Abstract
Safe drinking water is inaccessible for about 1.1 billion people globally. This study scrutinizes the physicochemical characteristics, pollution sources, suitability for drinking and agricultural purposes, and associated health risks of groundwater in the Thar Desert of Pakistan. Groundwater collected from 15 villages was analyzed for physical and chemical contaminants We found that a significant number of sampled wells exceeded the World Health Organization’s (WHO) guidelines for various contaminants: F− in 9 wells, Cl− in 12 villages, SO42− in 10 villages, and NO3− in 11 wells. The groundwater was predominantly classified as Na+ > Ca2+ > Mg2+ > K+ and Cl− > SO42− > HCO3− > NO3− > F−. Elevated concentrations of F− (0.24–29.9 mg/L), Cl− (20.6–8310 mg/L), NO3− (2.73–196 mg/L), and SO42− (7.74–1158 mg/L) exceeded WHO safe limits in most samples. Factor analysis identified both geogenic and anthropogenic activities as significant contributors to the observed groundwater chemistry. Based on the water quality index, 87% of samples were unsuitable for drinking, and a non-carcinogenic risk assessment deemed groundwater from 80% of the sites unacceptable for adults and entirely unsuitable for infants. Our findings indicate a high risk of F− and NO3− exposure for Thar Desert inhabitants. To ensure safe water consumption, we propose community-level implementation of treatment methods like distillation, reverse osmosis, and electrodialysis. Our study elucidates an urgent need for multi-stakeholder efforts toward sustainable water resource management and effective remediation. Implementing our proposed interventions and ongoing monitoring will be vital for protecting this vulnerable population's health and wellbeing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Access to safe drinking water remains a pressing global concern, affecting 1.1 billion people worldwide (Bain et al. 2012). Only a small portion of the Earth's water supply can be utilized as freshwater, and groundwater sources are particularly crucial for drinking water in many regions, including the Thar Desert in Pakistan (Ayoob and Gupta 2007). Anthropogenic activities, such as cattle farming and improper well lining, contribute to high nitrate concentrations in the study area (Giammarino and Quatto 2015; Rezaei et al. 2018, 2019a, b). On the other hand, fluoride (F−), calcium (Ca2+), and potassium (K+) concentrations in the Thar Desert are primarily geogenic in origin (Rafique et al. 2008). Both fluoride (F−)and nitrate (NO3−)contaminants disrupt the local ecosystem and pose risks to human health (Schullehner et al. 2018; Giddings et al. 2012).
While one of the major issues related with potable or drinking water is bacteriological parameters, chemical parameters are just as vital. Climate change is recognized as another driver for water pollution, exacerbating the degradation of water quality. The global phenomenon of water quality degradation, including recalcitrant chemical contamination, eutrophication, hazardous algal blooms, and microbial contamination, are likely exacerbated by climate change and extreme events resulting in negative trend in water quality (Barbieri et al. 2023). Despite the fact that approximately 71% of the Earth is covered in water, only 2.4% of the global water supply is distributed on land, and only a small portion of that can be utilized as freshwater (Ayoob and Gupta 2007; Ward et al. 2018).
Studies across diverse geographical regions highlight the prevalence and impacts of high NO3− and F− concentrations in groundwater. Elevated NO3− levels in arid regions of India and Pakistan have become a widespread concern (Ward et al. 2018). Similarly, F− contamination has been observed in various countries, including India, China, Mexico, and East African Rift Valley countries (Onipe et al. 2020; Rasool et al. 2018; Yadav et al. 2019). NO3− is widespread in the global drinking water supply. It has been identified as a probable carcinogen in humans by the International Agency for Research on Cancer (IARC) (Grosse et al. 2006). Excessive exposure to NO3− has been linked to an increased risk of colorectal cancer (CRC) (Schullehner et al. 2018). This is particularly concerning as South Asians have been experiencing a significant increase in CRC incidence (Giddings et al. 2012).
Fluoride occurs naturally in minerals and is highly reactive and corrosive (Ward et al. 2018). When F− rich minerals encounter water with high alkalinity, they release fluorides into groundwater through hydrolysis (Apambire et al. 1997; Buxton and Shernoff 1999; Ward et al. 2018). Fluoride contamination in groundwater is influenced by local and regional geological conditions (Ward et al. 2018). Areas of the Thar Desert in Sindh province are among those in Pakistan which are experiencing endemic fluorosis on a regional scale (Demelash et al. 2019; Rafique et al. 2015). The Thar Desert (Tharparkar District) is located in the eastern part of Pakistan with a population of approximately 1.6 million individuals (Final results (Census-2017)). The communities of the desert rely on minimal agriculture and animal herding for sustenance. The region has sand dunes and vegetation typical of desert ecosystems (Khuhawar et al. 2019). The Thar Desert often faces droughts, with limited rainfall occurring mainly during the monsoon season from June to September (Stowers et al. 2020). The people living in the Thar Desert generally face poverty, limited education, and poor health conditions (Wang et al. 2022).
While F− is considered beneficial to human health if taken in limited quantities up to 0.5–1.0 mg/L, excessive F− exposure is known to cause dental and skeletal fluorosis, osteosclerosis, thyroid, and kidney problems if its concentration exceeds 1.5 mg/L in drinking water (Choubisa 2012; Srivastava and Flora 2020). Chronic intake of excessive F− leads to severe and permanent bone and joint deformations in skeletal fluorosis, for which early symptoms include sporadic pain and stiffness of joints, and finally the spine, major joints, muscles, and nervous systems are damaged (Bharati et al. 2017). Given the serious adverse health outcomes associated with excessive F− exposure, it is important to assess F− concentrations in the drinking water supply of Thar Desert communities. These findings underscore the urgent need for a comprehensive evaluation of groundwater quality and associated health risks in this region.
A comprehensive understanding of the implications of F− contamination requires a more holistic examination, one that considers the impact on human health and agricultural productivity, as well as possible remediation strategies. The novelty of our study lies in its integrative approach: it not only elucidates the severity of the F− contamination problem but also examines the health risks posed to local inhabitants, the implications on agricultural practices, and the potential strategies for fluoride removal from groundwater.
This study becomes an imperative addition to the current body of knowledge, presenting a broader perspective on F− contamination's effects in the Thar Desert region. Moreover, our research sets the groundwork for future studies to further explore sustainable water management strategies and optimize remediation techniques, vital for ensuring the region's inhabitants' safety and well-being. It is our hope that this study will also inform policymaking and stimulate multi-stakeholder engagement towards mitigating fluoride contamination in the Thar Desert and similar regions globally.
The aim of this study is to assess the groundwater quality of villages in the Thar Desert and determine if intervention is necessary. Major ion analysis will assess the sources of high concentrations of F− and NO3− ions (Guo 2021). The suitability of groundwater for drinking and irrigation purposes will be evaluated using the Water Quality Index (WQI), Sodium Absorption Ratio (SAR), Percent Sodium (%Na), Magnesium Hazard (MH), Kelly's ratio (KR), and Potential Salinity (PS) (Yu et al. 2019). Additionally, a health risk assessment will be conducted to estimate the potential health risks associated with fluoride and nitrate exposure using Hazard Quotients (HQ) (both oral and dermal), Total hazard index (THI), and Carcinogenic Risk (CR) (Goswami et al. 2020). The WQI provides a single value to represent water quality, making it easier to interpret and communicate the status of water for drinking and irrigation purposes. The health risk assessment helps us examine the potential risks faced by humans. These indices and assessments are critical in ensuring water quality standards, protecting public health, and maintaining sustainable agricultural practices.
Materials and methods
Study area
The desert of Thar forms the south-eastern part of Sindh province and consists of Tharparkar and nearly half of the Umarkot districts. The land area is spread over about 22,000 sq. km and has population more than one and half million. The socioeconomic condition of Thari people is poor, with meager levels of education, health, and other necessities of basic life. People of this area rely on the groundwater for drinking as well as livestock purposes and it is available at a depth of 6–90 m which is obtained through wells (Rafique et al. 2008, 2009). Cattle farming and agriculture based on rainfall are the only sources of livelihood in this area.
The study area has a tropical desert climate. The months of April, May, and June are the hottest ones during the day. The average minimum and maximum temperatures during this period remain between 24 and 48 °C, respectively, while December, January, and February are comparatively colder months with average minimum and maximum temperatures 9–28 °C, respectively. Rainfall varies from year to year with mean annual rainfall of 200–300 mm. Most of the rain falls in the monsoon months between June and September, whereas the winter rains are insignificant (Rafique et al. 2008).
