Abstract
Water quality is used to evaluate the overall health status of a particular water body. It can also be used to identify possible sources of pollution due to anthropogenic activities and develop strategies for improving the water body. It is a measure of the quality of water based on physical, chemical, and biological parameters. The water quality index considers several parameters, including pH, dissolved oxygen, temperature, alkalinity, acidity, and total dissolved solids. Esa-Odo reservoir serves both industrial and domestic purposes for the people. However, there is dearth of information on the quality of the waterbody. The study’s objective was to use the water quality parameters to determine the reservoir’s water quality index. Thirteen (13) water quality parameters were selected between October 2017 and September 2018 across the three zones of the reservoir, covering the dry and rainy seasons, using standard methods. The results of the water quality parameters revealed significant differences (p < 0.05) in water temperature, alkalinity, sulphate and acidity. The correlation coefficient showed a strong correlation between K + and sulphate; conductivity and total dissolved solids. PCA and cluster analysis showed the importance of water quality properties and revealed a close relationship between transition and riverine zones when compared to the dam site. The water quality index (WQI) across the three zones indicated poor water quality that ranged between 75.8 and 77.9. Human activities such as farming along the shoreline could have contributed to the poor water quality of the reservoir. Regular monitoring of water quality status by the stakeholders in charge of water resources should be encouraged in order to protect the waterbody from further anthropogenic activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water quality refers to the physical, chemical, and biological parameters. Other properties are radiological, and aesthetic parameters of its quality (Alobaidy et al. 2010; Ombaka and Gichumbi 2012). It could also mean the properties of water that influence its useful applications and an ecosystem’s sustainability (Sudarshan et al. 2019). It is a vital factor for improving the level of public human health projects in an area (Qishlaqi et al. 2017). Any ecosystem’s water quality provides important information on the resources available for sustaining life and whether they are suitable for human use (Lianthuamluaia et al. 2013). Due to the high demand, contamination risk, and potential depletion of freshwater resources in developing countries, the evaluation of freshwater water quality is essential (Yan et al. 2015). A water quality index (WQI) evaluates the water quality state, which is single with no unit value derived from a complex mathematical measurement based on a lot of water quality parameters (Pesce and Wunderlin 2000). The water quality index (WQI) is one of the most important techniques for informing policymakers and community members about water quality (Toma 2012). It therefore becomes a significant tool for evaluating and sustaining inland waterbodies. Also, effective decision-making tools based on statistical techniques are applied to aspects that influence the evaluation of the water quality in multiparametric statistical analysis (Varol and Davraz 2015). Multivariable statistical tests such as principal component analysis (PCA) can be used to minimize data when a large amount of data is obtainable (Noori et al. 2010). Also, cluster analysis is one of the multivariable statistical analyses that is usually employed to estimate relative similarities in observed parameter uniformity (Shrestha and Kazama 2007). In Nigeria, several studies have been conducted on the water quality status of some bodies of water. Rabiu et al. (2018) assessed the water quality parameters of Watari Dam, Kano State. The authors analyzed water quality properties, which included conductivity, pH, oxygen, and nutrient parameters. These parameters in the water body were within the recommended limits but posed a threat of pollution due to some nutrient parameters. A previous study in the Ikpoba River, as reported by Okonofua et al. (2019) using the water quality index approach, showed that the multivariable statistical method of water quality properties revealed poor water quality in the water body. Reservoirs and other fresh waterbodies are the chief sources of water for household, agriculture, and industries (Shil et al. 2019) as they are the most important resources for life (Bouaroudj et al. 2019; Han et al. 2020; He et al. 2020). These water bodies are degraded mostly by natural activities and human processes such as waste from households, industries, and agricultural run-off into the waterbody (Singh and Kumar 2017). Other processes are geochemical influences, chemical properties of water bodies (Giridharan et al. 2010), and occurrences as a result of nature, such as water interaction with the earth’s crust structure that the water body is flowing through (Şener et al. 2017; Subramani et al. 2010). Thus, making it unsuitable for agricultural usage, manufacturing, or other applications (Kazi et al. 2009; Sánchez et al. 2007). Contamination as a result of microplastics have been a major challenge in our water body in which it enters the environment through domestic and industrial activities and contain chemicals which can affects the quality of the water body (Kye et al. 2023). Similarly, the pollution of the environment through household chemicals particularly the water bodies could cause health hazards to man who depends on the water body for domestic purposes especially children (Khalil et al. 2022). Some materials such as fish bones have been documented to reduce the effect of Lead uptake by plants in soil contaminated with Lead-acid batteries thereby resulting in the decrease of Lead absorption in the plant shoots, roots, and grain (Tauqeer et al. 2022). Rasool et al. (2022) was of the opinion that soil contaminated with Lead-acid batteries endangers the environment including groundwater, soils, waterbodies and reduces plant therapeutic qualities through Lead transfer.
Hence, constant monitoring and assessment of water quality are crucial to preserve, regulate and manage waterbodies from degradation (Barakat et al. 2016). Esa-odo reservoir, which was dammed on the River Osun (a UNESCO-recognized site downstream of the reservoir), is an important reservoir in Osun State. It provides water for industrial activities and source of drinking. The study was conducted on the Esa-Odo reservoir because the river in which the reservoir was dammed is of local and international importance with dearth of information on its quality of water and usefulness. For this reasons, this study on the Esa-Odo reservoir has to be carried out.
The objectives of the study are to;
-
(i)
Evaluate the physicochemical parameters of the reservoir and,
-
(ii)
Assess the water quality of the reservoir based on multivariate analysis and through water quality index analysis.
