org/10.1016/j.jenvman.2020.111248
Sun Y, Gao B, Yao Y, Fang J, Zhang M et al (2014) Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem Eng J 240:574–578. https://doi.org/10.1016/j.cej.2013.10.081
Wang Z, Guo H, Shen F, Yang G, Zhang Y, Zeng Y et al (2015) Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3–), and phosphate (PO43–). Chemosphere 119:646–653. https://doi.org/10.1016/j.chemosphere.2014.07.084
Wang Z, Shen D, Shen F, Li T (2016) Phosphate adsorption on lanthanum loaded biochar. Chemosphere 150:1–7. https://doi.org/10.1016/j.chemosphere.2016.02.004
Weber K, Quicker P (2018) Properties of biochar. Fuel 217:240–261. https://doi.org/10.1016/j.fuel.2017.12.054
Yang YY, Lusk MG (2018) Nutrients in urban stormwater runoff: current state of the science and potential mitigation options. Curr Pollution Rep 4(2):112–127. https://doi.org/10.1007/s40726-018-0087-7
Zeng Z, Zhang SD, Li TQ et al (2013) Sorption of ammonium and phosphate from aqueous solution by biochar derived from phytoremediation plants. J Zhejiang Univ Sci B 14(12):1152–1161. https://doi.org/10.1631/jzus.B1300102
Zhang G, Liu H, Liu R, Qu J (2009) Removal of phosphate from water by a Fe–Mn binary oxide adsorbent. J Colloid Interface Sci 335(2):168–174. https://doi.org/10.1016/j.jcis.2009.03.019
Zhang L, Zhou Q, Liu J, Chang N, Wan L, Chen J (2012) Phosphate adsorption on lanthanum hydroxide-doped activated carbon fiber. Chem Eng J 185:160–167. https://doi.org/10.1016/j.cej.2012.01.066
Zhang M, Song G, Gelardi DL, Huang L, Khan E et al (2020) Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res 186:116303. https://doi.org/10.1016/j.watres.2020.116303
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
SY: Conceptualization, execution, draft writing and editing. HP: Conceptualization, execution, draft writing and editing. AKH: Conceptualization, supervision, reviewing and editing. KRR: Conceptualization, supervision, reviewing and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to Publish
Authors provide the consent to publish the manuscript.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yadav, S., Pipil, H., Haritash, A.K. et al. Fe(III)-modified bamboo biochar for the removal of phosphate from synthetic and field stormwater runoff. Sustain. Water Resour. Manag. 10, 140 (2024). https://doi.org/10.1007/s40899-024-01123-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-024-01123-1