Stable groundwater is lacking in the area; however, several brackish to saline open dug wells are present in low-lying inter-dune playa flats. The source of water is perched aquifers at the contact of subrecent deposits and overlying sand dunes. These aquifers are found throughout the Thar Desert. No natural and man-made drainage system exists in the area and whenever monsoon rains fall, the water is immediately absorbed by the sand or flows to the nearest topographic low where it eventually evaporates or infiltrates (Islam et al. 2018).
Hydrology
The water table ranges between 52 and 93 m depth from the surface. Water table varies between 0.61 and 7.62 m. There is no natural and man-made drainage system developed in the Thar area. When the monsoon rains fall, the water is immediately absorbed by the sand, or rarely during heavy downpours, flows very short distances down into the low-lying interdune areas where it is quickly absorbed. Practically, no river or streams exist in the Thar and the drainage is totally internal. Rainwater flows to the nearest topographic low, as sheet flow that eventually either evaporates or infiltrates.
Sample collection
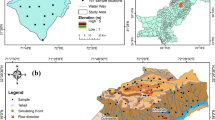
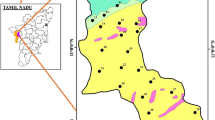
Extensive fieldwork was carried out for the collection of groundwater samples and geological and hydrogeological information in the study area (Fig. 1). A global positioning system (GPS), Garmin, Model eTrex Summit was used for location and elevation readings. This was supported by topographic sheets made available from the Survey of Pakistan.
Fifteen groundwater samples were randomly collected from the shallow- and deep-dug wells directly above the outlet after withdrawing the sampled water several times from the water table with a stainless-steel container tightened with a fiber rope. This was done to remove groundwater stored in the well itself and to obtain representative samples. In all cases, pre-washed, nitric acid (1%) rinsed 1.5-L polyethylene bottles were used (Shen et al. 2003; Kazi et al. 2018). At the sampling sites, these bottles were again rinsed with the groundwater to be sampled, filled, tightened with sealed caps, and immediately transported to the laboratory for analyses. Temperature, pH, conductivity (EC), total dissolved solids (TDS) and dissolved oxygen (DO) were measured at the sampling site.
Method
Standard methods were used for the determination of the chemical characteristics of the water samples. pH and EC of the groundwater samples were measured with a portable pH meter (Hanna pH Meter, Model: HI98107) and EC meter (Hanna DisT4 Conductivity Meter Model: HI98304), respectively. The TDS of the filtered samples was determined by the evaporation method. A HANNA model HI 9142 m was used for DO measurements in the field. Two mL of the ortho phosphoric acid was added to 30 mL of sample and the solution was kept in dark for 5–10 min, then 2–3 drops of starch indicator were added and titrated with sodium thiosulfate solution 0.1 N at the end point the blue color disappeared.
F− concentrations were measured electrochemically using an ion-selective electrode method using the Orion 5 Star. Standard F− solutions (1.0 and 10 mg/L) were prepared from commercially available stock solution (1000 mg/L). 25 mL of TISAB was added in 25 mL of 10 mg/L F−standard solution. The ion meter was calibrated by immersing both reference and F− electrodes into solution for a slope of 56 ± 2 mv. The composition of TISAB solution was 242 g of TRIS (hydroxymethy amino methane), 230 g of sodium tartrate (Na2C4H4O6. 2H2O), and 84 mL of concentrated hydrochloric acid per 500 mL of deionized water. Analysis was carried out in triplicate, and the results were found reproducible with ± 5% error. Determination of Cl−, NO3−, and SO42− were conducted by Dionex ICS-2500 ion chromatograph (Dionex Corporation, Sunnyvale, CA, USA) coupled with anion separation column, conductivity detector, and an autosampler. Prior to analysis of samples, the linear calibration curves of anions were plotted between known concentrations of standards and instrument response with correlation coefficient r > 0.995. The accuracy of calibration curves was evaluated by analyzing quality control standards. The percent standard deviation of measurements evaluated on duplicate samples was found to be better than ± 10%, while spike recoveries ranged between 90 and 104%. Concentration of HCO3− was analyzed by acid titration using bromocresol green as the indicator. Sodium and K+ were determined by flame photometry. Calcium and Mg2+ were measured by a titration method using a standardized solution of ethylenediaminetetraacetic acid (EDTA).
Results and discussion
Geological characteristics of wells
The description and location of each sampling location are shown in Table 1. All the wells we sampled are in the Thar Desert in the Sindh province of southern Pakistan. They service 15 villages, providing water to 1.6 million individuals, with density of 84 persons per square kilometer, making it the world’s most populated desert (Kumar et al. 2023). All the wells had clear water with a normal odor. Eleven of the wells had water with a brackish taste while only three (Kosbo, Sobhaharo, and Jhurmurio) had a sweet taste, and one (Roshan Ji Dhani) had a saline taste. Groundwater samples were collected for shallow wells from three villages (Mehran Vero, Gori, and Sajai).
Physico-chemical characteristics of groundwater
Table 2 provides a statistical overview of the physico-chemical parameters. The pH levels, which varied between 7.10 and 8.54, indicate the groundwater's slightly alkaline nature. The exceptionally high EC values, ranging from 402 to 18,640 µS/cm (mean = 6898 µS/cm), imply high ionic concentrations. The TDS were similarly high, ranging from 354 to 12,098 mg/L. The maximum EC and TDS measurements were found in Roshan ji Dhani.
The elevated TDS concentrations can be attributed to several factors, including high evaporation rates that concentrate soluble salts on the surface and the prolonged residence time of water in the aquifer, which allows for more extensive dissolution of soluble materials. Both EC and TDS in most samples exceed the WHO's acceptable limit of 400 µS/cm and 300 mg/L, respectively. The observed DO range (2.64–7.14 mg/L) fell short of the WHO recommended value of 4.0–6.0 mg/L.
Figure 2 displays the concentration of F− in groundwater samples from each village. The highest concentration of F− (29.9 mg/L) was detected in a well in Mehran Vero. F− plays a dual role, being vital for health in small amounts but harmful in excess. It is essential for dental health in concentrations of 0.7–1.0 mg/L, yet concentrations exceeding 1.5 mg/L can induce severe fluorosis.
Nearly 80% of rural villages throughout Asia and Africa are affected by skeletal fluorosis, impacting over 100 million people globally (Nishimura et al. 2013). The classic symptoms of skeletal fluorosis include stained teeth, bone diseases, stooped backs, crooked hands and legs, blindness, and other handicaps (B et al. 2021). Fluoride concentrations in groundwater can vary, with values normally ranging from 0.5 to 1.0 mg/L in natural water bodies (Schouteden et al. 2015). Studies conducted in different regions have reported varying F− concentrations in groundwater. For example, a study in India found F− concentrations ranging from 0 to 16 mg/L with a mean of 0.84 mg/L (Adkins and Brunst 2021). In Malaysia, the mean fluoride concentration in drinking water ranged from 0.71 ± 0.12 mg/L (Adimalla et al. 2019). In Myanmar, the mean fluoride concentration in groundwater was 1100 µg/L, with some wells exceeding safe levels (Grandjean 2019). In Thailand, F− levels in drinking water varied across regions, with concentrations ranging from 0.01 to 0.92 mg/L (Dec et al. 2020). Studies in Pakistan have also assessed F− levels in drinking water, with varying results. One study found that 84% of the samples contained less than 0.7 mg/L of F− (Luo et al. 2018). Another study reported that 16% of monitored water sources in Pakistan exceeded the WHO's permissible safe limit for F− (Zhao et al. 2022). Endemic fluorosis has been observed in the Thar Desert areas of Pakistan, with significantly higher urinary, plasma, and serum F− levels in affected individuals compared to controls (Dharmaratne 2015). The presence of high F− concentrations in groundwater can be attributed to factors such as regional and local geological settings, hydrological conditions, and weathering and leaching of rocks and minerals (Carwile et al. 2020; Podgorski et al. 2018; Xiuping et al. 2023). Fluoride exposure has been associated with various health effects, including neurotoxicity, skeletal fluorosis, dental fluorosis, and adverse birth outcomes (Ghosh and Mondal 2019; Putraphan et al. 2022; Riddell et al. 2021; Till et al. 2018). It is important to assess and monitor F− levels in drinking water to prevent health risks associated with excessive F− exposure.