Materials and methods
Study area description
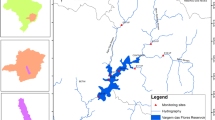
The study area is the Esa-Odo reservoir in the small community of Esa-Odo, Osun State. The area lies approximately on latitudes 007°75′0′ N to 007°77′18′ N and longitudes 04°81′0′ E to 04°82′12′. The reservoir was built on the Osun River, which took its source from Igede-Ekiti and flows through Osun State to the Lagos State lagoon. The area experienced dry season between November and February, and rainy season varied from March to October. The data collected were compared with published findings by other authors.
Water sampling
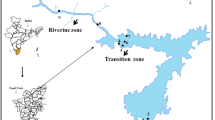
Samples of water were collected for one annual cycle on monthly basis across three zones (Fig. 1) of the reservoir. The sampling was done in each of the sampling zones using GPS (Table 1) between October 2017 and September 2018. Most activities along the reservoir were cash crop farming and fishing, which some local fishermen do for a living. The water samples collected were preserved at 4 °C with immediate analysis according to (APHA 1999). Parameters such as water temperature was determined on the field using a mercury-in-glass thermometer; pH, conductivity, and total dissolved solids were analyzed using PCE-PHD Version 1.1 Model Q656697); dissolved oxygen and biochemical oxygen demand were analyzed using Winkler’s reagents. In order to analyze other water quality parameters such as total suspended solids, total hardness, chloride, alkalinity, sulphate, nitrate, calcium, magnesium, phosphate, potassium, and acidity; water samples were analyzed based on suitable titrimetric or instrumentation method in the Hydrobiology Laboratory of the Department of Zoology, Obafemi Awolowo University, Ile-Ife.
Of the seventeen parameters selected for water analysis, only thirteen were used to calculate the water quality index. These are pH, conductivity, total dissolved solids, total suspended solids, total hardness, chloride, DO, and BOD. Other physico-chemical parameters are alkalinity, sulphate, nitrate, calcium, and magnesium.
Multivariate analysis
Multivariate techniques have been broadly employed to analyze multiple data and variables under conditions that are interrelated (Ramos et al. 2022). It has also been used to analyze water quality characteristics (Nnorom et al. 2019). Water parameters were analyzed using SPSS software version 24 (SPSS, USA). ANOVA was used to determine the spatial differences, while the correlation coefficient matrix was used to examine the relationship among the water parameters using PAST version 4.10. Principal components analysis (PCA) was used to analyze the relationship between water quality variables in the three zones of the reservoir, and a visual representation was done using graphs performed with PAST software version 2.12. Cluster analysis showed the relationship based on the clustering of different zones of the reservoir. The results of the physical and chemical properties of the water were expressed as a mean ± standard deviation. Also, ArcGis 10.8 software was used to create spatial map distribution of selected water quality parameters.
Water quality index
The water quality index is the most simple and widely employed water index for determining the total quality of water bodies (Gad et al. 2021; Ma et al. 2020). It involves the use of the weighted index method, in which the estimated unit weight assigned to each of the physicochemical parameters of water was considered for the calculation. Therefore, by assigning unit weights, all the selected water properties of different units and dimensions were changed to a common scale. The equation for WQI in this study was based on modified version of several equations that has been in use since its first proposal by Horton (1965).
The water quality index in this study was calculated mathematically as
where Qi is the quality rating scale for ith parameter, Vi is the estimated concentration of ith parameter in the scale, Si is the recommended standard value for ith parameter and Vo is the ideal value of the ith parameter. Here, for all the parameters ideal value, Vo is taken as zero except for pH, which is 7.0 while DO and BOD is 14.6 (Khan and Tahsin 2020).
where Wi = unit weight for ith Parameter and K = proportionality constant = \(\frac{1}{\Sigma \frac{1}{ Si}}\)
(Khan and Tahsin 2020) The obtained value of the water quality index determines the quality of the water, which can be very excellent (WQI = 0–25), good (WQI = 26–50), poor (WQI = 51–75), very poor (WQI = 76–100) or unsuitable (WQI = above 100) (Sudarshan et al. 2019).
Results
Water quality parameters
The summary of environmental parameters of water samples collected at different zones of the reservoir is presented in Table 2. The water quality showed that the highest mean water temperature was observed at the riverine when compared with the other zones, with a significant difference (p < 0.05) between the rainy and dry seasons. The mean level of pH in the reservoir was higher in the dry season (7.11 ± 0.03) than 7.09 ± 0.027 recorded in the rainy season. Also, the mean conductivity of the reservoir varied from 119 ± 10.6 µS/cm at the dam site to 122 ± 9.37 µS/cm at the transition zone. On the other hand, TDS had the highest mean value of 81.1 ± 0.90 mg/L during the dry season and 80.2 ± 1.44 mg/L in the rainy season, with no significant difference (p > 0.05) between the seasons. In this study, the highest mean TSS concentration (29.7 ± 4.35 mg/L) was recorded at the riverine, and the lowest value (28.3 ± 5.25 mg/L) was observed at the dam site. The total hardness of the Esa-Odo reservoir was higher during the dry season with a mean concentration of 59.6 ± 2.05 CaCO3mg/L while the mean value of 58.4 ± 1.25 CaCO3mg/L recorded in the rainy season. Chloride levels in the reservoir water had a mean value of 7.35 ± 2.25 mg/L, 7.63 ± 2.10 mg/L and 8.40 ± 2.79 mg/L for dam site, transition and riverine zones, respectively. A higher mean DO value of 5.37 ± 0.27 mg/L was recorded during the dry season when compared with 4.95 ± 0.22 mg/L observed in the rainy season, with no significant difference (p > 0.05) between the seasons. The BOD of the reservoir was highest at the riverine (2.85 ± 1.03 mg/L) than 2.71 ± 1.24 mg/L recorded at the dam site. Also, the highest mean alkalinity of 49.3 ± 1.17 CaCO3mg/L was observed during the rainy season while 42.2 ± 1.31 CaCO3mg/L was recorded during the dry season, with a significant difference (p < 0.05) between the two seasons. The mean sulphate level of the reservoir varied from 3.46 ± 1.25 mg/L at the riverine to 3.85 ± 1.17 mg/L and 3.85 ± 0.91 mg/L at both the transition and dam sites respectively. A high mean nitrate value of 1.73 ± 0.63 mg/L was recorded at the transition zone while the lowest value of 1.56 ± 0.53 mg/L was observed at the dam site during this study. The mean level of calcium in the reservoir was higher in the rainy season (16.5 ± 0.47 mg/L) than 15.7 ± 0.58 mg/L recorded during the dry season. Maximum potassium value was observed at the riverine, and there was no significant difference (p > 0.05) between the seasons. The mean levels of phosphate across the zone differ significantly and the highest mean concentration was recorded at the dam site. Acidity had the highest mean concentration of 13 ± 0.51 mg/L in the rainy season, while the dry season recorded a value of 10.5 ± 0.92 mg/L with a significant difference (p < 0.05) between the seasons. ANOVA showed that there was no significant difference (p > 0.05) among the water parameters across the zones.
