Abstract
An important consequence of groundwater overexploitation is worsening water quality. Water that is extracted at greater depths may contain higher concentrations of chemical elements, most of which come from the aquifer bedrock. This situation can cause health problems especially when the water is destined for drinking use. The study area is known as Silao-Romita aquifer (SR), which is located in the centre–west of the state of Guanajuato. The objective of this project was to determine the origins of the total inorganic arsenic (iAs) and fluoride (F−) present in the groundwater of SR through spatial distribution and hydro-chemical analysis techniques. Physicochemical parameters from the data base of Guanajuato’s State Water Commission (CEAG) were used for 25 well water samples. It was determined that the highest concentrations of iAs and F− were located in the south of SR. Approximately 28% of the samples exceeded the Maximum Permissible Limit (MPL) given for iAs in the Mexican regulations and 8% of the samples exceeded the MPL given for F−. High concentrations of iAs and F− were found on altered rhyolitic rocks with the presence of hydrothermalism. The water had temperatures of between 22 and 36 °C and pH values between 7.1 and 8.1. The predominant type of the water was bicarbonate-calcium and bicarbonate-sodium. The correlation between physic-chemical parameters and geology suggested that the presence of iAs and F− is due to the dissolution of acidic volcanic rocks and to the extraction of ancient/deeper waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is an extremely important resource in countries with arid and semi-arid regions, as it becomes the main source of supply for the population. In Mexico, there is a marked difference in rainfall due to seasonality and topography. In the north of Mexico, arid and semi-arid regions are predominated with annual rainfall less than 500 mm/year. While in the south of Mexico, temperate and humid climates predominate with annual rainfall that exceeds 2000 mm/year. Approximately, 16% of the total aquifers in Mexico are overexploited. This situation becomes a very important problem, because this overexploitation occurs in the center and north of the country, where most of the population is located and provides the highest contribution to the country’s Gross Domestic Product (CONAGUA 2018a).
The intense groundwater extraction during the last 3 decades has caused important alterations in the hydrogeological balance. The main consequences of intense overexploitation of water are the lowering of the water level, the reduction of spring and river discharges, a deterioration of the ecosystems that depend on the shallow phreatic level and increasing intensity and extent of the subsidence phenomenon (Navarro de León et al. 2005).
Another important consequence of overexploitation is a worsening of the quality of water available for human consumption, because of extracting water from greater depths implies that the water is mostly mineralized due to the time of contact that it has had with the geological material present and the temperature of the environment. Due to their concentrations in the Earth's crust, As and F are elements that are usually present in high concentrations in groundwater. The health problems associated by consuming water with high concentrations of As are generally dermal lesions such as hyperpigmentation, skin cancer, cardiovascular, renal, hematological and respiratory problems. Epidemiological evidence of consuming water with high concentrations of F−, carry an increasing risk of dental fluorosis and that progressively higher concentrations lead to increasing risks of skeletal fluorosis. In addition to other conditions such as development of a greater susceptibility to kidney diseases and cancer as well as an effect on the development of the human brain, reducing, among other effects, the IQ of children of school age (Smedley and Kinniburgh 2002; WHO 2017).
To reduce the negative effect of the high concentrations of heavy metal or other elements and offer drinking water with quality, many water purification project have been involved in drinking water system (Wang et al. 2019; Sandoval et al. 2019; Panagopoulos 2021), which are normally high cost for installation and operation. And many areas are looking for alternative water resources like desalination sea water, etc. (Wang et al. 2021). While the purification systems need energy and will leave more environmental problems as residuals, chemicals, gases and so on (Panagopoulos and Haralambous 2020; Panagopoulos et al. 2019). Even solar energy can be used in the purification systems (Wang et al. 2020, 2021), it is a much better option if we can slow down the water drawdown by a better management of water use. For a better management of water resource, the first stage is to diagnostic the problem with physical and chemical studies.
