Abstract
Appraising weathering intensity and rate in areas subjected to a weathering-limited regime—i.e. Achala batholith (Sierras Pampeanas de Córdoba, Argentina)—is complicated due to regolith scarcity and the absence of soil profiles. The A-type granite exhibits characteristic landforms patchily mantled with coarse-grained (i.e. grus) and poorly sorted regolith in topographic lows. Physical weathering is substantial; chemical weathering of granite and derived debris is incipient. Rainfall and scarce snowfall carry continental chemical signatures due to aerosols, largely supplied by extensive neighboring playas and arid/semiarid environments. Initial assessments of Achala’s embryonic chemical weathering revealed that the chemical index of weathering (CIW) showed a significant difference between the granitic source and fine-grained regolith, triggered by mineral dissolution/hydrolysis. The δ18O and δ2H signatures of springs/streams are close to local rainfall, and show a significant deuterium excess (i.e. > 10 per mil). Stream chemistry suggested the contribution of plagioclase, and biotite, with calcite and gypsum (i.e. partly supplied as aerosols?), and likely pyrite oxidation adding to the dissolved pool. PHREEEQC inverse modeling applied in ephemeral/perennial springs, identified illite (~ 32 µmol kgw−1), Ca-montmorillonite (~ 43 µmol kgw−1), and sepiolite (~ 22 µmol kgw−1) as main transferred phases. First-order streams broadly concurred with previous findings, with gibbsite (~ 23 µmol kgw−1), chalcedony (~ 87 µmol kgw−1), and kaolinite (~ 43 µmol kgw−1) as frequent and more abundant crystalline phases. Modeling suggests that most dissolution and mineral formation occur within the realm of springs, with first-order streams largely expressing a reduced role in mole transferring.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thousands of scientific articles have addressed rock weathering in the evolution of modern Earth Sciences, and the one published in the Geological Magazine by GH Kinahan, over 150 years ago (Kinahan 1866), is among the early ones. Wedephol’s (1995) and Nesbitt and Young’s (1982) classic papers belong to the most cited group. It follows that many authors have dealt –and still do- with the subject matter, expanding the knowledge on the physicochemical characteristics and dynamics of weathering (e.g. Drever 2005, and references therein).
About fifteen percent of all the documents published on weathering have been devoted to probing into the mechanisms occurring in granites, in different climatic and tectonic settings (e.g. Oliva et al 2003; Lee et al. 2008; Vázquez et al. 2016; Kanamaru et al. 2018). Weathered in similar climates, different granite types would deliver contrasting water types. For example, a calc-alkaline granite would supply a dissolved weathering product that would differ from its alkaline counterpart, which is depleted in Ca2+, and Mg2+, and enriched in Na+ and SiO2 concentrations (Meybeck 2005). Likewise, two geochemically similar granites in contrasting climates would also supply divergent water types. The literature on rock weathering is indeed abundant, and the one addressing the weathering of granites continues to attract scientific attention (e.g. Wang et al. 2023; Liu et al. 2024).
The articles focusing on the features and changing aspects of early weathering are a constrained subset among the thousands of papers generally dealing with weathering (e.g. White 2005). Although it is not among the most cited papers, Garrels and Mackenzie (Drever 1997) published an article on the characteristics of early weathering (i.e. through the chemistry of springs and lakes) in the Sierra Nevada (California and Nevada, USA), seeking to relate major dissolved chemical components with the original minerals using an ingenious approach.
Several authors probed into the characteristics of weathering in the Sierras Pamepanas of Córdoba, Argentina, mainly in its two foremost mountain ranges: Sierra Grande and Sierra de Comechingones (e.g. Gaiero et al. 1998; Pasquini et al. 2002; Kirschbaum et al. 2005; Lecomte et al. 2005, 2009; García et al. 2007). The aim, here, is to review the characteristics of early chemical weathering in a uniform lithology scenario (i.e. aluminous A-type granite), controlled by weathering-limited denudation. This article revisits different approaches—mainly using previously published data -, succinctly comparing the characteristics of incipient weathering by probing into the chemistry of high-altitude (i.e. ~ 1100–2100 m a.s.l.) perennial/ephemeral springs and streams in the AchalaFootnote 1 batholith (Sierras Pampeanas, Córdoba, Argentina).
Study area and methods
Geography and geology
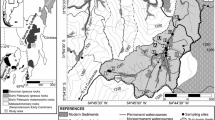
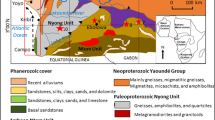
Sierras Pampeanas designates a mountainous and partly semiarid region that occupies portions of seven Argentine provinces in central-west Argentina (~ 0.3 106 km2). It comprises several mountain chains, generally following a north–south orientation, roughly parallel to the Andes. In this article, the attention will be placed on Sierra Grande and Sierra de ComechingonesFootnote 2 (i.e. the northern and southern sectors, respectively), where the Achala batholith is a central element composed of a group of plutons with a mapped area of over 2500 km2, thus constituting the largest group of intrusive rocks exposed in the Sierras Pampeanas (Fig. 1).
Schematic geologic map of a sector of Argentina’s Sierras Pampeanas. The Achala batholith (“Batolito de Achala”), with an area of ~ 2500 km.2, is the central feature in the map, modified from Lira and Sfragulla (2014). The South American map (inset) shows the approximate location of the Sierras Pampeanas. The figure includes the sampled areas referred to in the text. The samples for stable isotopes were collected in the same area sampled by Pasquini et al (2002). The GNIP station (i.e. La Suela) is within the area sampled by Lecomte et al. (2011)
The Sierra Grande and Sierra de Comechingones belong in Argentina’s Córdoba Province (i.e. Sierras de Córdoba). Jointly, they are ~ 490 km long and ~ 150 km wide. With a maximum altitude of 2884 m a.s.l., the Cerro ChampaquíFootnote 3 is the highest peak in Sierra de Comechingones, whereas the Cerro Los Gigantes (2350 m a.s.l.) is the highest mountain in Sierra Grande.
The action of polar and subpolar fronts dominates climate in the area of interest. It lies in Argentina’s temperate zone, exhibiting active atmospheric dynamics; the regional climate is typically continental, with irregularly distributed rainfall, which occurs mostly during (austral) summer and early autumn (e.g. Pasquini et al. 2006). About 75% of the total annual precipitation (i.e. historical regional mean ~ 1100 mm y−1, record period 1943–2005, Martínez et al 2016) occurs between November and March. Mean monthly atmospheric precipitation in winter is ~ 40 mm (April-September), including occasional snowfall (e.g. Pasquini et al. 2006, and references therein). With considerable frequency, Achala’s highest ranges remain above the cloud level. They are thus subjected to 100% humidity (i.e. total cloud cover prevails 52% of the time, mainly during austral winter), thus water-feeding perennial/ephemeral springs (e.g. Pasquini et al. 2006).
The annual mean isotherm of ~ 16 °C encloses the area, falling to ~ 10 °C at 2000 m elevation. The maximum mean isotherm is ~ 20 °C, whereas in the higher areas, it is about ~ 14 °C. In contrast, the lowermost areas exhibit a minimum mean isotherm of ~ 9 °C whereas it is ~ 5 °C in the highest elevations. During winter, minimum temperatures can be − 10 ºC or lower (e.g. Pasquini et al. 2006, and references therein).