Multivariate analysis of the water quality
The correlation matrix table was used to describe the inter-relationship between different water quality parameters of the reservoir. The result of this study showed that there was a strong correlation between the water quality parameters of the reservoir (Table 3). Conductivity showed a highly significant correlation (p < 0.01) with TDS. However, a negative correlation was recorded between water temperature and magnesium. Potassium and sulphate revealed a strong positive correlation. Also, both TDS and calcium, as well as conductivity, showed a similar positive correlation. The component analysis showed the water quality parameters had more influence in different zones of the reservoir. It revealed water quality parameters that clustered with different reservoir zones (Fig. 2). It also revealed differences between the riverine, transition, and dam sites. The variables showed that there was a close relationship between acidity, alkalinity, and TSS, which clustered at both the riverine and transition zones, while magnesium, conductivity, pH, DO, BOD, TDS, sulphate, nitrate, chloride, water temperature, potassium, phosphate, and calcium are related at the dam site and clustered together. The water variables showed good clustering of physicochemical parameters at the dam site while water hardness showed not too close clustering with the dam site when compared with riverine and transition zones.
The clustering of the water qualities based on the zone is presented in Fig. 3. The similarity among each of the zones in the clusters recorded from cluster analysis was the result of correlation and autocorrelation among the water quality properties of the reservoir. As shown in Fig. 3, two clusters were extracted based on the distance. The first cluster combined the transition and riverine zones, while the second cluster was that of the dam site. Figure 4 showed the spatial map distribution of selected water quality parameters of the reservoir. The water quality parameters were illustrated by different colour combinations for their respective concentration ranges.
Water quality index
The calculation of WQI was based on an estimate of the unit weight assigned to each physicochemical parameter taken into consideration for the mathematical calculation; the weighted arithmetic index technique was used. A minimum weight of 0.0033 Wn was assigned to TDS, TSS, and hardness, while a maximum weight of 0.3317 Wn was assigned to both DO and BOD. Therefore, indicating the key importance of these properties in water quality evaluation of the environment and their significant influence on the water index. Thirteen (13) water quality parameters were carefully selected. The result of the WQI obtained from all the sampling zones and seasons is shown in Table 4. The transition zone recorded the highest value of WQI (77.3), while the lowest WQI (75.8) was observed in the riverine zone. The values of WQI increased considerably from one zone to another and improved considerably at the dam site. Seasonally, the dry season recorded the highest WQI value of 77.9 when compared with the rainy season, which recorded 76.4 as its WQI value. Irrespective of the zones and seasons, the values recorded indicated poor water quality based on the drinking water index.
A comparison of the water quality index with other inland water bodies is presented in Table 5. The WQI recorded in this study was between 75.8 and 77.9. It showed a lower value in comparison with Beheshtabad River, the Adolfo López Mateos Dam, and the Ogbese River which recorded 62.2–71, 73.5 and 44.9–46.6 respectively. However, Hebbal Lake and the Mahananda River revealed WQI values higher than those in the Esa-Odo reservoir.
Discussion
The water temperature in this study area varied from one zone to another. The mean temperature varied from 25.7 °C to 26 °C with higher water temperature recorded during the dry season. The rainy season had a mean temperature of 25.5 ± 0.19 °C while the dry season recorded a mean value of 26.1 ± 0.07 °C and this variation could be due to the weather conditions that usually occurred in the two seasons. The temperatures recorded were within the recommended limit (WHO 2004) and the observation was similar to the results recorded by Omoboye et al. (2022) in Owalla Reservoir. The findings of pH during the dry season were higher than the value recorded in the rainy season. Also, the mean value varied from 7.08 at the dam site to 7.11 at the transition zone. The values of pH recorded were above the values of FAO (2012), and it was slightly neutral. In comparison with other inland waters, this finding was lower than the pH values recorded at Oyan Dam by Osibona and Ayoola (2020). Also, this was consistent with the results of Onwugbuta-Enyi et al. (2008) in Bodo Creek in the Lower Niger Delta Basin. Electrical conductivity (EC) is the ability of water to conduct current; it also serves as a tool to determine the number of dissolved salts or ions in the solution (Pal et al. 2015). The mean conductivity values across the zones varied from 119 to 122 µS/cm with 120 ± 1.72 and 121 ± 1.47 µS/cm recorded during the rainy and dry seasons respectively. These findings were similar to the reports of Eneogwe et al. (2022) that recorded conductivity values which ranged between 21.5 and 156.4 µS/cm. However, the mean conductivity values recorded in this study were below the 86.3 µS/cm reported by Atwebembeire et al. (2018). Higher conductivity in the dry season could probably be due to the reduced volume of the water and the increase in temperature that is associated with ionization (Chikogu et al. 2012). TDS had a mean value of 80.2 ± 1.44 mg/L in the rainy season and 81.1 ± 0.90 mg/L during the dry season. The concentration of TDS was below the WHO (2004) concentration of 250 mg/L and the FAO (2012) concentration of 2000 mg/L. The result of the mean TSS value of 29.8 ± 0.86 mg/L recorded in the dry season was higher than the value of 28.2 ± 0.59 mg/L during the rainy season. The increased water flow that might carry more suspended particles was responsible for the higher results during the rainy season (Kitonga et al. 2018). The total hardness had a mean value that varied from 58.0 CaCO3mg/L at the transition zone to 59.5 CaCO3mg/L at the riverine zone; the values across the zones were below the mean value of 33.7 CaCO3mg/L recorded in Bontanga Reservoir.