Figure 3 demonstrates the Cl− concentration in the water from all 15 villages sampled, with concentrations ranging from 20.6 to 8310 mg/L. Most villages had Cl− concentrations surpassing the WHO's standard of 250 mg/L. High Cl− concentrations could be due to human activities, including irrigation or wastewater, or the weathering and leaching of sedimentary rocks.
Chloride is a common anion found in tap water, often combining with other elements to form salts (Sikdar and Kundu 2018). High Cl− concentrations in groundwater can be attributed to factors such as wastewater from human activities, irrigation, and weathering and leaching of sedimentary rocks (Sikdar and Kundu 2018). While there is limited research on the direct health effects of Cl− in drinking water, high levels can cause gastrointestinal problems, irritation, diarrhea, dehydration, and potentially impact the kidney and liver (Xie et al. 2014). Studies in different regions have reported varying Cl− concentrations in groundwater. For example, in Anekal Taluk, India, the mean Cl− concentration was 218.2 mg/L, with some samples exceeding WHO guidelines (Susaiappan et al. 2021). In the Peshawar valley of Pakistan, Cl− concentrations ranged from 0.00 to 26 mg/L, lower than the concentrations observed in the Thar Desert (Malla et al. 2015). Chloride levels higher than 250 mg/L can result in detectable differences in taste and odor (Lestari et al. 2023). It is important to monitor Cl− levels in drinking water to ensure compliance with health guidelines and prevent potential health risks associated with high concentrations (Houria et al. 2020; Kelly et al. 2018).
Figure 4 presents the variation of NO3− concentration (2.73–196 mg/L). Eleven of the villages’ drinking water supplies contained NO3− levels exceeding the WHO’s standard of 10 mg of NO3−–N/L. Nitrate originates from decaying plant or animal material, agricultural fertilizers, and domestic sewage. Overexposure can cause health problems such as methemoglobinemia, gastrointestinal cancers, and adverse birth outcomes, among others.
Concentration of NO3− found in our study was significantly higher compared to a monitoring study conducted in Anekal Taluk, Bangalore Urban district, India (range = 0.01–6.72 mg/L; mean = 2.00 mg/L) (Prakash and Somashekar 2006). NO3is commonly found in groundwater supplies, originating from decaying plant or animal material, agricultural fertilizers, and domestic sewage (Terblanche 1991). It is relatively non-toxic; however, excessive NO3− levels in drinking water can cause many health problems such as methemoglobinemia in infants, gastrointestinal cancers, Alzheimer’s disease, vascular dementia, multiple sclerosis, adverse birth outcomes including preterm birth, and intrauterine growth restriction (Brender et al. 2013; Shuval and Gruener 2013; Munoth et al. 2015; Yasin et al. 2015; Parvizishad et al. 2017; Qasemi et al. 2018; Adimalla 2019; Ahmed et al. 2019; Blaisdell et al. 2019; Coffman et al. 2021; Sherris et al. 2021). It is a colorless, odorless, and tasteless hence, individuals can be unknowingly exposed to extremely high levels of NO3−, unless they perform a chemical analysis. Due to prolonged exposure individuals may develop gastric problems due to the formations of nitrosamines. In our study, 11 of the village wells contained NO3− concentrations exceeding the WHO’s permissible limits. This is of particular concern to residents of Thar Desert. However, water treatments including distillation, reverse osmosis, ion exchange, and blending can effectively reduce drinking water NO3− concentrations.
Figure 5 illustrates the levels of SO42−, which ranged from 7.74 to 1158 mg/L. The village of Roshan ji Dhani had the highest concentration of SO42− (1158 mg/L), followed by Morasio (956 mg/L), Gogasar (815 mg/L), Gungio (734 mg/L), and Kharyoun (650 mg/L). Sulfate concentrations in 67% (10 out of 15) of groundwater samples collected from Thar Desert area were above the WHO permissible limit (250 mg/L).
SO42− is commonly found in water bodies and originates from various sources such as the oxidation of sulfite ores, the presence of shales, and the dissolution of gypsum and anhydrite (Chowdhury et al. 2016). The high concentrations of SO42− in groundwater observed in our study may be attributed to factors such as the discharge of untreated sewage and industrial effluents (Butu et al. 2022).
Sulfur deficiencies are rare, but excessive exposure from drinking water can lead to health problems like diarrhea and dehydration in humans, as well as harm to animals, including potential fatality (Mohsin et al. 2013). Sulfur concentrations above 250 mg/L can also affect water taste. High sulfide levels are associated with gastrointestinal issues, making drinking water sulfide concentrations a public health concern (Heizer et al. 1997). Research indicates that ion exchange and nanofiltration can effectively reduce sulfate in drinking water (Darbi et al. 2003). A study in Thailand showed SO42− concentrations in drinking water to be within the WHO standards, whereas evaluations in Pakistan found concentrations exceeding WHO limits, implying potential health risks for local residents (Thangiah 2019; Mohsin et al. 2013). Similarly, a study in Anekal Taluk identified sulfur levels in some samples above permissible standards (Prakash and Somashekar 2006).
Concentrations of HCO3− in our study ranged from 155 to 913 mg/L with the mean value of 459 mg/L. Though the concentrations of PO42− are low, they were detected in all the collected groundwater samples (except Sobharo and Bhojrajio) in the range of < DL–0.44 mg/L (Table 2). Anions dominance followed the order Cl− > SO42− > HCO3− > NO3− > F− and their contribution ratios were 73.5%, 12.5%, 12.3%, 1.5%, and 0.14%, respectively. Hardness is caused largely by compounds of Ca2+ and Mg2+ ranging from 4.0 to 377 mg/L and 5.0 to 452 mg/L, respectively. The cation abundance measured in this study followed the order Na+ > Ca2+ > Mg2+ > K+ with contribution ratios of 81.8%, 8.2%, 7.8%, and 2.2%, respectively.
Sources of ions in groundwater
According to the Pearson Correlation matrix (Table 3), an inverse relation was found between pH and EC, TDS, Cl−, SO42−, Na+, Ca2+, and Mg2+. This inverse correlation suggests that as the pH decreases, becoming more acidic, the concentration of these parameters tends to increase, likely due to the enhanced solubility of minerals at lower pH values. On the other hand, a strong positive correlation exists between pH and F− at a significance level of p < 0.05 (Khatri and Tyagi 2015). This indicates that as the pH of the water becomes more alkaline, the leaching of fluoride ions is facilitated, subsequently impacting the groundwater fluoride concentration (Khatri and Tyagi 2015). The alkaline nature of the groundwater in the study area, with pH values ranging from 7.10 to 8.54, hence favors the dissolution of fluoride ions (Khatri and Tyagi 2015).
Fluoride ions are known to be associated with certain types of rocks, particularly granitic rocks and their alteration products (Lachassagne et al. 2021). The study area's geology is characterized by the presence of a variety of these rocks, which may be contributing to the high fluoride concentrations observed in the groundwater (Lachassagne et al. 2021). These concentrations range up to 29.9 mg/L with a mean value of 5.35 mg/L, significantly exceeding the World Health Organization's permissible limit for F− in drinking water (1.5 mg/L) (Lachassagne et al. 2021).
This observation, coupled with the strong positive correlation between pH and F−, underlines the geochemical influence of the region's alkaline groundwater conditions and granitic geology on the high F− contamination levels (Cherukumilli et al. 2017). Future studies should explore these relationships further to improve our understanding of the geochemical processes that govern F− contamination in the region's groundwater, thereby aiding in the development of effective remediation strategies (Cherukumilli et al. 2017).
EC and TDS demonstrated a significant positive correlation with several ions (Cl−, SO42−, Na+, Ca2+, and Mg2+), suggesting anthropogenic sources for these ions in groundwater. In contrast, F− showed an inverse relationship with most major ions, but a positive correlation with HCO3−, typical in shallow groundwater (Robertson et al. 2016). No significant correlation was observed between NO3− and other ions, suggesting different sources of NO3− in the groundwater. A significant positive correlation was noted between SO42− and Ca2+, indicative of gypsum dissolution.
Cations (Ca2+, Mg2+, and Na+) were positively correlated with major anions (Cl− and SO42−) at a significance level of p < 0.01, suggesting their derivation from silicate weathering and ion-exchange processes in groundwater. Notably, K+ showed no association with major ions.