There are different regulations that determine the concentrations of elements contained in water, which is destined for drinking use, for example the Maximum Permissible limits (MPL) established by the World Health Organization. In Mexico, the corresponding regulations is the NOM-127-SSA1-1994 modified in the year 2000. Concentrations above those given in the Mexican water quality regulations for some elements such as As (25 μgL−1) and F− (1.5 mgL−1) have been detected in some aquifers in northern and central Mexico. In the Comarca Lagunera Region, which is part of the states of Coahuila and Durango, the presence of As has been detected in the groundwater. Evaporation is thought to be the main mechanism responsible for increasing the concentration of this element, worsened by intensive groundwater exploitation and the construction of dams that prevent the flow of the main rivers in the region (Gutiérrez-Ojeda 2008). In the state of Chihuahua, the presence of As is related to the recharge zone, which is constituted by andesitic-basaltic volcanic rocks (Reyes-Cortés et al. 2012). In Mexico, it is common to find As associated with Ag, Pb and Zn sulfides. In addition, in some areas there are significant amounts of As minerals such as arsenopyrite, scorodite, mimetite, adamite and tenantite, as in Zimapán and San Luis Potosí (Armienta and Rodríguez 1996). In Baja California, the presence of As is controlled by adsorption processes in iron oxyhydroxides, and the precipitation and dilution of calcite (Carrillo-Chávez et al. 2000). As is also present in the groundwater of several geothermal areas of Mexico, such as Puebla, Jalisco, and Michoacán. Concentrations in geothermal fluids are related to dissolution and exchange processes between hot fluids and the rock matrix (Birkle and Merkel 2002). In the state of Morelos, high concentrations of F− occur due to the chemical weathering of the intermediate and felsic igneous rocks that make up the region (Huízar et al. 2016). In the city of Durango, high concentrations of F− have been related to the mineralogical composition of the aquifer and to industrial activity. In San Luis Potosí, the concentration of F− in water is due to the solubility of fluorite, which is present in volcanic rocks (Carrillo-Rivera et al. 2002). In the state of Guanajuato, high concentrations of F− and As in groundwater have been associated with the erosion of volcanic rocks present in aquifers, the dissolution of fluorite, the oxidation of arsenic-bearing sulfide minerals and intensive pumping (Mahlknecht et al. 2004a; Ortega-Guerrero 2009; Morales-Arredondo et al. 2016; Knappett et al. 2018, 2020).
Due to its geographical position and climatologic conditions, in the state of Guanajuato, groundwater is the main source of supply for the population. In the state of Guanajuato, 14 of the 18 aquifers established by CONAGUA are overexploited (CONAGUA 2018b). According to Guanajuato’s State Water Commission (Comisión Estatal del Agua de Guanajuato, CEAG), there are 15,297 active wells, of which 84% are for agricultural use, 13% for urban public use and 3% for industrial and commercial use. Approximately, 3,824 million cubic meters of water are extracted per year, with 2783 million cubic meters recharge. This implies a deficit of 1041 million cubic meters of water annually (CEAG 2016). In some regions of the state of Guanajuato, the average water table has decreased between 2 and 3 m per year (Wester et al. 2011), and the mean water drawdown in all the state has been reported as 1.57 m per year (Li et al. 2020).
As mentioned previously, the presence of As and F− has been reported in some regions of the state of Guanajuato. However, the existence of them in regions like Silao-Romita aquifer has not been studied, less the mechanism of the presence of the high concentrations. Silao-Romita aquifer is a very important aquifer in the Guanajuato State for it supplies water for more than one million inhabitants (INEGI 2020) and supports the pillar industry, auto production and service. Therefore, the objectives of this study were (i) quantify the concentration of the total inorganic arsenic (iAs) and fluoride (F−) in the groundwater of the Silao-Romita aquifer, (ii) identify the spatial distribution of the concentration of these elements in groundwater, (iii) determine the main hydro-chemical processes that define the origin of these elements in the groundwater of the study aquifer.