The mean chloride of 7.68 ± 0.39 mg/L reported in the rainy season was lower than the level observed during the dry season (7.95 ± 0.41 mg/L). Low chloride contents in this study indicated low pollution level around the reservoir (Kumari et al. 2019). The mean DO varied from 4.99 mg/L at the dam site to 5.35 mg/L at the riverine zone. This agreed with the study of Iliya et al. (2019) in the Gurara River. DO values recorded in this study were below the (NSDWQ 2007; WHO 2004) recommended limit of 10 mg/L. Reduced DO during the rainy season may be caused by a significant influx of waste, which would also reduce the reservoir’s biological life, in accordance with the findings of Hassan et al. (2013). This showed that the water might be unsuitable for domestic purposes and reduced the activities of aquatic organisms in the reservoir. BOD is the level of oxygen necessary for the biological oxidation of organic materials in water. The mean BOD values in the dry season (4.95 ± 0.22 mg/L) were slightly lower than 5.37 ± 0.27 mg/L recorded during the rainy season. The result of this current study was in contrast with the findings of Akaahan and Azua (2016), who recorded BOD values that ranged between 1.21 and 2.18 mg/L. The low amount of BOD in the reservoir could probably be a result of low levels of organic matter and pollution. High levels of BOD during the rainy season might be due to runoff and organic materials.
The result of alkalinity during the rainy season with a value of 49.3 CaCO3mg/L was higher than the value recorded in the dry season (42.2 CaCO3mg/L). The mean value varied from 45 CaCO3mg/L at the dam site to 47.5 CaCO3mg/L at the riverine zone. The values of alkalinity recorded in Esa-Odo were above the values of FAO (2012). Similar findings were observed by Aliu et al. (2020) in the Obudu River, where the alkalinity level was 44.2 CaCO3mg/L. Also, this was consistent with the findings of Hyeladi and Nwagilari (2013) that reported an alkalinity level of 36–72 CaCO3mg/L in Alau Dam. Sulphate naturally occurs in groundwater as a result of sulfides, such as pyrite, being dissolved by percolating water, which releases sulphate ions from the interstratified minerals (Olobaniyi and Owoyemi 2010). In this study, sulphate (SO42−) levels ranged from 3.46 ± 1.25 mg/L in the riverine to 3.85 ± 1.17 mg/L at the dam site. The nitrate level in the reservoir could be due to infiltration from farmland as a result of fertilizer application to the farm. Nitrate had a mean value of 1.73 ± 0.09 mg/L in the rainy season and 1.55 ± 0.1 mg/L during the dry season. The nitrate levels in this current study were below (WHO 2004) concentration of 50 mg/L and (FAO 2012) level of 10 mg/L. These findings were in contrast with the results of Adedeji et al. (2020) that recorded nitrate levels between 0.19 and 0.22 mg/L in the Opa reservoir. High nitrate water consumption results in birth disorders of the central nervous system, diabetes, stomach carcinomas, methemoglobinemia illness, aberrant pain, and gastric cancers in infants (Varol and Davraz 2015; Vasanthavigar et al. 2010).
The growth and metabolism of aquatic organisms are significantly influenced by the hardness of the calcium (Vankar et al. 2019). The result of mean calcium of 16.5 ± 0.47 mg/L observed in the rainy season was higher than the level of 15.7 ± 0.58 mg/L in the dry season. All the levels of calcium across the zones were within the recommended limits for drinking water (WHO 2011). Similar observations were reported by Adesakin et al. (2016) and Olanrewaju et al. (2017) in the Opa and Eleyele reservoirs respectively. The magnesium values recorded in this study had a mean value that varied from 4.10 ± 1.56 mg/L at the transition zone to 4.75 ± 1.63 mg/L at the riverine zone; the values across the zones were consistent with the values recorded by (Pulugandi 2014) who recorded magnesium values that ranged between 5.3 and 11.2 mg/L in Vembakottai reservoir. Despite its abundance in nature, potassium is only found in trace amounts in the majority of natural fluids, owing to weathering, which converts it into secondary minerals that are insoluble (Mallick 2017). The mean potassium in Esa-Odo varied between 5.97 mg/L at the riverine site and 6.69 mg/L at the dam site. This was in agreement with the findings of Saxena (2012), who reported potassium levels that varied from 4.25 to 7.53 mg/L and a mean concentration of 5.93 ± 0.23 mg/L at Raipur reservoir. Potassium values recorded in this study were below (BIS 2003) permissible limit of 10 mg/L. The mean phosphate value in the dry season (1.39 ± 0.16 mg/L) were higher than 1.05 ± 0.07 mg/L reported in the rainy season. Increased level of phosphates in the dry season was similar to the study by Lanmandjèkpogni et al. (2018). Phosphates may be associated with buffer zones’ influence on phosphorus retention in the waterbody and run-off infiltration of water trapped at the sediments of the reservoir. The mean acidity varied from 11.33 mg/L at the dam site to 12.30 mg/L at the riverine zone. This result was higher than the concentrations recorded by Hameed et al. (2019) at Ifewara Reservoir.