Water quality assessment of drinking purposes
Calculation of WQI by \({\text{WQI}} = \sum_{t = 1}^n {{\text{SI}}_t }\) (Table 4) was done to assess the suitability of groundwater for drinking purposes. We assigned weights (wi) to each of the parameters (e.g., pH, TDS, Na+, Mg2+, Ca2+, Cl−, SO42−, HCO3−, and K+) based on their apparent health impact (Supplemental Table 1) (Akter et al. 2016). TDS, SO42− NO3−, and F− were given a maximum weight of 5 due to their significant role in assessment, while SO42− was given 4, pH and Cl− is given 3, Th, Ca2+, Mg2+, Na+, K+, and HCO3− is given a minimum weight of 2 because of its insignificant importance (Dahariya et al. 2016; Ahamad et al. 2018). According to our WQI values, only Jhurmurio had excellent drinking water quality (WQI = 45). Kasbo and Sobharo were rated as good (WQI = 65 and 81, respectively). However, 87% of samples were deemed unsuitable for drinking, with Roshan ji Dhani presenting the poorest quality (WQI = 1145) and hence, harmful for consumption (Akter et al. 2016).
Water quality assessment of irrigation purposes
Table 5 shows the water quality of each of the villages. Sodium absorption ratio (SAR) is a measure of the amount of sodium (Na+) relative to calcium (Ca2+) and magnesium (Mg2+) in the water extract from saturated soil paste. It is expressed as Na+/√ (Ca2+ + Mg2+) /2 (Sposito and Mattigod 1977). SAR values in our study ranging from 0 to 10 meq/L were classified as excellent, 10–18 meq/L as good, 18–26 meq/L as fair, and > 26 was identified as poor quality for irrigation purposes. As shown in Table 5, all the villages had poor water quality for the purposes of agricultural irrigation with Roshan ji Dhani (484 meq/L), Mithi City (405 meq/L), and Janjihar (388 meq/L) having the highest SARs.
Another criterion for the quality of irrigation water is percent sodium (%Na = [(Na+ + K+)/(Na+ + K+ + Ca2+ + Mg2+)] × 100) because of its reaction with the soil, which consequently reduces the permeability. Sodium has historically been a major problem in dug wells and shallow pumps in the Thar desert region (Memon et al. 2011). Water containing 0–20% Na is excellent for irrigation, 20–40% is good, 40–60% is permissible, 60–80% is of doubtful quality, and > 80% is unsuitable for irrigation. In the well we sampled, % Na ranged from 38.2 to 95.4%. Only Jhurmurio, Kasbo, and Sobharo had good and permissible levels of Na in their wells. Eighty percent of our samples had Na levels that were either suspect or unsuitable for consumption as they exhibited higher concentrations higher than the MDL of 200 mg/L Na in drinking water (Kumar and Puri 2012).
Magnesium hazard (MH = [(Mg2+)/(Ca2+ + Mg2+)] × 100) is utilized to check the fitness of water for irrigation. Magnesium and calcium bicarbonates make water hard, hence making water unsuitable for drinking and potentially causing gastric diseases. While there is no specified limit for HCO3− s set by the WHO, it is considered that it should not exceed 50 meq/L (Mohsin et al. 2013). The value of MH ranges between 8.14 and 89.6 meq/L (Table 5). 20% of the wells had a suitable level of MH while 80% had harmful levels of MH. Sajai had the highest MH level at 89.6 meq/L, and Kasbo had the lowest value of 8.14 meq/L. These concentrations are several folds higher than Mg2+ levels measured in Iran’s Rayen groundwater basin (Rezaei et al. 2020).
Kelly’s ratio (KR = [(Na+)/(Ca2+ + Mg2+)]) evaluates suitability of water for irrigation purposes. It should not exceed unity for irrigation water. Water samples with KR values < 1 are suitable for irrigation uses (Kadri et al. 2022). The KR levels measured in this study ranged from 0.56 to 20.3 meq/L (Table 5). Among the wells sampled, 80% of the samples had KR values > 1, making them unsuitable for agricultural irrigation purposes.
As low solubility salts precipitate and accumulate in the soil, it is important to measure the potential salinity of drinking water supplies. We calculated potential salinity (PS) using the following formula: Cl− + √SO42− (Xu et al. 2021). PS values < 3.0 meq/L are considered to represent excellent to good quality water, 3.0–5.0 meq/L ranges from good to injurious, and > 5.0 meq/L indicates injurious to unsatisfactory water quality. 80% of samples (Table 5) were > 5.0 of PS, making them unsatisfactory for irrigation. Roshan ji Dhani had the highest PS value of 234.5 meq/L and Jhurmurio had the lowest PS value of 0.56 meq/L.
Rawat et al. (2018) have given the permeability index (PI) (PI = [(Na+ + √HCO3−)/(Ca2+ + Mg2+ + Na+)] × 100) for examining the fitness of groundwater for agricultural uses. PI is categorized into three classes, class I (> 75%, suitable), class II (25–75%, good), and class III (< 25%, unsuitable) (Doneen 1964). In the present study, PI ranged from 36.9 to 95.6 (Table 5). 47% of the wells were classified as class I and 53% were class II, signifying that water is suitable for irrigation.
In summary, SAR, %Na, MH, KR, and PS values indicated that most well water sampled in the Thar Desert was unsuitable for irrigation. This poses significant concern for residents and suggests probable over-fertilization pollution, exacerbated by the desert's dry environment and associated challenges to achieving high crop yield (Rawat et al. 2018).
Health risk assessment of F− and NO3 −
For non-cancer health risks of groundwater, hazard quotients (HQ) for F− (HQ-F−) and NO3− (HQ-NO3−) were assessed for two target human populations (adults and infants). Ingestion is a major pathway of contaminants’ exposure in water and the dermal exposure to chemicals is generally considered to be low. HQ through oral intake (HQ oral) and dermal contact (HQ dermal) were estimated for both F− and NO3− according to the United States Environmental Protection Agency (USEPA) standards (USEPA 2004, 2005; Wu et al. 2019):
where C refers to F− and NO3− concentrations in water (mg/L); OI stands for the ingestion rate (L/day); EF0 is the exposure frequency (days/year); ED0 is defined as the exposure duration (years); BW represents the body weight of studied age group (kg); AT means the average lifespan (days); RfD stands for the reference dosage of F− and NO3− (mg/kg/day); Kp refers to the dermal permeability coefficient (cm/hour); CF is the unit conversion factor (L/cm3); tevent represents the event duration (hour/event); EV stands for the event frequency (event/day); EDd means the dermal contact exposure duration (years); EFd is the dermal contact exposure frequency (days/year); SA refers to human skin surface area (cm2); and GIABS is the fraction of F− and NO3− absorbed in gastrointestinal tract (unitless). Values and units of these parameters for adults and infants are summarized in Supplemental Table 2.
The total hazard index (THI) of non-carcinogenic F− and NO3− was computed for each age category according to the following equation:
Non-carcinogenic risk is considered as unacceptable when HQ or THI ≥ 1, but if the HQ or THI < 1, then it is regarded as within the permissible limit (USEPA 2014).
The HQ-F− for adults ranged from 0.13 to 15.8 (mean = 2.83) and from 0.67 to 83.3 (mean = 14.9) for infants (Table 6). HQ-F− for infants were 5.3 times higher than those for adults. Oral intake was found to be the main pathway for F− exposure for both adults and infants. We found that only three villages (Roshan ji Dhani, Jhurmurio, and Morasio) had drinking water acceptable (HQ > 1) for infants. The drinking water in the villages of Mehran Vero and Sajai were the most harmful for both adult and infant consumption. For NO3−, HQ values ranged 0.05–3.89 (mean = 1.14) for adults and 0.29–20.5 (mean = 6.00) for infants (Table 7). Given the heightened risk for adults, only four wells we sampled (Kasbo, Sobharo, Jhurmurio, and Gungio) had acceptable drinking water quality (HQ < 1) for infants. The water sampled in Morasio village was the most toxic for infant consumption with a HQ − NO3−of 20.5.