The analysis of recent rainfall and runoff data series from central Argentina has shown that there is, in general, a significant positive trend in rainfall, which has been verifiable since the second half of the twentieth century. Moreover, the spectral analysis also showed an apparent El Niño-Southern Oscillation (ENSO) limited influence south of ~ 31ºS (Pasquini et al. 2006).
Despite significant annual rainfall, it turns out that relatively steep slopes, and shallow regolith accumulations, yield scattered vegetation of grassland, and wide-ranging shrubbery. Morphological features typical of exposed granite, and gently undulating or relatively flat terrain are dominating features in the Achala batholith above ~ 1000 m elevation.
The Achala batholith (~ 31º07’S–32º00’S; ~ 64º30’W–65º15’W) is a Devonian-Carboniferous granitic body (e.g. Lira and Sfragulla 2014). The U–Pb SHRIMP crystallization ages of the main monzogranitic facies (379 ± 4 Ma) and younger intrusive tonalites (369 ± 3 Ma) denote that the main central zone of the batholith is of Upper Devonian age (Rapela et al. 2008). Silica-rich monzogranites (70–74% SiO2) are dominant although the batholith exhibits a wider SiO2 range (60–76%), plotting inside the fields of the calc-alkaline to alkali-calcium series. They are aluminous A-type granites (i.e. peraluminous) and show characteristic enrichments in F, Ga, Nb, high Ga/Al, and low K/Rb ratios. Rapela et al. (2008) proposed that the Achala granites would represent variable proportions of a juvenile mantle, with εNd from -1.1 to -4.0, along with the inherited zircons.
The dominant lithofacies is a fractured porphyritic monzogranite. It is a holocrystalline rock composed of quartz, K-feldspar, plagioclase, white mica, and biotite. K-feldspar is present as large phenocrysts (~ 2 to 20 mm) set in a medium to the fine quartz-feldspar matrix. Zircon, apatite, rutile, and opaque minerals occur as accessory minerals, whereas clays, sericite, chlorite, and muscovite represent the secondary minerals set. Román-Ross et al. (1998) described a similar mineral assemblage, along with alteration products in the prevailing lithofacies. More recently, Lira and Sfragulla (2014) reviewed Achala’s Devonian-Carboniferous magmatism.
Methodology
The water chemical data, published elsewhere and relevant to this paper, is included in tables inserted in the text. All cited articles involving water chemistry used similar field (i.e. sample collection, filtration, conductivity, pH, and alkalinity titration), and analytical methodology (i.e. chemically suppressed ion chromatography with conductivity detection for anions, and ICP-MS for major cations and trace elements).
Using published data, the procedure proposed by Tardy (1971), and modified by Boeglin and Probst (1998), was initially used to probe into Achala’s weathering in first-order streams. With molar proportions, it involves the calculation:
The coefficients in the above equation are contingent on the country rock’s major primary minerals and correspond to typical granitic mineral composition, with micas and feldspars.
This paper includes previous unpublished data on stable isotopes (i.e. δ2H, and δ18O). A sample set (i.e. seven samples) collected in the year 2000 was determined in Achala’s springs and streams. The samples were stored in 50 ml vials, tightly sealed without leaving air bubbles, and sent to Actlabs (Ancaster, Ontario, Canada) for subsequent analysis. Precision was 0.2 per mil for δ18O and 3.0% for δ2H. Vienna Standard Mean Ocean Water (VSMOW) was the standard selected for the analysis.
Campodonico et al. (2014) published the parent rock and regolith data recorded in a sample set collected in Achala’s batholith, describing the chemical and petrographic methodologies employed in their study. Using such data, a set of relative weathering indices (e.g. Price and Velbel 2003) was calculated to assess the chemical stripping exhibited by the mineral debris:
a) Chemical index of weathering {CIW = (100)[Al2O3/(Al2O3 + CaO* + Na2O)]};
b) Chemical index of alteration {CIA = (100)[Al2O3/(Al2O3 + CaO* + Na2O + K2O)]};
c) Vogt’s residual index [V = (Al2O3 + K2O)/(MgO + CaO* + Na2O)];
d) Weathering index of Parker {WIP = (100)[(2Na2O/0.35) + (MgO/0.9) + (2K2O/0.25) + (CaO*/0.7 + Na2O)]; and,
e) Plagioclase index of alteration {PIA = [(Al2O3—K2O)/ (Al2O3 + CaO* + Na2O—K2O)]}; CaO*is the CaO content included in the silicate fraction.Footnote 4
The resulting geometric means were statistically evaluated. The F test was employed to assess the hypothesis H0:σ12 = σ22 against the alternative H1:σ12 ≠ σ22. Similarly, the Student’s t-test was used to test the hypothesis H0:µ1 = µ2 against the alternative H1:µ1 ≠ µ2 (e.g. Davis 1986; Swan and Sandilands 1995). The comparison was between the source rock and the coarse- and fine-grained regolith.
The AqionFootnote 5 software (i.e. version 7.3.3) was employed to revalidate the aqueous solutions, verifying occasional charge balance errors (CBE) and establishing full charge balance (i.e. CBE ≈ 0), if required, making small adjustments by using dissolved inorganic carbon (DIC) as the correcting parameter. The Aqion software was also used to calculate important chemical parameters (e.g. ionic strength, PCO2, etc.).
PHREEQC Interactive (version 3.6.2.15100)Footnote 6 was the software employed to perform inverse modeling exercises, using rainfall data as the initial solution, and spring/stream chemical data as the evolved final solution (i.e. Table 9). The databases were those distributed with the program by the United States Geological Service (i.e. wateq4f.dat, minteq.dat, etc.), whereas the selected mineral phases are listed in the results (i.e. Table 10). All models ran with 0.025 (2.5%) uncertainties.
Reviewing previous studies
Achala’s regolith: main weathering features
Published research frequently approaches the assessment of weathering intensity and rate by contrasting the constituents of the fresh parent material with those of the weathered rock or the resulting unconsolidated mineral debris. This is a straightforward procedure in fully developed soil profiles (e.g. Krauskopf and Bird 1995; Drever 1997), usually found in transport-limitedFootnote 7 denudation scenarios. Vázquez et al. (2016) provide a good example of transport-limited erosion in granitic rocks cropping out in the Coastal Range of central Chile. However, an indirect approachFootnote 8 is indicated when the composition of the parent material is not evident, as commonly happens in weathering-limitedFootnote 9 denudation regimes. Such scenarios usually prevail in mountainous catchments, where regolith is thin or soils are poorly developed.Footnote 10 Relative methods are employed, therefore, to determine weathering intensity and rate (e.g. Bland and Rolls 1998). In such environment-type, primary-weathering reactions control stream chemistry, also markedly influenced by the chemistry of atmospheric precipitation.
The evaluation of rock weathering is a complicated case when occurring in a weathering-limited regime, even with uniform lithology. Mineral debris linked to an unmistakably identified provenance allows approaching the characteristics of weathering more truthfully, as it occurs with absolute methodologies. Weathering appraisal in a weathering-limited granite batholith is a good example, generally receiving significant attention.