The water quality index (WQI) of Esa-Odo reservoir was determined mathematically using Khan and Tahsin (2020) methods from thirteen water quality parameters for rainy and dry seasons. Parameters such as pH, conductivity, TDS, TSS, total hardness, chloride, DO and BOD were determined for WQI. Other parameters namely alkalinity, sulphate, nitrate, calcium and magnesium, were also considered for estimating the WQI value for the seasons and zones. In order to determine the unit weights, the minimum and maximum weights for each parameters due to the vital significance of these properties in water quality were used in water quality index evaluation. Total dissolved solids, total suspended solid and total hardness had the least unit weight of 0.0033 while dissolved oxygen and biochemical oxygen demand recorded maximum unit weights of 0.3338. The WQI varied from 75.8 at the riverine to 77.9 at the transition zone. Hence, the WQI of the dam site was of better quality when compared to the riverine and transition zones. This could be due to reduced anthropogenic activities at the dam site. High WQI recorded at the transition zone was as a result of intense fishing activities at the open water part of the reservoir. The finding in this study was similar to the observation of Aigberua and Tarawou (2019) at the inland freshwaters of Taylor Creek. The WQI ranged between 75.8 and 77.9 recorded in Esa-Odo reservoir could be compared with other waterbodies. The WQI was higher in comparison with the findings of Fathi et al. (2018) at Beheshtabad River; Akinbile and Omoniyi (2018) at Ogbese River and Quevedo-Castro et al. (2018) at Adolfo López Mateos Dam. However, the WQI of these current findings was lower than the concentration recorded by Sudarshan et al. (2019) at Hebbal Lake and Shil et al. (2019) at Mahananda River. The overall WQI of Esa-Odo reservoir could be classified as of poor quality. Seasonally, the WQI value of 77.9 recorded during the dry season was higher than the 76.4 observed in the rainy season. The reduction in WQI in the rainy season could probably be as a result of high humidity since it increases the amount of water entering the reservoir, this was also the view of Sánchez et al. (2007) and significant level of pollution in the rainy season when compared to the dry season.
Also, similar findings were recorded by Aliu et al. (2020) in the Obudu River, where the alkalinity level was 44.2 CaCO3mg/L. Also, the results of this study was consistent with the findings of Hyeladi and Nwagilari (2013) that reported an alkalinity level of 36–72 CaCO3mg/L in Alau Dam. Sulphate naturally occurs in groundwater as a result of sulfides, such as pyrite, being dissolved by percolating water, which releases sulphate ions from the interstratified minerals (Olobaniyi and Owoyemi 2010). In this study, the mean sulphate (SO42−) level ranged from 3.46 ± 1.25 mg/L in the riverine to 3.85 ± 1.17 mg/L at the dam site. The nitrate level in the reservoir could be due to infiltration from farmland as a result of fertilizer application to the farm. Nitrate had a mean value of 1.73 ± 0.09 mg/L in the rainy season and 1.55 ± 0.1 mg/L during the dry season. The levels were below the WHO (2004) concentration of 50 mg/L and the FAO (2012) level of 10 mg/L. These findings were in contrast with the results of Adedeji et al. (2020) that recorded nitrate levels between 0.19 and 0.22 mg/L in the Opa reservoir. Birth disorders of the central nervous system, diabetes, stomach carcinomas, methemoglobinemia illness, aberrant pain, and gastric cancers in infants are all associated with high nitrate water consumption (Varol and Davraz 2015; Vasanthavigar et al. 2010).
The growth and metabolism of aquatic organisms are significantly influenced by the hardness of calcium (Vankar et al. 2019). The results of the mean calcium level of 16.5 ± 0.47 mg/L observed in the rainy season was higher than the level of 15.7 ± 0.58 mg/L in the dry season. All the levels of calcium across the zones were within the recommended limits for drinking water (WHO 2011). Similar observations were reported by Adesakin et al. (2016) and Olanrewaju et al. (2017) in the Opa and Eleyele reservoirs respectively. The magnesium values recorded in this study had a mean value that varied from 4.10 ± 1.56 mg/L at the transition zone to 4.75 ± 1.63 mg/L at the riverine zone; the values across the zones were consistent with the values recorded by Pulugandi (2014), who recorded magnesium values that ranged between 5.3 and 11.2 mg/L in Vembakottai reservoir. Government at all levels especially the state government should give maximum attention to the reservoir by regulating human activities around the reservoir such as mining of fine sediments and disposal of palm oil effluents by the locals and regulating the use of chemicals by farmers along the shoreline of the reservoir. Also, regular monitoring of the health status of Esa-Odo reservoir and its tributaries through deployment of new technologies.
Conclusion
The evaluation of the water quality index of the Esa-Odo reservoir revealed that the water quality was poor and unfit for domestic use if not appropriately treated. The application of WQI to the reservoir indicated the significance of this index in classifying the reservoir under study. As a result, the reservoir’s water quality was observed to be influenced by anthropogenic activities, with the dry season having the highest WQI. Pollution along the shoreline of the reservoir could have contributed to the physico-chemical parameters and quality of the reservoir. Also, human activities such as farming along the shoreline could have deteriorated the water quality of the Esa-Odo reservoir. Therefore, periodic protection actions relating to the use of the reservoir water should be encouraged. It is recommended for all stakeholders involve in the management of the reservoir to intensify their efforts in monitoring of the quality of the reservoir water through constant investigation of the reservoir and reducing human activities around the reservoir.
Data availability
The data of this study will be available based on a request from the corresponding author.