Materials and methods
Study area
The study area, Silao-Romita aquifer (SR), is located in the west of the central region of the state of Guanajuato (Fig. 1). It covers an approximate area of 1899 km2. The area includes the municipalities of Guanajuato, Romita, Silao and part of Irapuato. SR is hydrologically delimited by the Sierra de Guanajuato (north-east and east), Valle de León (north-west), Sierra de Pénjamo (south) and Cerro El Veinte and Cerro Arandas (south-east), as well as hills in the west. There are some hills of volcanic origin within the valley (Lesser 1998). The elevations within the area range from 1700 to 2900 m above sea level (m a.s.l.) (Horst et al. 2007; INEGI 2011). The main rivers are the Guanajuato River and Silao River, which drain from the north to the south of SR. The Guanajuato River originates approximately 10 km from the city of Guanajuato and its waters are retained and regulated by the La Purísima Dam. The Silao River originates in the northern portion of the aquifer and intersects with the Guanajuato River in the north-west of Irapuato. The mixture flow draw into the El Conejo Dam (Lesser 1998; CONAGUA 2018c). Due to the increasing overexploitation of regional aquifers and the semi-humid conditions, some rivers in SR have become intermittent (Lesser 1998; Horst et al. 2007). Recharge to the aquifer occurs principally through natural rainwater infiltration, with recharge zones located in the main mountain ranges (Sierra de Guanajuato). Horizontal recharge also occurs in mountainous areas to the east and west of SR. Rivers and reservoirs also contribute a small amount to recharge, as does irrigation return in the more granular areas of the aquifer. Discharge from the aquifer is mainly associated with the extraction and is also influenced a bit by springs (Lesser 1998; Horst et al. 2007; CEAG 2018).

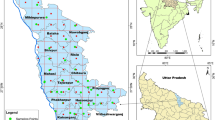
Source: Sampling sites taken from own data of 2019, and geological units taken from the Mexican Geological Service (SGM 1997, 1999)
Geographic location of the Silao-Romita aquifer (SR) with municipal capitals, water bodies, streams, geological faults, mines, sampling sites, and geological units. Ultramafic unit (JsUm), Diorita Tuna Mansa (Js/D), granitic (TpgGR), gabrotic (ToGa), Tonalite Cerro Pelón (JsTn), Formación la Esperanza (JsVs), Formación Guanajuato (TpaeCgp), andesites (ToA), latites (ToLa), rhyolites (ToR), rhyolitic tuffs (ToTR), rhyolite-ignimbrite (ToR-Ig) and rhyolite-rhyolitic tuff (ToR-TR), andesitic and basaltic lavas (TmA-B), sands-silts-conglomerates (TmAr-Cgp), spills-pyroclasts of basaltic composition (QB and QptTB-Ar).
Thirteen climatological stations, monitored by the National Meteorological System (SMN for its acronym in Spanish) of CONAGUA, are located in the study area. According to the Normal values of these station, the north-east of SR (near to Sierra de Guanajuato) experiences the highest annual precipitation in this area, 734.06 mm and the temperatures varies between 10.99 and 24.66 °C. In the north-west of the region, the temperature varies between 10.1 and 26.3 °C, with an annual precipitation of 615 mm. Between Silao and Romita, the temperature varies between 10.75 and 26.85 °C, with an annual precipitation of 640 mm. In the south-east, the temperature varies between 9.7 and 27.8 °C, with an annual precipitation of 699 mm. The lowest rainfall is recorded in the east of the valley, with a value of 354 mm, the temperature varies between 10.4 and 27.5 °C (SMN 1981—2010). In general, the annual temperature in SR is between 18.5 and 19 °C, the annual precipitation is between 622 and 640 mm, the annual evapotranspiration is 516 mm, and the average annual potential evaporation is between 1890 and 1923 mm (Horst et al. 2007; CONAGUA 2018c). Semi-arid temperate and sub-humid temperate are the predominant climate types in SR. The semi-arid temperate climate type is present in the valley of SR, and the sub-humid temperate climate type is predominant in the Sierra de Guanajuato and Cerro El Veinte (INEGI 2005).
According to the edaphological chart, the soil types present in SR, in order of greatest predominance, are: Vertisol, located in the south, center and east; Phaeozems, with Haplic and Luvic subunits—the first located in the center, west and north of SR, and the second predominantly in the east; Chromic Luvisol located in the north; Eutric Cambisol in north-west and north-east; and Kastanozems with Calcium and Luvic subunits, located in center-west and north-east, respectively (INEGI 2004). The main land use in SR is agriculture, which is reflected in the percentage of groundwater destined for agriculture. Of the total volume of groundwater extraction in SR, 90% is destined for agricultural use, 9% for urban public supply use and 1% is for self-supplied industry use (CEAG 2018). The predominant vegetation is pine and oak forests, crasicaule scrub, natural and induced grassland and deciduous forest. This vegetation features mainly in the Sierra de Guanajuato in the north, and in the Cerro Arandas and Cerro El Veinte in the south (INEGI 2017).