THI (HQ − F− + HQ − NO3−) ranged from 0.29 to 16.8 (mean = 4.0) for adults and from 1.53 to 88.4 (mean = 20.9) for infants. Except Sobharo (0.40), Jhurmurio (0.29), Gogasar (0.91), Gungio (0.51), and Janjihar (0.80), THI values for adults exceeded the acceptable limit of 1. The non-carcinogenic risks (THI) to infants were found to be very high in all the villages (range = 1.53–88.4). Furthermore, THI levels in infants were 5.26 times higher than those of adults in the study area. Results of HQ and THI demonstrated that infants are more susceptible to non-cancer risk related to F− and NO3− toxicity in the Thar Desert region. Overall, F− seems to contribute more to the non-cancer risk than NO3− in the study area.
Numerous studies have found heightened health risks, particularly fluorosis, in various global regions due to F− and NO3− in drinking water. For example, Brahman et al. (2014) revealed more severe risks in younger age groups in Pakistan, similar to Wongsanit et al. (2015) in Thailand and Martinez-Acuna et al. (2016) in Mexico. These findings are echoed in studies across China, Tunisia, Iran, and India (Wu and Sun 2016; Guissouma et al. 2017; Chen et al. 2017; Yousefi et al. 2018; Adimalla et al. 2018, 2021; Ahada and Suthar 2018; Rezaei et al. 2019a, b; Qasemi et al. 2019; Wu et al. 2019; Mukherjee et al. 2020; Adimalla and Qian 2020; Hu et al. 2021; Duvva et al. 2022). These investigations collectively demonstrate a pattern of higher health risks in infants and children compared to adults.
Our study in the Thar Desert region found notably higher levels of F− and NO3− compared to other regions, with infants exhibiting more significant Hazard Quotient values for F− (HQ-F−) and NO3− (HQ-NO3−) than adults. Drinking water was identified as the primary exposure pathway. High concentrations of these elements in local groundwater could pose severe non-cancer health risks to Thar Desert residents. Thus, immediate action to improve water quality is crucial for this population's health. Management strategies that are tailored to the geological and hydrogeological characteristics of the Thar Desert are required. This includes implementing effective remediation techniques and sustainable water management practices. Considering the geogenic nature of F− contamination, strategies should involve localized treatment methods or aquifer management approaches (Apambire et al. 1997; Ward et al. 2018). Similarly, to address the NO3− pollution, it is important that we consider targeted and multi-level measures such as improved agricultural practices, land-use regulations, and groundwater recharge initiatives (Ward et al. 2018).
The carcinogenic risk assessment (CR) for NO3− through oral intake was calculated for adults and infants in our study by the following equation:
where CSF refers to carcinogenic slope factor. The CSF value for NO3− is 10–5 mg/kg/day (Darvishmotevalli et al. 2019). Cancerous risk is regarded as acceptable when CR ≤ 1 × 10–6 (Toolabi et al. 2021). NO3− carcinogenic risk estimates are presented in Table 7. The CR values for adults and infants ranged from 5.41 × 10–7 to 3.89 × 10–5 and from 2.85 × 10–6 to 2.05 × 10–4, respectively. Maximum CR was identified at Morasio village (3.89 × 10–5 for adults and 2.05 × 10–4 for infants). As evident from Table 7, the CR posed by NO3− exceeded the safety limit in most of the villages, highlighting a greater risk of developing a carcinogenic disease among residents of the Thar desert region. Groundwater sampling artesian wells in agricultural areas in the Philippines and Thailand showed evidence of pollution with nitrates (Tirado 2007). Thirty percent of the wells had concentrations exceeding WHO’s safety limit of 50 mg /L of NO3. The study suggests that large quantities of nitrogen fertilizers were responsible for this contamination, posing a serious health concern for the babies and infants living around agricultural areas and who drink water from wells.
Our study stands apart from previous research in several significant ways. Firstly, while most existing studies focus primarily on identifying the extent of F− contamination, we delve deeper into understanding the source of F− ions, attributing them to natural mineral weathering and anthropogenic activities. Secondly, we carry out a comprehensive health risk assessment considering both adults and infants, which is often overlooked in existing literature. Lastly, our research explores the socio-economic implications of F− contamination on local communities, underlining the impact on agricultural practices and livelihoods, which is a largely under-explored area in existing studies. Thus, our research not only offers a more holistic understanding of the F− contamination issue but also paves the way for future work aimed at devising sustainable water management strategies.
Conclusions
Our study, examining F− contamination within the groundwater of the Thar Desert, has revealed alarming levels exceeding the WHO’s permissible limit for safe consumption. Concentrations of F− ranged from 0.24 to 29.9 mg/L, significantly surpassing the WHO threshold of 1.5 mg/L. The prevalent source of high F− levels appears to stem from natural weathering processes of F− -bearing minerals in the earth's crust, exacerbated by potential anthropogenic sources such as improper disposal of agricultural waste. This contamination poses a severe threat to public health, particularly impacting the local population reliant on groundwater as their primary drinking water source. This vulnerability is heightened among infants and children who are significantly susceptible to dental and skeletal fluorosis due to continual ingestion of F− -contaminated water. Furthermore, this contamination adversely affects the region's agricultural sector, impeding productivity and quality.
Addressing this extensive issue requires a multi-faceted approach. Traditional water treatment methods have proven inadequate in mitigating high F− concentrations. We suggest alternative methods such as activated alumina defluoridation, bone char defluoridation, and reverse osmosis ad potential solutions. These suggestions are not without constraints such as cost, resource availability, and technological complexity. Ongoing research efforts, continue to explore innovative approaches to combat F− contamination and enhanced water treatment methodologies, more suitable to rural environments lacking resources. To address this complex matter effectively, it is vital to consider region-specific constraints and needs when implementing remediation strategies.
To conclude, the problem of F− contamination in the groundwater of the Thar desert region presents a significant public health challenge requiring urgent attention. Our study emphasizes the need for a comprehensive, multi-pronged approach that includes the implementation of effective remediation techniques, continuous monitoring of water quality, and extensive public education campaigns to raise awareness about the dangers of fluoride contamination. Future research and efforts should also aim at creating sustainable water management strategies to ensure the safety and well-being of the region's inhabitants.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Adimalla N, Qian H (2020) Spatial distribution and health risk assessment of fluoride contamination in groundwater of Telangana: a state-of-the-art. Geochemistry 80:125548. https://doi.org/10.1016/j.chemer.2019.125548
Adimalla N, Li P, Venkatayogi S (2018) Hydrogeochemical evaluation of groundwater quality for drinking and irrigation purposes and integrated interpretation with water quality index studies. Environ Process 5:363–383. https://doi.org/10.1007/S40710-018-0297-4/FIGURES/7
Adimalla N, Qian H, Tiwari DM (2021) Groundwater chemistry, distribution and potential health risk appraisal of nitrate enriched groundwater: a case study from the semi-urban region of South India. Ecotoxicol Environ Saf 207:111277. https://doi.org/10.1016/J.ECOENV.2020.111277