References
Adedeji AA, Adesakin TA, Bolawa OP (2020) Assessment of the ecological status of Opa Reservoir, Ile-Ife, Nigeria based on a comparative study of its planktonic community and water quality parameters. Open Acad J Adv Sci Technol 4(1):1–14. https://doi.org/10.33094/5.2017.2020.41.1.14
Adesakin TA, Adedeji AA, Taiwo YF (2016) Temporal and spatial fluctuations of heavy metals in Opa Reservoir, Ile-Ife Nigeria. Afr J Aquat Sci 41(4):435–443. https://doi.org/10.2989/16085914.2016.1246355
Aigberua A, Tarawou T (2019) Water Quality Index (WQI) assessment along inland fresh waters of taylor creek in bayelsa state Nigeria. J Environ Treat Tech 7(3):260–269
Akaahan TJA, Azua ET (2016) Assessment of seasonal variation of oxygen demands as pollution indicators of River Benue, Nigeria. Eur J Earth Environ 3(3):1–9
Akinbile CO, Omoniyi O (2018) Quality assessment and classification of Ogbese river using water quality index (WQI) tool. Sustai Water Resour Manag 4(4):1023–1030. https://doi.org/10.1007/s40899-018-0226-8
Aliu OO, Akindele EO, Adeniyi IF (2020) Biological assessment of the headwater rivers of Opa Reservoir, Ile-Ife, Nigeria, using ecological methods. J Basic Appl Zool. https://doi.org/10.1186/s41936-020-00151-5
Alobaidy AHMJ, Al-Sameraiy MA, Kadhem AJ, Majeed AA (2010) Evaluation of treated municipal wastewater quality for irrigation. J Environ Prot 01(03):216–225. https://doi.org/10.4236/jep.2010.13026
APHA (1999) Standard methods for the examination of water and wastewater, vol 20. American Public Health Association, Washington, p 2671
Atwebembeire J, Bazira J, Kagoro G, Yatuha J, Andama M, Bunny JL (2018) The physico-chemical quality of streams and channels draining into River Rwizi, South Western Uganda. J Water Resour Prot 10(09):817–845. https://doi.org/10.4236/jwarp.2018.109047
Barakat A, El Baghdadi M, Rais J, Aghezzaf B, Slassi M (2016) Assessment of spatial and seasonal water quality variation of Oum Er Rbia River (Morocco) using multivariate statistical techniques. Int Soil Water Conserv Res 4(4):284–292. https://doi.org/10.1016/j.iswcr.2016.11.002
BIS (2003) Bureau of Indian Standards (BIS). In Bureau of Indian Standards Specification for drinking water. IS: 10500:91 revised 2003, Bureau of Indian Standards, New Delhi. https://doi.org/10.1016/j.ajodo.2020.08.002
Bouaroudj S, Menad A, Bounamous A, Ali-Khodja H, Gherib A, Weigel DE, Chenchouni H (2019) Assessment of water quality at the largest dam in Algeria (Beni Haroun Dam) and effects of irrigation on soil characteristics of agricultural lands. Chemosphere 219:76–88. https://doi.org/10.1016/j.chemosphere.2018.11.193
Chikogu V, Adamu I, Vivan EL (2012) Public health effects of effluent discharge of kaduna refinery into River Romi Greener. J Med Scie 2(3):2276–7797
Eneogwe C, Mohammed Sanni II, Umar Abubakar AI, Apeh Abraham II (2022) Seasonal variation of reservoir water quality: a case study of Kubanni reservoir, Zaria, Nigeria. Environ Health Eng Manag J 9(2):125–134. https://doi.org/10.34172/EHEM.2022.14
FAO (2012) Coping with water scarcity. An action framework for agriculture and food security. FAO reports. https://doi.org/10.1097/00010694-199203000-00010
Fathi E, Zamani R, Rafat A, Bidaki Z (2018) Water quality evaluation using water quality index and multivariate. Appl Water Sci 8(7):1–6. https://doi.org/10.1007/s13201-018-0859-7
Gad M, El-Safa MMA, Farouk M, Hussein H, Alnemari AM, Elsayed S, Khalifa MM, Moghanm FS, Eid EM, Saleh AH (2021) Integration of water quality indices and multivariate modeling for assessing surface water quality in qaroun lake. Egypt Water (switzerland) 13(16):1–22. https://doi.org/10.3390/w13162258
Giridharan L, Venugopal T, Jayaprakash M (2010) Identification and evaluation of hydrogeochemical processes on river Cooum South India. Environ Monit Assess 162(1–4):277–289. https://doi.org/10.1007/s10661-009-0795-y
Hameed IO, Adeniyi IF, Adesakin TA, Aduwo AI (2019) Phytoplankton diversity and abundance in relation to physico-chemical parameters of Ifewara Reservoir, Southwestern Nigeria. World News Nat Sci 24(April):251–268
Han Q, Tong R, Sun W, Zhao Y, Yu J, Wang G, Shrestha S, Jin Y (2020) Anthropogenic influences on the water quality of the Baiyangdian Lake in North China over the last decade. Sci Total Environ 701:134929. https://doi.org/10.1016/j.scitotenv.2019.134929
Hassan MA, Voepel H, Schumer R, Parker G, Fraccarollo L (2013) Displacement characteristics of coarse fluvial bed sediment. J Geophys Res Earth Surf 118(1):155–165. https://doi.org/10.1029/2012JF002374
He J, Wu X, Zhang Y, Zheng B, Meng D, Zhou H, Lu L, Deng W, Shao Z, Qin Y (2020) Management of water quality targets based on river-lake water quality response relationships for lake basins – a case study of Dianchi Lake. Environ Res. https://doi.org/10.1016/j.envres.2020.109479
Horton RK (1965) An index number system for rating water quality. Water Pollut Control Fed 37:300–306
Hyeladi A, Nwagilari JE (2013) Assessment of drinking water quality of Alau Dam Maiduguri, Borno State, Nigeria. Int J Sci Res Publ 4(10):1795–1803