The characteristics of Achala’s regolith
The synergic action of physical, biological, and chemical weathering generates widespread tors,Footnote 11solution pits and pans,Footnote 12 and tafonis, as dominant morphological features in a granitic landscape. Another important attribute is the patchy distribution of grus (i.e. the loose collection of weathered mineral grains) on the Achala batholith, on top of exposed rounded granite boulders (i.e. subjected to spheroidal weathering), or coarse regolith mixed with sand/silt grain-size detritus, accumulated in topographic lows, rock joints or edges. Campodonico et al. (2014) analyzed the characteristics of Achala’s regolith. Near the source rock, mineral debris is equivalent to the original granite, with grain size fluctuating between very coarse sand (~ 2 mm mean grain size) and silt (~ 62-µm mean grain size). Campodonico et al. (2014) supplied a detailed description of the dominant grus: abundant quartz (~ 1 to 5 mm), relatively unaltered K-feldspar grains (~ 0.5 to 11 mm), muscovite (~ 0.1 to 1.5 mm), and plagioclase (~ 0.1 to 1 mm) exhibiting an alteration comparable with the one verifiable in the outcropping monzogranite. Plagioclase shows alteration to sericite, clay minerals, and secondary muscovite. Likewise, biotite (~ 0.2 to 2 mm) exhibits minor alteration to chlorite and secondary muscovite.
The fine-grained mineral debris is composed of variable proportions of fine/very fine sand (250 to 62.5 µm), silt (62.5 to 3.9 µm), and clay (< 3.9 µm). According to Campodonico et al. (2014), plagioclase (~ 0.2 to 2 mm) is more altered (i.e. virtually replaced by clays and sericite) than K-feldspar grains (~ 0.2 to 5 mm), which only show minor alteration. Fine-grained biotite (~ 0.2 to 0.7 mm) exhibits intense segregation of Fe-oxides. Inclusions in biotite, reported as accessory mineral phases, were apatite, rutile, zircon, and opaque minerals. The X-ray diffraction analysis of the < 2 µm size-fraction showed clay mineral assemblages with illite > kaolinite > smectite, with traces of albite, K-feldspar, and quartz (Campodonico et al. 2014).
Table 1 shows the geometric means (GM) and the corresponding geometric standard deviations (SDG)Footnote 13 of compositional data collected in the Achala batholith. Table 1 includes the granite’s most frequent chemical composition, the CIPW-calculated normative mineralogy for the porphyritic monzogranite, and the coarse- and fine-grained regolith (Campodonico et al. 2014). Rapela et al. (2008), Lira and Sfragulla (2014), Campodonico et al. (2014), and references cited therein, supplied additional petrographic information on the Achala granite.
The cursory inspection of Table 1 shows slight differences between the chemical and mineralogical characteristics prevailing in the country rock (i.e. monzogranite) and the coarse- and fine-grained regolith. Campodonico et al. (2014) showed –using mass balance calculations- that there are no statistically significant chemical differences between granite and coarse-grained regolith. There were statistically significant losses of MgO, MnO, and P2O5 in the fine-grained regolith (i.e. concerning the unaltered granite), which Campodonico et al. (2014) ascribed to the early chemical weathering of biotite and apatite. Plagioclase alteration was apparent through petrographic observations. CIPW norm calculations (Table 1) supported these observations. Regolith sorting may be the cause behind other differences, like those of quartz or K-feldspar.
3.1.2 The REE signature of Achala’s regolith. Geochemical research has shown that rare earth elements (REEs) are among the least mobile chemical elements in weathering. The variations in REE concentrations in natural waters have been explained by the presence of colloids that increase their apparent solubility (e.g. Gaillardet et al. 2005, and references therein). Different studies have determined that fractionation between light (LREE) and heavy REEs (HREE) is the outcome of variances in their affinity for being adsorbed onto surfaces. Accordingly, REEs are mobilized during weathering but are primarily recycled within the weathered environment rather than conveyed substantial distances in solution. This conclusion is important because it confirms the proposition that, for most weathering environments, REEs are transported by particles from the weathered milieu (e.g. Gaillardet et al. 2005, and references therein).
Achala’s country rock-normalized spidergrams (Fig. 2) showed a discernible HREE enrichment, endorsed by the LaN/YbN ratios,Footnote 14 with the following geometric means (GM) ± geometric standard deviations (SDG): 0.72 ± 1.112 for the coarse-grained regolith, and 0.54 ± 1.326, for the fine-grained regolith (Campodonico et al. 2014). Such previous study also showed Eu anomalies (i.e. EuN/EuN*) in the coarse-grained regolith samples (GM ± SDG: 1.40 ± 1.183), and in the fine-grained regolith (GM ± SDG: 1.52 ± 1.075), which can be attributed to the retention of detrital feldspar in the coarse fraction (Campodonico et al. 2014).
The Upper Continental Crust (UCC)-normalized extended diagram (Fig. 2) shows that regolith—regardless of its dominant grain size—preserves a signature similar to the original granite, with LREEs dominating over HREEs. However, finer-grained-sized standards (e.g. wide-ranging provenance PAAS and NASC), show an enriched signature, with HREEs slightly richer than LREEs. Fu et al. 2019 have shown that A-type granites –as Achala’s- may have different REE enrichment situations in comparison with I- or S-type granites.
3.1.3 The assessment of weathering in Achala’s granite using relative indices. The ratio between the more and the less stable oxides allows the assessment of weathering in rocks, sediments, or soils. This is the most frequent approach when the parent material is unknown or uncertain.Footnote 15 The result, expressed as an index, does not identify the minerals or minerals subjected to chemical stripping. The scientific literature describes several diverse weathering indices, most of which are based on the assumption that Al2O3 remains immobile during weathering (e.g. Price and Velbel 2003).
Several weathering indices for Achala’s country rock and the derived mineral debris are included in Table 2. A hasty inspection of each index's means and the corresponding standard deviations show small differences between the source rock and the coarse- (i.e. grus) or fine-grained mineral debris.
The resulting analysis showed that the null hypothesis had to be rejected when CIW (Harnois 1988) was used to compare the variances of fine-grained regolith with the source rock and with the coarse-grained regolith (p < 0.01) (Table 3). The remaining indices did not allow the rejection of the null hypotheses using the F test. The subsequent use of the Student’s t-test (e.g. Davis 1986) in both rejected instances led to the additional conclusion that there is no evidence to suggest that the samples (i.e. granite, coarse- and fine-grained regolith) came from populations having different means (Table 3). Therefore, standard statistical methods showed that CIW was the only index in the chosen set, which, in terms of weathering, separated fine-grain regolith from granite and grus. Furthermore, it is worth noticing that CIW is the only index in the used set that does not include K2O in the calculation.
Weathering in the Achala batholith: a brief overview of its dissolved chemical signature
Several authors have examined the dissolved geochemical characteristics of mountainous rivers draining the Sierra Grande and Sierra de Comechingones in Argentina’s Córdoba Province. Lecomte et al. (2009), and Martínez et al. (2016), considered first-order streams in AchalaFootnote 16 without fully exploring the linkage between the streams and the water-supplying springs. Both papers completed the respective investigations by probing through inverse modeling into the occurring weathering processes. On the other hand, Pasquini et al. (2002) studied the chemistry of Achala’s springs and first-order streams but did not use PHREEQC inverse modeling in the analysis. Such a procedure is presented in this article and the results are discussed in the following section.
Table 4 shows chemical data of a group of ephemeral-perennial springs and adjoining first-order streams draining a portion of Achala’s batholith (Pasquini et al. 2002). All streams are in the proximity of springs—although they do not necessarily have a direct connection with them—and they have a reduced interaction with fine-grained regolith, since most flow mainly in direct contact with bare granite (Fig. 3), often through rock fractures (e.g. temporary springs).