Adimalla N, Venkatayogi S, Das SVG (2019) Assessment of fluoride contamination and distribution: a case study from a rural part of Andhra Pradesh, India. Appl Water Sci. https://doi.org/10.1007/S13201-019-0968-Y
Adkins EA, Brunst KJ (2021) Impacts of fluoride neurotoxicity and mitochondrial dysfunction on cognition and mental health: a literature review. Int J Environ Res Public Health. https://doi.org/10.3390/IJERPH182412884
Ahada CPS, Suthar S (2018) Groundwater nitrate contamination and associated human health risk assessment in southern districts of Punjab, India. Environ Sci Pollut Res 25:25336–25347. https://doi.org/10.1007/S11356-018-2581-2
Ahamad A, Madhav S, Singh P et al (2018) Assessment of groundwater quality with special emphasis on nitrate contamination in parts of Varanasi City, Uttar Pradesh, India. Appl Water Sci 8:1–13. https://doi.org/10.1007/S13201-018-0759-X
Ahmed N, Bodrud-Doza M, Towfiqul Islam ARM et al (2019) Appraising spatial variations of As, Fe, Mn and NO3 contaminations associated health risks of drinking water from Surma basin, Bangladesh. Chemosphere 218:726–740. https://doi.org/10.1016/J.CHEMOSPHERE.2018.11.104
Akter T, Jhohura FT, Akter F et al (2016) Water quality index for measuring drinking water quality in rural Bangladesh: a crosssectional study. J Health Popul Nutr 35:1–12. https://doi.org/10.1186/S41043-016-0041-5/TABLES/5
Apambire WB, Boyle DR, Michel FA (1997) Geochemistry, genesis, and health implications of fluoriferous groundwaters in the upper regions of Ghana. Environ Geol 33:13–24. https://doi.org/10.1007/S002540050221/METRICS
Ayoob S, Gupta AK (2007) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36:433–487. https://doi.org/10.1080/10643380600678112
Bain RES, Gundry SW, Wright JA et al (2012) Accounting for water quality in monitoring access to safe drinking-water as part of the millennium development goals: lessons from five countries. Bull World Health Organ 90:228–235. https://doi.org/10.2471/BLT.11.094284
Barbieri M, Barberio MD, Banzato F et al (2023) Climate change and its effect on groundwater quality. Environ Geochem Health 45:1133–1144. https://doi.org/10.1007/S10653-021-01140-5
Bharati P, Kubakaddi A, Rao M, Naik RK (2017) Clinical symptoms of dental and skeletal fluorosis in Gadag and Bagalkot districts of Karnataka. Kamla Raj Enterp 18:105–107. https://doi.org/10.1080/09709274.2005.11905816
Blaisdell J, Turyk ME, Almberg KS, Jones RM, Stayner LT (2019) Prenatal exposure to nitrate in drinking water and the risk of congenital anomalies. Environ Res 176:108553. https://doi.org/10.1016/j.envres.2019.108553
Brahman KD, Kazi TG, Baig JA et al (2014) Fluoride and arsenic exposure through water and grain crops in Nagarparkar, Pakistan. Chemosphere 100:182–189. https://doi.org/10.1016/J.CHEMOSPHERE.2013.11.035
Brender JD, Weyer PJ, Romitti PA, Mohanty BP, Shinde MU, Vuong AM, Sharkey JR, Dwivedi D, Horel SA, Kantamneni J, Huber JC, Zheng Q, Werler MM, Kelley KE, Griesenbeck JS, Zhan FB, Langlois PH, Suarez L, Canfield MA (2013) Prenatal nitrate intake from drinking water and selected birth defects in offspring of participants in the national birth defects prevention study. Environ Health Perspect 12:1083–1089. https://doi.org/10.1289/ehp.1206249
Butu AW, Emeribe CN, Muoka IO et al (2022) Downstream effects of industrial effluents discharge on some physicochemical parameters and water quality index of river Rido, Kaduna State, Nigeria. Tropical Aquatic Soil Pollut 2:90–108. https://doi.org/10.53623/TASP.V2I2.100
Buxton HT, Shernoff PK (1999) Ground-water resources of Kings and Queens counties, Long Island, New York. Water Supply Paper. https://doi.org/10.3133/WSP2498
Carwile JL, Ahrens KA, Seshasayee SM et al (2020) Predictors of plasma fluoride concentrations in children and adolescents. Int J Environ Res Public Health 17:1–16. https://doi.org/10.3390/IJERPH17249205
Chen J, Wu H, Qian H, Gao Y (2017) Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a Semiarid region of Northwest China. Expo Health 9:183–195. https://doi.org/10.1007/S12403-016-0231-9/FIGURES/5
Cherukumilli K, Delaire C, Amrose S, Gadgil AJ (2017) Factors governing the performance of bauxite for fluoride remediation of groundwater. Environ Sci Technol 51:2321–2328. https://doi.org/10.1021/ACS.EST.6B04601
Choubisa SL (2012) Fluoride in drinking water and its toxicosis in tribals of Rajasthan, India. Proc Natl Acad Sci India Sect B Biol Sci 82:325–330. https://doi.org/10.1007/s40011-012-0047-8
Chowdhury S, Mazumder MAJ, Al-Attas O, Husain T (2016) Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Total Environ 569–570:476–488. https://doi.org/10.1016/J.SCITOTENV.2016.06.166
Coffman VR, Jensen AS, Trabjerg BB, Pedersen CB, Hansen B, Sigsgaard T, Olsen J, Schaumburg I, Schullehner J, Pedersen M, Stayner LT (2021) Prenatal nitrate exposure to nitrate from drinking water and markers of fetal growth restriction: a population-based study of nearly one million Danish-born children. Environ Health Perspect 129:27002. https://doi.org/10.1289/EHP7331
Dahariya NS, Ramteke S, Sahu BL et al (2016) Urban groundwater quality in India. J Environ Prot (irvine, Calif) 7:961–971. https://doi.org/10.4236/JEP.2016.76085
Darbi A, Viraraghavan T, Jin YC et al (2003) Sulfate removal from water. Water Qual Res J 38:169–182. https://doi.org/10.2166/WQRJ.2003.011
Darvishmotevalli M, Moradnia M, Noorisepehr M et al (2019) Evaluation of carcinogenic risks related to nitrate exposure in drinking water in Iran. MethodsX 6:1716–1727. https://doi.org/10.1016/J.MEX.2019.07.008
Dec K, Łukomska A, Skonieczna-żydecka K et al (2020) Chronic exposure to fluoride affects GSH level and NOX4 expression in rat model of this element of neurotoxicity. Biomolecules. https://doi.org/10.3390/BIOM10030422
Demelash H, Beyene A, Abebe Z, Melese A (2019) Fluoride concentration in ground water and prevalence of dental fluorosis in Ethiopian Rift Valley: systematic review and meta-analysis. BMC Public Health 19:1–9. https://doi.org/10.1186/S12889-019-7646-8/FIGURES/3
Dharmaratne RW (2015) Fluoride in drinking water and diet: the causative factor of chronic kidney diseases in the North Central Province of Sri Lanka. Environ Health Prev Med 20:237–242. https://doi.org/10.1007/S12199-015-0464-4
Doneen LD (1964) Notes on water quality in agriculture published as a Water Science and Engineering Paper 4001, Department of water Science and Engineering, University of California
Duvva LK, Panga KK, Dhakate R, Himabindu V (2022) Health risk assessment of nitrate and fluoride toxicity in groundwater contamination in the semi-arid area of Medchal, South India. Appl Water Sci 12:1–21. https://doi.org/10.1007/S13201-021-01557-4/FIGURES/15
Final Results (Census-2017) Pakistan Bureau of Statistics. (n.d.). https://www.pbs.gov.pk/content/final-results-census-2017
Ghosh A, Mondal S (2019) Application of multivariate statistics towards the geochemical evaluation of fluoride enrichment in groundwater at Shilabati river bank, West Bengal, India. Environ Eng Res 24:279–288. https://doi.org/10.4491/EER.2018.178
Giammarino M, Quatto P (2015) Nitrates in drinking water: relation with intensive livestock production. J Prev Med Hyg 56:E187. https://doi.org/10.15167/2421-4248/jpmh2015.56.4.457
Giddings BH, Kwong SL, Parikh-Patel A et al (2012) Going against the tide: Increasing incidence of colorectal cancer among Koreans, Filipinos, and South Asians in California, 1988–2007. Cancer Causes Control 23:691–702. https://doi.org/10.1007/S10552-012-9937-6/TABLES/7
Goswami B, Rankawat R, Gadi BR (2020) Physiological and antioxidative responses associated with drought tolerance of Lasiurus sindicus Henr. endemic to Thar desert, India. Brazil J Botany 43:761–773. https://doi.org/10.1007/S40415-020-00666-9
Grandjean P (2019) Developmental fluoride neurotoxicity: an updated review. Environ Health. https://doi.org/10.1186/S12940-019-0551-X
Grosse Y, Baan R, Straif K et al (2006) Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol 7:628–629. https://doi.org/10.1016/S1470-2045(06)70789-6