Iliya A, Ojutiku R, Kolo R, Arimoro F, Mohammed A, Musa B (2019) Assessment of the Spatio-temporal environmental parameters of surface water in Gurara reservoir, Kaduna state, Nigeria. Int J Fish Aquat Stud 7(1):198–204
Kazi TG, Arain MB, Jamali MK, Jalbani N, Afridi HI, Sarfraz RA, Baig JA, Shah AQ (2009) Assessment of water quality of polluted lake using multivariate statistical techniques: a case study. Ecotoxicol Environ Saf 72(2):301–309. https://doi.org/10.1016/j.ecoenv.2008.02.024
Khalil M, Iqbal M, Turan V, Muhammad H, Farhad M (2022) Household chemicals and their impact. Environmental micropollutants. Elsevier Inc, Amsterdam. https://doi.org/10.1016/B978-0-323-90555-8.00022-2
Khan MAI, Tahsin A (2020) Evaluation of drinking water quality in terms of water quality index for Faridpur Sadar Upazila. 5th International Conference on Civil Engineering for Sustainable Development, KUET, Khulna, Bangladesh. p. 16, ICCESD-202. https://doi.org/10.5281/zenodo.5145035
Kitonga LM, Moturi WN, Mwonga SM, Taabu I (2018) Assessment of physico-chemical characteristics of groundwater among different farm sizes in Ainabkoi sub-county, Uasin Gishu County, Kenya. Afr J Environ Sci Technol 12(11):408–416. https://doi.org/10.5897/ajest2018.2497
Kumari S, Khan JA, Thakur MS, Lal H (2019) Study of physico-chemical characteristics of water and soil in relations to fish production in Motia Lake Reservoir. HSOA J Atmos Earth Sci 2(1):1–9. https://doi.org/10.24966/aes-8780/100006
Kye H, Kim J, Ju S, Lee J, Lim C, Yoon Y (2023) Microplastics in water systems: a review of their impacts on the environment and their potential hazards. Heliyon 9(3):e14359. https://doi.org/10.1016/j.heliyon.2023.e14359
Lanmandjèkpogni MPS, De Paule Codo F, Yao BK, Aina MP (2018) Seasonal variation of nutrient pollution and suspended solids in the drainage network of Okpara Basin in Parakou (North-East of Benin). J Environ Prot 09(13):1359–1371. https://doi.org/10.4236/jep.2018.913084
Lianthuamluaia ATL, Purushothaman CSDG, Ramteke KK (2013) Assessment of seasonal variations of water quality parameters of Savitri Reservoir, Poladpur, Raigad District Maharashtra. Bioscan 8(4):1337–1342
Ma Z, Li H, Ye Z, Wen J, Hu Y, Liu Y (2020) Application of modified water quality index (WQI) in the assessment of coastal water quality in main aquaculture areas of Dalian, China. Marine Pollut Bull. https://doi.org/10.1016/j.marpolbul.2020.111285
Mallick J (2017) Hydrogeochemical characteristics and assessment of water quality in the Al-Saad Lake Abha Saudi Arabia. Appl Water Sci 7(6):2869–2882. https://doi.org/10.1007/s13201-017-0553-1
Nnorom IC, Ewuzie U, Eze SO (2019) Multivariate statistical approach and water quality and other drinking water sources in Southeastern Nigeria. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e01123
Noori R, Khakpour A, Omidvar B, Farokhnia A (2010) Comparison of ANN and principal component analysis-multivariate linear regression models for predicting the river flow based on developed discrepancy ratio statistic. Expert Syst Appl 37(8):5856–5862. https://doi.org/10.1016/j.eswa.2010.02.020
NSDWQ (2007) Nigerian standard for drinking water quality. Nigerian Stand Drink Water Qual 52:19–24
Okonofua ES, Nwadialo IB, Ekun MO (2019) Modeling Ikpoba river water quality using principal component analysis (PCA) method and water quality. J Civil Eng Sci Technol 10(1):59–74
Olanrewaju A, Ajani E, Kareem O (2017) Physico-chemical status of Eleyele Reservoir, Ibadan Nigeria. J Aquacu Res Dev. https://doi.org/10.4172/2155-9546.1000512
Olobaniyi S, Owoyemi F (2010) Characterization by factor analysis of the chemical facies of groundwater in the deltaic plain sands aquifer of Warri, western Niger delta, Nigeria. Afr J Sci Technol 7(1):73–81. https://doi.org/10.4314/ajst.v7i1.55201
Ombaka O, Gichumbi JM (2012) Water quality assessment of Ruguti river in Meru South, Kenya. Int J Water Res Environ Eng 4(12):404–414. https://doi.org/10.5897/IJWREE12.093
Omoboye HY, Aduwo AI, Adewole H, Adeniyi IF (2022) Water quality and planktonic community of Owalla Reservoir, Osun State, Southwest Nigeria. Acta Limnol Bras. https://doi.org/10.1590/s2179-975x1820
Onwugbuta-Enyi J, Zabbey N, Erondu ES (2008) Water quality of Bodo creek in the lower Niger delta basin. Anim Sci 2(3):132–136
Osibona AO, Ayoola SO (2020) Physico-chemical parameters and ichthyofauna composition of Oyan dam area, Ogun State, Nigeria. West Afr J Fish Aquat Sci 1(1):17–25
Pal M, Samal NR, Roy PK, Roy MB (2015) Electrical Conductivity of lake water as environmental monitoring – a case study of Rudrasagar Lake. IOSR J Environ Sci Ver I 9(3):2319–2399. https://doi.org/10.9790/2402-09316671
Pesce SF, Wunderlin DA (2000) Use of water quality indices to verify the Córdoba City (Argentina ) on Suquía River. Wat Res 34(11):2915–2926
Pulugandi C (2014) Analysis of water quality parameters in Vembakottai water reservoir, Virudhunagar district, Tamil Nadu – a report. Res J Recent Sci 3:242–247