Images of characteristic high-altitude (~ 1800 m a.s.l.) first-order streams in the Achala batholith. Notice water flowing over bare rock (left), and tafoni and solution pans (right), typical of the granitic scenario (Jennings 1968). Photographs are ~ 3 m wide
The streams exhibit a higher content of dissolved species than springs: springs belong to the very dilute water type (i.e. TZ+ ≈350 ± 40.8 µeq L−1)Footnote 17 whereas streams are classified as dilute by a small margin (i.e. TZ+ ≈400 ± 118.7 µeq L−1). Ionic strength and, in general, other chemical variables (i.e. nutrients included), also show this difference. As expected, springs are more significant sources of dissolved inorganic carbon (DIC) than streams. Low-order streams (i.e. first-order, in particular) play an important role in the evasion of CO2 from inland waters, a feature revealed during the assessments of the global carbon budget (e.g. Butman and Raymond 2011). The decreasing order of ionic abundance (i.e. Na+ > Ca2+ > Mg2+ > K+, for cations, and HCO3− > Cl− ≥ SO42− for negatively charged species) matches the sequence determined in Achala’s high-altitude granite-draining streams (e.g. Lecomte et al. 2005, 2009; Martínez et al. 2016).
Lecomte et al. (2009) further probed into the physicochemical characteristics of chemical weathering occurring in the Achala batholith, showing the connection existing with geomorphological features. The data gathered in first-order order streams draining exposed granite was separated (Table 5) from the broader set -including higher-order streams- compiled by Lecomte et al. (2009). Contrasting with the preceding case, most first-order streams in the set drain areas have a substantial accumulation of fine-grained regolith and exhibit, therefore, an extended mineral–water contact.
Like in the preceding case, water in the high-altitude granite-dominated environment fluctuates between very dilute (i.e. which could be chemically close to atmospheric precipitations) and dilute chemical types (185 < TZ+ < 750 µeq L−1) (Table 5) (Lecomte et al. 2009). Following the chemical signature imposed by the country rock (Campodonico et al. 2014), the most frequent order of molar abundance among cations and anions was identical to the one determined in springs and streams (Pasquini et al. 2002).
Martínez et al. (2016) published a detailed study performed during a hydrological year in a small A-type granite-draining catchment in Sierra de Comechingones. The water chemistry determined in the first-order headwater was separated from the higher-order streams in the catchment, and the set is reproduced in Table 6. Mean TZ+ ≈388 ± 201.6 µeqL−1 allowed its classification within the dilute water type, in agreement with the formerly described examples.
Results and discussion
Achala’s atmospheric precipitations: the isotopic, and the Na+ /Cl− ratio signature
The chemical composition of atmospheric precipitation falling over a specific area may vary through time, with the distance from the oceanic source, and with anthropogenic/natural inputs, through aerosols. The water supplied by rain- or snowfall interacts with plants, regolith—or soil, if present -, and bedrock. Therefore, the geochemical attributes of water flowing in a drainage basin usually depend more on the flow paths followed by water through regolith and soils than on the chemical characteristics of atmospheric precipitation. However, exceptionally abundant rainfall events may result in riverine chemical signals that may be close, in chemical terms, to those exhibited by precipitation. Further, the dissolved chemical signal of mountainous streams draining catchments with scant regolith and/or immature soils may be more affected by precipitation chemistry than other river types, where prolonged and intimate contact with regolith/soils occurs (e.g. Meixner et al. 2000).
To probe with stable isotopes into the characteristics of Achala’s springs and rivers, δ18O and δ2H were determined simultaneously with the sampling of other parameters (Table 7). An excess of 2H (or D) relative to 18O in the vapor is caused during evaporation by the diffusion of water molecules across a density gradient; the more diffusion (i.e. kinetic fractionation) that occurs, the higher the deuterium excess observed in vapor (i.e. evaporated moisture) (e.g. Bershaw 2018). The data shows that deuterium excess (De)Footnote 18 is significant (i.e. De > 10 per mil) in springs and streams of the Achala batholith. Such increased De in precipitation can arise from the important addition of re-evaporated moisture from continental sources to the water vapor traveling inland. Suppose moisture from precipitation with an average excess of 10 per mil is re-evaporated. In that case, the lighter 2H1H16O molecule may again contribute preferentially to the isotopic composition of the water vapor, and this, in turn, leads to an enhanced deuterium excess in precipitation.
Figure 4 shows the δ18O and δ2H diagram. The Local Meteoric Water Line (LMWL) and the Global Meteoric Water Line (GMWL)were included for comparison. In addition to local springs and streams data, GNIPFootnote 19 isotope data for rainfall collected at nearby La Suela station (~ 31°S, ~ 64°W, 892 m a.s.l.) was included in the graph, showing that some rainfall events plot close to the isotopic signature of Achala’s springs and streams. Likely, chemical signatures in springs and first-order streams are significantly associated with the volume and intensity of atmospheric events.
Stable isotopes in springs, streams, and rainfall in Achala. Rainfall data from La Suela station [i.e. Global Network of Isotopes in Precipitation (GNIP)] was included for comparison. The Global Meteoric Water Line (GMWL) [δ2H = 8δ18O + 10], and the Local Meteoric Water Line (LMWL) [i.e. orthogonal regression equation included in the graph (Dapeña 2008) is plotted for reference]. The sampled streams are not hydrologically connected to the sampled springs. See text for additional information
The orthogonal regression for the LMWL (Fig. 4) has been calculated employing average weighted precipitation data (Dapeña 2008).Footnote 20 The plotted data shows that the δ18O composition of springs appears somewhat less negative than the samples collected in first-order streams. Besides the significance of De described above, there is an aspect that must be underlined here, and it is that the sampled springs do not water-supply the sampled streams, being separated entities. A second relevant aspect is that springs are water-supplied by rainfall and winter-spring meltwater that is sometimes subjected to sublimation in the mountainous windy environment, thus affecting the isotopic composition. The third factor that likely impacts the isotopic signature of springs and streams is the frequent low cloud level (i.e. below 2000 m a.s.l.) that prevails in spring and autumn, frequently maintaining a water-saturated environment in the highest parts of the mountainous chain.
To probe into the geochemical linkage existing between atmospheric precipitations and first-order streams, Fig. 5 shows a scatter diagram of Na+ vs. Cl− concentrations, as determined in streams draining the Achala batholith. Only one sample plot within the field defined by Möller (1990) for continental rain (i.e. Na+ /Cl−ratios between 1.1 and 1.8, not influenced by excess Na+ or Cl−). The slope of the lines for other Na+ /Cl− ratios of local rainfall (i.e.1.24, Lecomte et al. 2009), and the rainfall plus snowfall mean for the area (i.e. 2.0, Lecomte et al. 2009) are included for comparison.
Scatter diagram of Na+ vs. Cl− in first-order streams and springs draining the Achala batholith. The Na+/Cl− ratios (y/x = 1.1–1.8) are defined by Möller (1990). Local rainfall (y/x = 1.24), and the mean of local rainfall-snowfall (y/x = 2) are from Lecomte et al. (2005, 2009). Data from springs and first-order streams (Pasquini et al. 2002) show a considerable Na+ enrichment. See text for further explanation
Data suggests that there are two likely Na+ sources. One source is the aerosols from neighboring (i.e. to the N-NW of Achala, Fig. 1) salt flats/desert area, which supply allochthonous Na+ and Cl−. Salty dust can be delivered by dominant northern winds (i.e. as dry fallout) or by rainfall/snowfall. Spring and stream data (Pasquini et al. 2002) in Fig. 5, further indicates that the weathering of Na+-bearing minerals (e.g. plagioclase) is the additional likely source in springs for the Na+excess.