Guissouma W, Hakami O, Al-Rajab AJ, Tarhouni J (2017) Risk assessment of fluoride exposure in drinking water of Tunisia. Chemosphere 177:102–108. https://doi.org/10.1016/J.CHEMOSPHERE.2017.03.011
Guo Z (2021) Soil hydrology process and rational use of soil water in Desert regions. Water (basel). https://doi.org/10.3390/W13172377
Heizer WD, Sandler RS, Seal E et al (1997) Intestinal effects of sulfate in drinking water on normal human subjects. Digest Dis Sci 42:1055–1061. https://doi.org/10.1023/A:1018801522760
Houria B, Mahdi K, Zohra TF (2020) Hydrochemical characterisation of groundwater quality: Merdja Plain (Tebessa Town, Algeria). Civil Eng J 6:318–325
Hu Y, You M, Liu G, Dong Z (2021) Spatial distribution and potential health risk of fluoride in drinking groundwater sources of Huaibei, Anhui Province. Sci Rep 11:8371. https://doi.org/10.1038/S41598-021-87699-6
Islam MA, Rahman MM, Bodrud-Doza M et al (2018) A study of groundwater irrigation water quality in south-central Bangladesh: a geo-statistical model approach using GIS and multivariate statistics. Acta Geochimica 37:193–214. https://doi.org/10.1007/S11631-017-0201-3
Kadri A, Baouia, K, Kateb S, Al-Ansari N, Kouadri S, Najm HM, Mashaan NS, Eldirderi MMA, Khedher KM (2012) Assessment of groundwater suitability for agricultural purposes: a case study of South Oued Righ region, Algeria. Sustainability 14(14):8858. https://doi.org/10.3390/su14148858
Kazi TG, Brahman KD, Afridi HI et al (2018) Effects of high fluoride content in livestock drinking water on milk samples of different cattle in endemic area of Pakistan: risk assessment for children. Environ Sci Pollut Res 25:12909–12914. https://doi.org/10.1007/S11356-018-1563-8/TABLES/2
Kelly VR, Cunningham MA, Curri N et al (2018) The distribution of road salt in private drinking water wells in a Southeastern New York Suburban Township. J Environ Qual 47:445–451. https://doi.org/10.2134/JEQ2017.03.0124
Khatri N, Tyagi S (2015) Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front Life Sci 8:23–39. https://doi.org/10.1080/21553769.2014.933716
Khuhawar MY, Ursani H, Khuahwar TMJ, Lanjwani MF, Mahaser AA, Tunio IA, Soomro AG, Rind IK, Brohi R, Khuhawar AH, Solangi SH, Soomro R, Kandhro AJ, Pathan AS (2019) Assessment of water quality of groundwater of Thar desert. Pak J Hydrogeol Hydrol Eng 7:2. https://doi.org/10.4172/2325-9647.1000171
Kumar S, Singh JP, Machiwal D et al (2023) Traditional in-situ rainwater conservation method for sustaining threatened plant species: a case study from the Indian Thar Desert. https://doi.org/10.21203/RS.3.RS-2473437/V1
Kumar M, Puri A (2012) A review of permissible limits of drinking water. Indian J Occup Environ Med 16:40. https://doi.org/10.4103/0019-5278.99696
Lachassagne P, Dewandel B, Wyns R (2021) Review: hydrogeology of weathered crystalline/hard-rock aquifers—guidelines for the operational survey and management of their groundwater resources. Hydrogeol J 29:2561–2594. https://doi.org/10.1007/S10040-021-02339-7
Lestari MF, Al’Wahid MI, Fuady MIN et al (2023) Analysis of mineral water quality based on SNI 3553:2015 and its consequences from legal perspectives. IOP Conf Ser Earth Environ Sci 1190:012041. https://doi.org/10.1088/1755-1315/1190/1/012041
Luo W, Gao X, Zhang X (2018) Geochemical processes controlling the groundwater chemistry and fluoride contamination in the Yuncheng Basin, China—an area with complex hydrogeochemical conditions. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0199082
Malla R, Shrestha S, Chapagain SK et al (2015) Physico-chemical and oxygen-hydrogen isotopic assessment of Bagmati and Bishnumati rivers and the shallow groundwater along the river corridors in Kathmandu Valley, Nepal. J Water Resour Prot 07:1435–1448. https://doi.org/10.4236/JWARP.2015.717117
Martínez-Acuña MI, Mercado-Reyes M, Alegría-Torres JA, Mejía-Saavedra JJ (2016) Preliminary human health risk assessment of arsenic and fluoride in tap water from Zacatecas, México. Environ Monit Assess 188:1–13. https://doi.org/10.1007/S10661-016-5453-6/TABLES/9
Memon M, Soomro MS, Akhtar MS, Memon KS (2011) Drinking water quality assessment in Southern Sindh (Pakistan). Environ Monit Assess 177:39–50. https://doi.org/10.1007/s10661-010-1616-z
Mohsin M, Jamal F, Safdar S, Faryal Asghar P (2013) Assessment of drinking water quality and its impact on residents health in Bahawalpur City. Int J Hum Social Sci 3:114
Mukherjee I, Singh UK, Singh RP, Anshumali KD, Jha PK, Mehta P (2020) Characterization of heavy metal pollution in an anthropogenically and geologically influenced semi-arid region of east India and assessment of ecological and human health risks. Sci Total Environ 705:135801. https://doi.org/10.1016/j.scitotenv.2019.135801
Munoth P, Tiwari K, Goyal R (2015) Fluoride and nitrate ground water contamination in Rajasthan, India. In: Hydro 2015 International 20th International Conference on Hydraulics, Water Resources and River Engineering, IIT Roorkee, India
Nishimura T, Xu SY, Jiang YB et al (2013) A simple visual sensor with the potential for determining the concentration of fluoride in water at environmentally significant levels. Chem Commun 49:478–480. https://doi.org/10.1039/C2CC36107H
Onipe T, Edokpayi JN, Odiyo JO (2020) A review on the potential sources and health implications of fluoride in groundwater of Sub-Saharan Africa. J Environ Sci Health 55:1078–1093. https://doi.org/10.1080/10934529.2020.1770516
Parvizishad M, Dalvand A, Mahvi AH, Goodarzi F (2017) A review of adverse effects and benefits of nitrate and nitrite in drinking water and food on human health. Health Scope 6:14164. https://doi.org/10.5812/JHEALTHSCOPE.14164
Podgorski JE, Labhasetwar P, Saha D, Berg M (2018) Prediction modeling and mapping of groundwater fluoride contamination throughout India. Environ Sci Technol 52:9889–9898. https://doi.org/10.1021/ACS.EST.8B01679
Prakash KL, Somashekar RK (2006) Groundwater quality-assessment on Anekal Taluk, Bangalore Urban district, India. J Environ Biol 27:633–637
Putraphan B, Nantanapiboon D, Osathanon T (2022) Fluoride concentration in tap water from different regions in Thailand. Dentika Dental J 25:9–14
Qasemi M, Afsharnia M, Farhang M et al (2018) Health risk assessment of nitrate exposure in groundwater of rural areas of Gonabad and Bajestan. Iran Environ Earth Sci 77:1–9. https://doi.org/10.1007/S12665-018-7732-8/FIGURES/5
Qasemi M, Afsharnia M, Farhang M et al (2019) Spatial distribution of fluoride and nitrate in groundwater and its associated human health risk assessment in residents living in Western Khorasan Razavi. Iran Desalin Water Treat 170:176–186. https://doi.org/10.5004/DWT.2019.24691
Rafique T, Naseem S, Bhanger MI, Usmani TH (2008) Fluoride ion contamination in the groundwater of Mithi sub-district, the Thar Desert, Pakistan. Environ Geol 56:317–326. https://doi.org/10.1007/S00254-007-1167-Y/FIGURES/8
Rafique T, Naseem S, Usmani TH et al (2009) Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh, Pakistan. J Hazard Mater 171:424–430. https://doi.org/10.1016/J.JHAZMAT.2009.06.018
Rafique T, Ahmed I, Soomro F et al (2015) Fluoride levels in urine, blood plasma and serum of people living in an endemic fluorosis area in the Thar Desert, Pakistan. J Chem Soc Pak 37:1212–1219
Rasool A, Farooqi A, Xiao T et al (2018) A review of global outlook on fluoride contamination in groundwater with prominence on the Pakistan current situation. Environ Geochem Health 40:1265–1281. https://doi.org/10.1007/S10653-017-0054-Z
Rawat KS, Singh SK, Gautam SK (2018) Assessment of groundwater quality for irrigation use: a peninsular case study. Appl Water Sci 8:1–24. https://doi.org/10.1007/S13201-018-0866-8/FIGURES/18
Rezaei A, Hassani H, Hayati M et al (2018) Risk assessment and ranking of heavy metals concentration in Iran’s Rayen groundwater basin using linear assignment method. Stoch Env Res Risk Assess 32(5):1317–1336. https://doi.org/10.1007/S00477-017-1477-X/TABLES/13