Qishlaqi A, Kordian S, Parsaie A (2017) Hydrochemical evaluation of river water quality — a case study. Appl Water Sci 7(5):2337–2342. https://doi.org/10.1007/s13201-016-0409-0
Quevedo-Castro A, Rangel-Peraza JG, Bandala E, Amabilis-Sosa L, Rodríguez-Mata A, Bustos-Terrones Y (2018) Developing a water quality index in a tropical reservoir using a measure of multiparameters. J Water Sanit Hygiene Dev. https://doi.org/10.2166/washdev.2018.049
Rabiu HD, Umar L, Sulaiman I, Madina M, Abubakar AI (2018) Assessment of the water quality of watari dam, kano state using selected physicochemical parameters. Int J Adv Acad Res Sci 4(5):2488–9849
Ramos H, Luzia M, Dutra S, Lima NS, Melo G, Ara C, Regina A, Moura L, Monteiro ADS, Ferreira D (2022) Study of the influence of physicochemical parameters on the water quality index (WQI) in the Maranh ã o Amazon, Brazil. Water 14:1–13
Rasool B, Muhammad R, Muhammad Z, Khan A (2022) Synergetic efficacy of amending Pb - polluted soil with P - loaded Jujube (Ziziphus mauritiana) twigs biochar and foliar chitosan application for reducing Pb distribution in moringa leaf extract and improving its anti - cancer potential. Water Air Soil Pollut. https://doi.org/10.1007/s11270-022-05807-2
Sánchez E, Colmenarejo MF, Vicente J, Rubio A, García MG, Travieso L, Borja R (2007) Use of the water quality index and dissolved oxygen deficit as simple indicators of watersheds pollution. Ecol Ind 7(2):315–328. https://doi.org/10.1016/j.ecolind.2006.02.005
Saxena M (2012) Water quality and trophic status of Raipur reservoir in Gwalior, Madhya Pradesh. J Nat Sci Res 2(8):82–97
Şener Ş, Şener E, Davraz A (2017) Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Sci Total Environ 584–585:131–144. https://doi.org/10.1016/j.scitotenv.2017.01.102
Shil S, Kumar U, Pankaj S (2019) Water quality assessment of a tropical river using water quality index ( WQI ), multivariate statistical techniques and GIS. Appl Water Sci 9(7):1–21. https://doi.org/10.1007/s13201-019-1045-2
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin Japan. Environ Modell Softw 22(4):464–475. https://doi.org/10.1016/j.envsoft.2006.02.001
Singh UK, Kumar B (2017) Pathways of heavy metals contamination and associated human health risk in Ajay River basin, India. Chemosphere 174:183–199. https://doi.org/10.1016/j.chemosphere.2017.01.103
Subramani T, Rajmohan N, Elango L (2010) Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region Southern India. Enviro Monit Assess 162(1–4):123–137. https://doi.org/10.1007/s10661-009-0781-4
Sudarshan P, Mahesh MK, Ramachandra TV (2019) Assessment of seasonal variation in water quality and water quality index ( WQI ) of Hebbal Lake, Bangalore India. Environ Ecol 37(March):309–317
Toma JJ (2012) Water quality index for assessment of water quality of Duhok Lake, Kurdistan Region of Iraq. J Adv Lab Res Biol 3(3):119–124
Tauqeer HM, Basharat Z, Adnan Ramzani PM, Farhad M, Lewińska K, Turan V, Karczewska A, Khan SA, Faran GE, Iqbal M (2022) Aspergillus niger-mediated release of phosphates from fish bone char reduces Pb phytoavailability in Pb-acid batteries polluted soil, and accumulation in fenugreek. Environ Pollut 313:120064. https://doi.org/10.1016/j.envpol.2022.120064
Vankar J, Tatu K, Kamboj RD, Gupta R, Christian L (2019) Assessment of surface water quality of wadhwana irrigation reservoir, Gujarat, India. J Ecol 8(2):14–21
Varol S, Davraz A (2015) Evaluation of the groundwater quality with WQI (Water Quality Index) and multivariate analysis: a case study of the Tefenni plain (Burdur/Turkey). Environ Earth Sci 73(4):1725–1744. https://doi.org/10.1007/s12665-014-3531-z
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Rajiv Ganthi R, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2010) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess 171(1–4):595–609. https://doi.org/10.1007/s10661-009-1302-1
WHO (2004) World Health Organisation: guidelines for drinking water quality, third edition, vol. 1. Recommendations. In: World Health Organization. https://www.who.int/water_sanitation_health/dwq/GDWQ2004web.pdf. Accessed July 2022
WHO (2011) Water quality for drinking: WHO guidelines. Encycl Earth Sci Ser. https://doi.org/10.1007/978-1-4020-4410-6_184
Yan CA, Zhang W, Zhang Z, Liu Y, Deng C, Nie N (2015) Assessment of water quality and identification of polluted risky regions based on field observations & GIS in the Honghe River Watershed. China Plos One 10(3):1–13. https://doi.org/10.1371/journal.pone.0119130
Author information
Authors and Affiliations
Contributions
Conceptualization, OOK. Writing, OEO. Review, editing, and supervision, OOK. All authors have read and agreed to publish the version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Obayemi, O.E., Komolafe, O.O. Environmental assessment of Esa-Odo reservoir based on water quality index and multivariate analysis. Sustain. Water Resour. Manag. 9, 135 (2023). https://doi.org/10.1007/s40899-023-00913-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-023-00913-3