The data plotted in Fig. 5 includes samples collected during the rainy (i.e. December-March) and dry (May–August) seasons in the pilot drainage basin (Martínez et al. 2016). Data implies that wet conditions (i.e. summer rains) promote the lowering of the Na+ /Cl− ratio of local rainfall.
Observations on the chemistry of Achala’s springs and streams
The use of the RE parameter (Boeglin and Probst 1998) was the initial step to approach the nature of the likely weathering byproducts of silicate hydrolysis in Achala’s batholith. If RE = ~ 0, the leading weathering product should be gibbsite; if RE = ~ 2, the formation of kaolinite should prevail, and if RE = ~ 4, smectite should be the most frequent solid weathering product (Boeglin and Probst 1998).
The data set in Table 5 shows a significant linear correlation (not shown, r = 0.67, p < 0.001) between conductivity (i.e. TDS) and RE (i.e.i.e..e., with values fluctuating in the ~ 1 to 3 range), suggesting that the likelihood of obtaining smectite as a weathering product in the early alteration of granite, increases with increasing TDS concentration. In some small first-order streams,Footnote 21RE oscillates between ~ 0.4 and ~ 0.8, indicating the possible formation of Al(OH)3(i.e. gibbsite), an essential building block in the structure of clays, like kaolinite and illite. The RE parameter applied to the data of Table 6 shows a tendency to produce gibbsite and kaolinite because RE fluctuates between ~ 0 and ~ 2.
Figure 6 shows the relationships between Ca2+/Na+ (x-axis), HCO3−/Na+, and Mg2+/Na+ (y-axes) (i.e. data from Tables 4, 5, and 6). Besides the theoretical calcite dissolution line, the other lines in Fig. 6 were plotted with data published by Garrels and Mackenzie (Drever 1997) in their reconstruction of source minerals and weathering products for Sierra Nevada ephemeral springs, and water with deeper circulation (Nevada and California, USA). Garrels and Mackenzie’s model's weathering products included kaolinite and the dissolved pool. The reconstructed weathering reactions occurring in deeper water circulation included smectite and kaolinite as crystalline byproducts, and dissolved species, which exhibited higher concentrations than those determined in springs. Consequently, the evolution lines depicting deep water circulation were omitted in Fig. 6.
Ca2+/Na+ ratio plotted against Na+-normalized HCO3− and Mg2+ in springs and first-order streams Achala’s batholith. Upper graph: First-order streams sampled by Lecomte et al. (2009)a, and by Martínez et al. (2016)b. Lower graph: springs and streams sampled by Pasquini et al. (2002). Plotted for comparison is the theoretical chemical evolution of calcite dissolution. The data to draw the chemical evolution of ephemeral springs (Sierra Nevada, USA) is from Garrels and Mackenzie (Drever 1982). Data from deep water circulation would be plotted outside the graph, to the right (e.g. Ca2+/Na+ ratio ≈ 1.5). See text for additional explanation. Note logarithmic axes
The somewhat wider scattering of Ca2+/Na+ vs HCO3−/Na+, associated with the y = 2 × line suggests a contribution of calcite dissolution, which appears to be mostly associated with streams in all cases (i.e. Pasquini et al. 2002, Lecomte et al. 2011, and Martínez et al. 2016). Precipitation as a byproduct of plagioclase hydrolysis, and dry fallout (i.e. wind-transported dust) are likely dominant sources in first-order streams. Conversely, alkalinity in springs seems associated with silicate hydrolysis, articulating with the possible mineral sources (i.e. biotite, apatite, and plagioclase) indicated by the analysis of weathered mineral debris (i.e. Sect. "Achala’s regolith: main weathering features").
The variability of concentrations is significantly ampler in first-order streams than in springs. The source of Mg2+ seems consistently controlled by the hydrolysis of silicates in both, springs and streams.
The integral analysis of Fig. 6 shows that springs in Achala’s batholith are closer to temporary dynamics than to the deeper circulation of perennial springs (e.g. the Ca2+/Na+ molar ratio is ~ 0.6 in ephemeral springs and ~ 1.5 in the deeper circulation of the Sierra Nevada example.
Sulfate is a significant component among anions in the dissolved products of Achala’s early weathering. The geometric mean fluctuates significantly in granite draining waters: ~ 18.1 µM in springs, and 23.1 µM in streams (Table 4); ~ 46.3 µM (Table 5), and ~ 12.3 µM (Table 6). Sulfate in the mountainous springs and streams of Achala is supplied either through the oxidation of sulfides or by dry falloutFootnote 22/atmospheric precipitations. The maximum SO42− concentration of sulfide oxidation in surface waters saturated with oxygen is ~ 200 µM (Tranter 2005).
Pyrite (FeS2) is among the most common sulfide minerals on the Earth’s surface, and it plays an important role in geochemistry and biological/environmental processes. When exposed to the atmosphere, pyrite oxidizes, forming sulfuric acid in the presence of humidity (e.g. Stumm and Morgan 1996)
Fe2+ endures oxygenation to Fe3+, subsequently hydrolyzed to Fe(OH)3(s) liberating more acidity and coating mineral grains in the streambed. FeS2 can reduce Fe3+, pyrite oxidizes again, and releases more protons and Fe2+:
The net effect of this system is the production of soluble iron (i.e. subsequently oxidized to Fe3+), SO42−, and acidity, which attacks calcite and other minerals. Thus, a substantial amount of Fe2+ is lost from the solution via the precipitation of Fe(OH)3(s) (Stumm and Morgan 1996). This mechanism can be inferred from Fig. 7, which also leads to the assumption that a considerable proportion of SO42−, in Achala’s granite, is supplied by pyrite oxidation.
Exploring early weathering in springs/streams with PHREEQC.
PHREEQC is a computer program written in C programming language, conceived to execute varied aqueous geochemical calculations, and used to tackle an extensive variety of problems concerning water resourcesFootnote 23 (e.g. Parkhurst 1997; Parkhurst and Appelo 2013). The software is successfully used in exploring groundwater chemistry (e.g. Uliana and Sharp 2001) as well as probing into the dynamics of chemical reactions in the aqueous milieu (e.g. Marsac et al. 2011; Fu et al. 2019).
PHREEQC modeling seems more frequently used in connection with groundwater than in streams or rivers. However, the advantage of exploring the nature of weathering in a river catchment with a modeling approach is that it supplies a quantitative assessment of the ongoing hydrolysis/dissolution mechanisms, estimates the formation of new mineral phases, and allows a comparison with the traditional analytical approach.
PHREEQC inverse modeling was the tool used in the weathering assessment of Achala’s uppermost granite catchments (Lecomte et al. 2005, Martínez et al. 2018) (Table 8). In both instances, the starting solution was the mean regional rainfall chemical composition. The drainage basin modeled by Lecomte et al. (2005) covers an approximate area of 60 km2 (i.e. fourth-order stream). In contrast, the catchment studied by Martínez et al. (2018) has an estimated drainage area of ~ 0.032 km2 (i.e. first-order stream). A poorly sorted fine- and coarse-grained regolith mantles both drainage areas. Interstitial water had, in both instances, a significant residence time.
Both papers suggest the generation of similar precipitated weathering products (i.e. illite and kaolinite), although the smaller catchment produced more illite than kaolinite (i.e. ~ 160 against ~ 70 µmol kgw−1) both, in absolute terms as well as in an area-normalized approach. The simulations failed to identify apatite as a likely reactant because the analytical data did not include phosphorous.