Rezaei A, Hassani H, Hassani S et al (2019a) Evaluation of groundwater quality and heavy metal pollution indices in Bazman basin, southeastern Iran. Groundw Sustain Dev 9:100245. https://doi.org/10.1016/J.GSD.2019.100245
Rezaei H, Jafari A, Kamarehie B et al (2019b) Health-risk assessment related to the fluoride, nitrate, and nitrite in the drinking water in the Sanandaj, Kurdistan County, Iran. Human Ecol Risk Assess 25:1242–1250. https://doi.org/10.1080/10807039.2018.1463510
Rezaei A, Hassani H, Tziritis E et al (2020) Hydrochemical characterization and evaluation of groundwater quality in Dalgan basin SE Iran. Groundw Sustain Dev 10:100353. https://doi.org/10.1016/J.GSD.2020.100353
Riddell JK, Malin AJ, McCague H et al (2021) Urinary fluoride levels among canadians with and without community water fluoridation. Int J Environ Res Public Health. https://doi.org/10.3390/IJERPH18126203
Robertson A, Ranalli A, Austin S, Lawlis B (2016) The source of groundwater and solutes to many devils wash at a former uranium mill site in Shiprock New Mexico. Scientific Investigations Report
Sa B, Ganapathy D, Sasanka K (2021) Fluoride content in water bodies near Velliangiri Hills. J Contemp Issues Bus Gov. https://doi.org/10.47750/CIBG.2020.26.02.221
Schouteden C, Serwas D, Palfy M, Dammermann A (2015) The ciliary transition zone functions in cell adhesion but is dispensable for axoneme assembly in C. Elegans. J Cell Biol 210:35–44. https://doi.org/10.1083/JCB.201501013
Schullehner J, Hansen B, Thygesen M et al (2018) Nitrate in drinking water and colorectal cancer risk: a nationwide population-based cohort study. Int J Cancer 143:73–79. https://doi.org/10.1002/IJC.31306
Shen F, Chen X, Gao P, Chen G (2003) Electrochemical removal of fluoride ions from industrial wastewater. Chem Eng Sci 58:987–993. https://doi.org/10.1016/S0009-2509(02)00639-5
Sherris AR, Baiocchi M, Fendorf S, Luby SP, Yang W, Shaw GM (2021) Nitrate in drinking water during pregnancy and spontaneous preterm birth: a retrospective within-mother analysis in California. Environ Health Perspect 129:057001. https://doi.org/10.1289/EHP8205
Shuval HI, Gruener N (2013) Infant methemoglobinemia and other health effects of nitrates in drinking water
Sikdar S, Kundu M (2018) A review on detection and abatement of heavy metals. ChemBioEng Rev 5:18–29. https://doi.org/10.1002/CBEN.201700005
Sposito G, Mattigod SV (1977) On the chemical foundation of the sodium adsorption ratio. Soil Sci Soc Am J 41:323–329. https://doi.org/10.2136/SSSAJ1977.03615995004100020030X
Srivastava S, Flora SJS (2020) Fluoride in drinking water and skeletal fluorosis: a review of the global impact. Curr Environ Health Rep 7:140–146. https://doi.org/10.1007/S40572-020-00270-9
Stowers KC, Jiang Q, Atoloye A et al (2020) Racial differences in perceived food swamp and food desert exposure and disparities in self-reported dietary habits. Int J Environ Res Public Health 17:7143. https://doi.org/10.3390/IJERPH17197143
Susaiappan S, Somanathan A, Sulthan MT, Masilamani IP (2021) Groundwater quality variation and regression analysis: a case study around municipal dumpsite in India. Rev Chim 72:133–145. https://doi.org/10.37358/RC.21.1.8410
Terblanche APS (1991) Health hazards of nitrate in drinking water. Water SA 17:77–82
Thangiah AS (2019) Spectrophotometric determination of sulphate and nitrate in drinking water at Asia–Pacific international university campus, Muak Lek, Thailand. Rasayan J Chem 12:1503–1508. https://doi.org/10.31788/rjc.2019.1235201
Till C, Green R, Grundy JG et al (2018) Community water fluoridation and urinary fluoride concentrations in a national sample of pregnant women in Canada. Environ Health Perspect. https://doi.org/10.1289/EHP3546
Tirado R (2007) Nitrates in drinking water in the Philippines and Thailand. Greenpeace South East Asia, Greenpeace Res Lab 10:2007
Toolabi A, Bonyadi Z, Paydar M et al (2021) Spatial distribution, occurrence, and health risk assessment of nitrate, fluoride, and arsenic in Bam groundwater resource. Iran Groundw Sustain Dev 12:100543. https://doi.org/10.1016/J.GSD.2020.100543
USEPA (2004) Risk assessment guidance for superfund volume I: human health evaluation manual (Part E), Washington, DC, USA
USEPA (2005) Guidelines for carcinogenic risk assessment; EPA/630/P-03/001F, Risk Assessment Forum, Washington, DC, USA
USEPA (2014) Human health evaluation manual, supplemental guidance: update of standard default exposure factors, OSWER Directive 9200.1-120. Washington, DC, USA
Wang W, Chen Y, Wang W et al (2022) Assessing the influences of land use change on groundwater hydrochemistry in an Oasis-Desert region of Central Asia. Water 14:651. https://doi.org/10.3390/W14040651
Ward MH, Jones RR, Brender JD et al (2018) Drinking water nitrate and human health: an updated review. Int J Environ Res Public Health 15:1557. https://doi.org/10.3390/IJERPH15071557
Wongsanit J, Teartisup P, Kerdsueb P et al (2015) Contamination of nitrate in groundwater and its potential human health: a case study of lower Mae Klong river basin, Thailand. Environ Sci Pollut Res Int 22:11504–11512. https://doi.org/10.1007/S11356-015-4347-4
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, Mid-west China. Expo Health 8:311–329. https://doi.org/10.1007/S12403-015-0170-X/FIGURES/6
Wu J, Lu J, Wen X et al (2019) Severe nitrate pollution and health risks of coastal aquifer simultaneously influenced by saltwater intrusion and intensive anthropogenic activities. Arch Environ Contam Toxicol 77:79–87. https://doi.org/10.1007/S00244-019-00636-7/FIGURES/4
Xie Y, Dong H, Zhang S et al (2014) Effect of water on the density, viscosity, and CO2 solubility in choline chloride/urea. J Chem Eng Data 59:3344–3352. https://doi.org/10.1021/JE500320C
Xiuping H, Kang Y, Handong L, Xin W (2023) weathering of clay-pyrite of coal-bearing formation in the endemic fluorosis area of southwest China. Pol J Environ Stud 32:1433–1445. https://doi.org/10.15244/PJOES/155963
Xu P, Zhang Q, Qian H, Zheng L (2021) Spatial distribution characteristics of irrigation water quality assessment in the Central-Western Guanzhong Basin, China. IOP Conf Ser Earth Environ Sci 647:012143. https://doi.org/10.1088/1755-1315/647/1/012143
Yadav KK, Kumar S, Pham QB et al (2019) Fluoride contamination, health problems and remediation methods in Asian groundwater: a comprehensive review. Ecotoxicol Environ Saf 182:109362. https://doi.org/10.1016/J.ECOENV.2019.06.045
Yasin M, Ketema T, Bacha K (2015) Physico-chemical and bacteriological quality of drinking water of different sources, Jimma zone, Southwest Ethiopia. BMC Res Notes 8:1–13. https://doi.org/10.1186/S13104-015-1376-5/TABLES/3
Yousefi M, Ghoochani M, Hossein Mahvi A (2018) Health risk assessment to fluoride in drinking water of rural residents living in the Poldasht city, Northwest of Iran. Ecotoxicol Environ Saf 148:426–430. https://doi.org/10.1016/J.ECOENV.2017.10.057
Yu Y, Kalashnikova OV, Garay MJ et al. (2019) A global analysis of dust diurnal variability using CATS observations. Atmos Chem Phys Discuss. DOI: 10.5194/ACP-2019-975
Zhao Y, Pu G, Li Y et al (2022) Serum levels of CXCR4, SDF-1, MCP-1, NF-κB and ERK1/2 in patients with skeletal fluorosis. Int J Environ Res Public Health. https://doi.org/10.3390/IJERPH192416555
Acknowledgements
We express our appreciation to the Wadsworth Center at New York State Department of Health for the support. We extend our thanks to Dr. Kim McClive-Reed for editing the manuscript.
Funding
Not applicable. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All the authors participated equally in the execution and production of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Begum, T., Shareef, A., Rafique, T. et al. Assessment of groundwater quality with special emphasis on fluoride contamination: a case study in Thar Desert, Pakistan. Sustain. Water Resour. Manag. 10, 53 (2024). https://doi.org/10.1007/s40899-023-01024-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-023-01024-9