In contrast, the largest catchment produced more kaolinite than illite (i.e. ~ 106 against ~ 10-µmol kgw−1) (Table 8). Hydrolysis seems more efficient in the smaller catchment, and reactions transfer more secondary minerals per unit area, probably due to higher PCO2, and a significantly higher water residence time. Data was collected during (austral) wintertime (i.e. stream baseline flow) when solutes exhibited the highest concentrations.
Additional hydrochemical data (Table 4) was employed to model and compare weathering in the Achala batholith, by using chemical data collected in ephemeral/perennial springs and related first-order streams. The geometric means of the chemical data (Table 4) were used to develop PHREEQC inverse models, using mean rainfall data as a starting solution in both instances (i.e. in springs as well as in first-order streams) (Table 9).
The software supplied seven feasible models in each case (i.e. rainfall against springs, and rainfall against first-order streams). Table 10 shows the variability (i.e. as mol kgw−1) of the mole transferred for each dissolved or altered mineral phase as well as the number of models (i.e. out of the total possible models) where de indicated phase contributed to the reaction.
In both approaches (i.e. rainfall/springs, rainfall/streams) the most ubiquitous hydrolyzing/dissolving reactants were plagioclase, biotite, halite (i.e. rainfall), and CO2 (g). Plagioclase was the most susceptible solid phase to dissolve (i.e. most frequent values ~ 89–97 µmol kgw−1; ~ 28–30% of the total dissolved pool), reaching similar values in both, springs and streams, whereas CO2 (g) was by far the most abundant phase, accounting for ~ 45 and ~ 48% of the dissolved pool in springs and first-order streams, respectively. Biotite was the second most abundant solid phase subjected to hydrolysis in both, springs and streams with ~ 2–3% of the total dissolved pool. Gypsum was a minor phase supplying Ca2+ and SO42− in springs and streams and may be a proxy of pyrite oxidation in oxygenated environments. Other solute-supplying phases—with marginal frequency- in springs and streams were calcite, K-feldspar, and K-mica (Table 10).
Alternatively, the phases most frequently found (i.e. as likely alteration products) in both, springs and streams, were illite (~ 13 and ~ 8%, respectively, of the total pool) and gibbsiteFootnote 24 (~ 24 and ~ 31%, respectively), with Ca-montmorillonite, kaolinite, chalcedony (i.e. Achala batholith’s tafoni regularly exhibit silica speleothems within hollows), and sepioliteFootnote 25 as less frequent reactants.
Gibbsite and chalcedony (~ 24 and ~ 30%, with lower frequencies) lead in the set of resulting alteration species, with significant phase transferred in both, springs (~ 60-µmol kgw−1) and streams (~ 90-µmol kgw−1). Illite (mean ~ 31 µmol kgw−1), and Ca-montmorillonite (mean ~ 43 µmol kgw−1), appear to reach higher values in springs than in streams (illite mean ~ 23 µmol kgw−1; Ca-montmorillonite mean ~ 34 µmol kgw−1).
Both mean solutions (i.e. springs and first-order streams) evolved from a common starting solution (i.e. mean rainfall chemical composition). The differences between the starting solution (i.e. rainfall) and springs or first-order streams are assumed as caused by reactions between water, and the interacting minerals and gases. Therefore, PHREEQC modeling implies that most mole transfer (i.e. over 95%) occurs within the spring realm, establishing the chemical fingerprint found downstream, in first-order streams. The simulation of the reactions occurring between springs –as the starting solution- and 1st order streams—as a final solution- delivered only two models: one with gibbsite as the only phase (~ 60 to 70 µmol kgw−1), and another with gibbsite (~ 60 µmol kgw−1)Footnote 26 along with sepiolite (~ 4.4 µmol kgw−1), as likely transferred phases.
The results listed above are coherent with the information obtained from the RE equation, as well as with the exploration of chemical data, which denotes the occurrence of silicate hydrolysis -mostly in ephemeral springs -, along with pyrite oxidation, and calcite/gypsum dissolution.
Summarizing and concluding comments
The Achala batholith is central Argentina's main peraluminous, A-type granitic core of the Sierras Pampeanas. This overview of its chemical weathering seeks to re-examine published and unpublished data, focusing on the early chemical weathering of large, silica-rich, granitic bodies. The mountainous region is subjected to a weathering-limited denudation regime (i.e. exposed mature weathering profiles are absent), revealing varied morphological features, typical of bare weathered granite. Abundant grus is ubiquitous on top of rounded and fractured boulders, and fine- and coarse-grained regolith has accumulated in topographic depressions.
Earlier published research and the approach followed in this contribution allow summarizing the following conclusions:
-
Weathering indices did not disclose significant chemical differences between source rock and coarse- and fine-grained regolith (Campodonico et al. 2014; Martínez et al. 2018). A larger set of relative weathering indices (i.e. CIA, CIW, Vogt’s residual index, WI, and PIA), basically concur with earlier findings, showing the nature of Achala’s chemical weathering. Due to the scant alteration of K-feldspar in the regolith, CIW is the only index that exhibits statistically verifiable geochemical differences between the fine-grained regolith, source rock, and grus.
-
The regolith preserves the UCC-normalized REE spidergram of Achala’s batholith A-type granite. As the regolith’s grain size decreases, so does the LREE/HREE fractionation, and the Eu anomaly. The outline of the coarse- and fine-grained regolith is markedly different from the pattern exhibited by standard mudstones (i.e. PAAS and NASC).
-
Earlier papers (e.g. Lecomte et al. 2005, 2011; García et al. 2007; Martínez et al. 2018) reported chemical data on rainfall and snowfall falling over the Sierras Grandes of Córdoba (Argentina), which differs significantly from atmospheric precipitations with a distinct oceanic origin. Stable isotopes (i.e. δ18O and δD) determined in Achala’s springs and streams are coherent with GNIP data, with important deuterium excess (De > 10 per mil), suggesting the significant addition of re-evaporated moisture from continental sources to the water vapor traveling inland. Frequent northern winds promote the effect of aerosols supplied by arid salt flats (i.e. situated to the N-NW of the Sierras Pampeanas), affecting the chemical quality of Achala’s stream water (e.g. García et al. 2022).
-
The concentration of major ions conveys the image that the dominating mineral sources in springs, and low-order streams are close to those denoted by the analysis of weathered mineral debris (Campodonico et al. 2014). The scattering in the plotting of Ca2+/Na+ vs HCO3−/Na+ or Mg2+/Na+ implies significant variability in the rock-water contact (i.e. suggesting circulation through alluvium or rock fractures). Data points plotted next to the ideal calcite dissolution line suggest the contribution of disseminated calcite to the solute pool, partly supplied as wind-transported aerosols or as a plagioclase hydrolysis byproduct.
-
Lecomte et al. (2005) led to the use of computer models (i.e. PHREEQC) to assess weathering intensity in the Achala batholith. Likewise, Martínez et al. (2018) evaluated exogenous processes in a small pilot catchment in the Sierra de Comechingones. Both approaches identified plagioclase, calcite, CO2(g), and biotite as the phases controlling the transfer of solutes. Martínez et al. (2018) also identified K-mica, fluorite, gypsum, and halite as subordinate reactants supplying solutes in a first-order granitic stream. Illite was the most abundant alteration product, followed by kaolinite. In contrast, such order was reversed in the fourth-order stream modeled by Lecomte et al. (2005), with far ampler and lengthy water-regolith contact. First-order streams appeared more efficient than the higher-order system in dissolving and supplying area-normalized mineral constituents (i.e. specific chemical yield decreases with increasing drained area).
-
Pyrite oxidation was not included in simulations even though it is a probable source of SO42− and total Fe as long as oxygenation remains operative. Iron is removed from the solution and coats mineral grains, immobilized as (oxy) hydroxides. Aerosol-supplied gypsum is a plausible additional source of SO42− in streams, just as Fe is a likely derivative of pyrite oxidation and biotite hydrolysis.
-
Data collected in ephemeral/perennial springs and associated streams draining the highest part of the Sierra de Comechingones (i.e. Achala batholith) (Pasquini et al. 2002) was employed to examine the geochemical characteristics of early weathering using PHREEQC inverse modeling. The models involved contrasting the mean chemistry of regional rainfall with the geometric means of the main chemical components of high-altitude springs and streams. Divergent from previous examples, water had a dominant contact with bare granite and lesser interaction with regolith in this scenario.
-
Plagioclase, biotite, halite, and CO2(g) were the leading reactants in this instance. Less frequently identified, as likely phases delivering dissolved constituents, were K-feldspar, calcite, and K-mica. Gypsum reached a discernible frequency of occurrence in streams, herein jointly interpreted as wind-supplied aerosols from neighboring playas, and as a proxy of pyrite oxidation.
-
Contrasting with earlier findings, illite (in springs) and gibbsite (in streams), were recognized as the more ubiquitous mineral phases. Less frequently, PHREEQC identified Ca-montmorillonite and sepiolite as transferred phases in springs, whereas kaolinite and chalcedony played a similar role in low-order streams.
-
This evaluation of early weathering in a granite batholith shows that inverse geochemical modeling applied in springs and streams may be a valuable addition when assessing weathering using a relative methodology in weathering-limited denudational regimes. It unveils mineral phases that likely participate as reactants or products in the process, which may be difficult to identify when exclusively investigating solid-weathered debris through instrumental methodologies. Mineral phases resulting from early weathering processes usually are in trace amounts and, hence, difficult to detect. Moreover, the exercise showed that rather than in low-order streams, most hydrolysis/dissolution takes place in springs (~ 95%) -whether ephemeral or perennial- as well as the transfer of mineral phases (~ 83%).
Data availability
Data is not deposited in a public repository.
Code availability
Not applicable.
Notes
From achallay, quechua for nice, good-looking.
Common term used to designate the aboriginal inhabitants of the region (i.e. hênîa and kâmîare).
“Water at the top” in the aboriginal language.
The indirect method, proposed by McLennan (1993), involves the subtraction of P2O5 from the molar proportion of total CaO.
Transport-limited denudation occurs where weathering processes are efficient at producing debris but where transport processes are inefficient at removing it (Carson and Kirkby 1972).
Usually, the calculation of the ratio between the more stable and less stable oxides.
Weathering- limited denudation occurs where erosion proceeds faster than debris-producing weathering (Carson and Kirkby 1972).
High-energy mountain streams and rivers have been identified as singular river types, deserving focused attention (Wohl 2010).
Isolated granite masses, consisting of either a single or of numerous combined blocks presenting variable degrees of angularity or roundness (Twidale 1968).
Pits are small pockmarks (i.e. few millimeters to some centimeters in diameter and depth); pans develop from pits by lateral extension in all directions but for the most part maintain a perfectly flat floor (Fairbridge 1968).
When a1, a2, … an > 0, GM = exp [1/n ln an]; likewise SDG = exp {SD [ln (an)]}.
N denotes normalization to mean granite composition.
The Achala batholith is subjected to a weathering-limited denudation regime and, hence, there are no fully-developed weathering profiles.
Lecomte et al. (2009) expanded the investigation by probing into higher-order streams and rivers.
TZ+ = 2Ca2+ + 2Mg2+ + Na+ + K+.
De = δD − 8 δ18O.
IAEA/WMO (2020). Global Network of Isotopes in Precipitation. The GNIP Database. Accessible at: https://nucleus.iaea.org/wiser.
As defined by Strahler (1964).
Extended salt flats (Salinas Grandes), to the NE of the Sierra Grande-Sierra de Comechingones are significant sources of wind-blown halite, gypsum/anhydrite, and carbonate.
Al(OH)3.
Mg4Si6O15(OH)2·6H2O.
Approximately equivalent to 78 µg kgw.−1.
References
Bershaw J (2018) Controls on deuterium excess across Asia. Geosciences 8, 257; https://doi.org/10.3390/geosciences8070257
Bland W, Rolls D (1998) Weathering. An introduction to the scientific principles. Arnold, London, 271 pp
Butman D, Raymond PA (2011) Significant efflux of carbon dioxide from streams and rivers in the United States; https://doi.org/10.1038/NGEO1294
Campodonico VA, Martínez JO, Verdecchia AI, Pasquini AI, Depetris PJ (2014) Weathering assessment in the Achala Batholith of the Sierra de Comechingones, Córdoba, central Argentina. I: Granite-regolith fractionation. Catena 123:121–134. https://doi.org/10.1016/j.catena.2014.07.016
Carson MA, Kirkby NJ (1972) Hillslope form and processes. Cambridge University Press, Cambridge
Dapeña C (2008 Light environmental isotopes: their application in hydrology and hydrogeology. Doctoral thesis, FCEN (Universidad de Buenos Aires). 442 pp (In Spanish)
Davis JC (1986) Statistics and data analysis in geology, 2nd edn. Wiley & Sons, New York, p 646
Drever JI (1982) The geochemistry of natural waters, 3rd edn. Prentice Hall, Upper Saddle River
Drever JI (1997) The geochemistry of natural waters. Surface and groundwater environments, 3rd edn. Prentice Hall, Upper Saddle River, p 436
Drever JI (2005) (Ed) Surface and ground water, weathering, and soils, Vol 5, Treatise on Geochemistry, Elsevier-Pergamon, Oxford, p 626
Fairbridge RW (1968) Solution pits and pans. In: Geomorphology. Encyclopedia of Earth Sciences. Springer, Berlin, Heidelberg. https://doi.org/10.1007/3-540-31060-6_342
Fu W, Li X, Feng Y, Feng M, Peng Z, Yu H, Lin H (2019) Chemical weathering of S-type granite and formation of Rare Element (REE)-rich regolith in South China- Critical control of lithology. Chem Geol 520:33–51
Gaiero DM, Pecsi HE, Depetris PJ (1998) Effects of quarry mining and other environmental impacts in mountainous drainage basins: the Chicama-Toctina example of Córdoba. Argentina Env Geol 34(2/3):159–216
García MG, Lecomte KL, Pasquini AI, Formica SM, Depetris PJ (2007) Sources of dissolved REE in mountainous streams draining granitic rocks, Sierras Pampeanas (Córdoba, Argentina). Geochim Cosmochim Acta 71:5355–5368
García MG, Lecomte KL, Depetris PJ (2022) Natural and anthropogenic sources of solutes in the wet precipitation of a densely populated city in Southern South America. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.132307
Harnois L (1988) The CIW index: a new Chemical Index of Weathering. Sediment Geol 55:319–322. https://doi.org/10.1086/648222
Jennings J.N. (1968) Tafonis. In: Geomorphology. Encyclopedia of Earth Science. Springer, Berlin, Heidelberg. https://doi.org/10.1007/3-540-31060-6_365
Kanamaru T, Suganuma Y, Oiwane H, Miura H, Miura M, Okuno J, Hayakawa H (2018) The weathering of granitic rocks in a Hyper-arid and hydrothermal environment: a case study from the Sør-Rondane mountains. Geomorphology 317:62–74
Kinahan GH (1866) The effect of weathering on rocks. Geol Mag 3(20):46–88
Kirschbaum A, Martínez E, Pettinari G, HeFerrero S (2005) Weathering profiles in granites, Sierra Norte (Córdoba, Argentina). Jour South Am Earth Sci 19:479–493
Krauskopf KB, Bird DK (1995) Introduction to geochemistry, 3rd edn. McGraw-Hill Inc, New York, p 647
Lecomte KL, Pasquini AI, Depetris PJ (2005) Weathering in a semiarid mountain river: Its assessment through PHREEQC inverse modeling. Aquatic Geochem 11:173–194
Lecomte KL, García MG, Fórmica SM, Depetris PJ (2009) Influence of geomorphological variables on mountainous stream water chemistry (Sierras Pampeanas, Córdoba, Argentina). Geomorphology 110:195–202
Lecomte KL, García MG, Fórmica SM, Depetris PJ (2011) Hydrochemistry of mountainous rivers (Sierras de Córdoba, Argentina): dissolved major elements. Lat Am Jour Sedim and Basin Anal 18:43–62
Lee SY, Kim SJ, Baik MH (2008) Chemical weathering of granite under acid rainfall environment, Korea. Environ Geol 55:853–862
Lira R, Sfragulla R (2014) The Devonian-Carboniferous magmatism: The Achala batholith and smaller plutons north of Cerro Champaquí. In Martino RD and Guereschi AB (ed) Geología y recursos naturales de la Provincia de Córdoba. Relatorio XIX Congreso Geológico Argentino, Tomo I, pp. 293–347 (In spanish)
Liu R, Chi Y, Xie Y, Kang C, Sun L, Wu P, Wei Z (2024) Characteristics and influencing factors of the granite weathering profile: A case study of a high latitude area in northern China. Minerals 14, 17. https://doi.org/10.3390/min14010017
Marsac R, Davranche M, Gruau G, Bouhnik-Le Coz M, Dia A (2011) An improved description of the interaction between rare earth elements and humic acids by modeling PHREEQC-Model IV coupling. Geochim Cosmochim Acta 75(19):5625–5637
Martínez JO, Campodonico VA, Fórmica SM, Depetris PJ (2016) Weathering assessment in the Achala Batholith of the Sierra de Comechingones, Central Argentina. II: Major hydrochemical characteristics and carbon dynamics. Environ Earth Sci 75:554. https://doi.org/10.1016/j.catena.2014.07.016
Martínez JO, Campodonico VA, Fórmica SM, Depetris PJ (2018) Weathering assessment in the Achala Batholith of the Sierra de Comechingones, Córdoba, Central Argentina. III: appraising chemical weathering. Env. Earth Sci 77:242. https://doi.org/10.1007/s12665-018-7417-3
McLennan SM (1993) Weathering and global denudation. J Geol 101:295–303
Meixner T, Bales RC, Williams DH, Campbell DH, Baron JS (2000) Stream chemistry modeling of two watersheds in the Front Range, Colorado. Water Resour Res 36:77–87
Meybeck M (2005) Global occurrence of major elements in rivers. In Drever JI (ed) Surface and ground water, weathering, and soils. Treatise on Geochemistry, Elsevier-Pergamon, Oxford, pp 207–223
Möller D (1990) The Na/Cl ratio in rainwater and the sea salt chloride cycle. Tellus 42B:254–262
Nesbitt HW, Young GM (1982) Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 299(5885):715–717
Oliva P, Viers J, Dupré B (2003) Chemical weathering in granitic environments. Chem Geol 2002:225–256
Parkhurst DL (1997) Geochemical mole-balance modeling with uncertain data. Water Resour Res 33(8):1957–1970
Parkhurst DL, Appelo, CAJ (2013) Description of input and examples for PHREEQC version 3—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: USGS Techniques and Methods, book 6, chap. A43, 497 p http://pubs.usgs.gov/tm/06/a43/
Pasquini AI, Lecomte KL, Piovano EL, Depetris PJ (2006) Recent rainfall and runoff variability in central Argentina. Quat Int 158:127–139
Pasquini AI, Grosso LB, Mangeaud AP, Depetris PJ (2002). Geochemistry of mountain rivers in the sierras Pampeanas: I. Spring and streams in the Achala Batholith, Córdoba province, Argentina. Rev Asoc Geol Arg 57(4):437–444 (In Spanish)
Price JR, Velbel MA (2003) Chemical weathering indices applied to weathering profiles developed on heterogeneous felsic metamorphic parent rocks. Chem Geol 202:397–416
Rapela CW, Baldo EG, Pankhurst RJ, Fanning CM (2008) The Devonian Achala batholith of the Sierras Pampeanas: F-rich, aluminous A-type granites. 6th South American Symposium on Isotope Geology, CD-ROM, 8p San Carlos de Bariloche, Argentina
Román-Ross MG, Kirschbaum AM, Ribeiro Guevara S, Arribere MA (1998) Weathering processes in the Achala Batholith, Sierra Grande de Córdoba: Chemical and mineralogical changes. Rev Asoc Geol Argen 54(4):480–488 ((In Spanish))
Strahler AN (1964) Quantitative geomorphology of drainage basins and channel networks. In: Chow V (ed) Handbook of Applied Hydrology. McGraw Hill, New York, pp 439–476
Stumm W, Morgan JJ (1996) Aquatic chemistry. Chemical equilibria and rates in natural waters, 3rd edn. Wiley-Interscience, New York, p 1022
Swan ARH, Sandilands M (1995) Introduction to geological data analysis. Blackwell Science, Oxford, p 446
Tranter M. (2005) Geochemical weathering in glacial and proglacial environments. In Drever JI (ed) Surface and groundwater, weathering, and soils. Treatise on Geochemistry 5, Elsevier-Pergamon, Oxford, pp 189–205
Twidale CR (1968) Granite landforms. In: Geomorphology. Encyclopedia of Earth Science. 2Springer, Berlin, Heidelberg. https://doi.org/10.1007/3-540-31060-6_168
Uliana MM, Sharp JM Jr (2001) Tracing regional flow paths to major springs in Trans-Pecos Texas using geochemical data and geochemical models. Chem Geol 179:53–72
Vázquez M, Ramírez S, Morata D, Reich M, Braun J-J, Carretier S (2016) Regolith production and chemical weathering of granitic rocks in central Chile. Chem Geol 446:87–98
Wang W, Feng J, Qui M (2023) Mineral weathering and elemental migration in granite weathering pits (Gnammas): A case study in eastern China. Minerals 13:70. https://doi.org/10.3390/min13010070
Wedephol H (1995) The composition of the continental crust. Geochim Cosmochim Acta 59(7):1217–1232
White AF (2005) Natural weathering rates of silicate minerals. In Drever JI (ed) Surface and groundwater, weathering, and soils. Treatise on Geochemistry 5, Elsevier-Pergamon, Oxford, pp 133–168
Wohl W (2010) Mountain rivers revisited. American Geophysical Union, Washington DC, p 573
Funding
The cited references identify funding sources. Field and analytical work performed in ephemeral/perennial springs and streams were supported by the Universidad Nacional de Córdoba (Córdoba, Argentina), and Argentina’s CONICET.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Depetris, P.J. Reviewing early chemical weathering in the Achala Batholith (Sierras Pampeanas, Córdoba, Argentina). Sustain. Water Resour. Manag. 10, 173 (2024). https://doi.org/10.1007/s40899-024-01115-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-024-01115-1