SR is located between the physiographic regions of the Mesa Central (MC) and the Trans Mexican Volcanic Belt (TMVB). During the Middle- and Late-Cenozoic, the structures developed were mainly faults and fractures. The relationships between these faults and some stratigraphic units allowed them to be distinguished as resulting from several faulting events in the Paleocene–Eocene to Pliocene–Pleistocene. In the Oligocene, an increase in the rate of volcanism occurred, related to subduction tectonics in the west of Mexico. These events led to the reactivation of faults and the generation of the grabens and pillars (Alanis-Ruiz 2002; Nieto-Samaniego 2005). SR is divided into two geological groups: the older one is formed of Jurassic to Cretaceous marine volcanic and sedimentary rocks, tonalite, diorite, basaltic and an outcrop of ultramafic rocks. These rocks are located in the Sierra de Guanajuato. The second one includes alluvial deposits and volcanic rocks with age of Tertiary to Quaternary. These rocks are predominantly located in the valleys and hills of the north, west and south, and the regional aquifer is also composed of these rocks (Nieto-Samaniego et al. 2016).
The geological succession begins with an Ultramafic unit, San Juan de Otates (JsUm), which consists of ultrabasic and basic rocks. They are formed by serpentinites, peridotites, serpentinized clinopyroxenites and gabbro with accumulation textures. The age of this unit corresponds to Upper Jurassic. This unit is followed by Diorite Tuna Mansa (Js/D), which designates different crystalline facies of rocks of dioritic composition, essentially granitic (TpgGR) and gabrotic (ToGa). The ages determined by k/Ar are between 101 ± 3 Ma and 122.5 ± 5.6 Ma. And its age corresponds to the Upper Jurassic. Guanajuato Conglomerate (TpaeCgp) consists of a polymictic conglomerate composed of conglomerates with quartz, limestone, granite, and andesite fragments, cemented by a clay matrix. Its thickness is estimated at 1500 m. The age for this unit varies from Paleocene to lower Oligocene. During the Oligocene, volcanic activity was generated that caused the deposit of two igneous sequences. The first of these is of intermediate composition, with the second of acid composition. The units corresponding to both sequences are: andesites (ToA), latites (ToLa), rhyolites (ToR), rhyolitic tuffs (ToTR), rhyolite-ignimbrite (ToR-Ig) and rhyolite-rhyolitic tuff (ToR-TR). The age of this units varies between 29 ± 0.8 Ma and 30 ± 1.5 Ma. During the Miocene, andesitic and basaltic lavas were present (TmA-B), and their age is related to the age of the Zamorano Volcano and Palo Huérfano volcano with ages of 10.9 ± 0.5 Ma and 16 Ma, respectively. Sands, silts and conglomerates with thicknesses of approximately 60 m (TmAr-Cgp) were deposited in the Neogene, located discordantly on andesitic spills and rhyolitic tuffs. The last stage of volcanism was of basaltic composition (QB and QptTB-Ar), with spills and pyroclasts. The last unit corresponds to lake deposits of the Quaternary. The epithermal auroargentiferous mineral deposits are characterized as being of the vetiform type in the Guanajuato Mining District, located north-east of SR. The main deposits contain gold and silver mineralization, followed in order of importance by Pb, Zn and Cu, as well as small deposits of W and Mn. The ore deposited was produced by geological events, characterized by volcanism to produce intense fracturing, setting the stage for hydrothermal activity and mineralization. Hydrothermal activity lasted for approximately two million years due to the magmatic episode that formed the Chichíndaro Rhyolite, which buried and trapped the very permeable breccias and faulted rocks hosting the Guanajuato Mining District. There are deposits of kaolin, which were formed from strong hydrothermal alterations, and are also associated with sites of rhyolitic domes (SGM 1997; Lesser 1998; SGM 1999; Nieto-Samaniego et al. 2016; CEAG 2018).
Data analysis
The water quality database used in this study were provided by the Guanajuato’s State Water Commission (Comisión Estatal del Agua de Guanajuato, CEAG). The CEAG has a groundwater quality monitoring network. The laboratory analysis is carried out by a certified laboratory, which is hired by the CEAG. The SR aquifer water quality database is made up of a monitoring network of 25 water wells distributed along the SR aquifer. For this study project, the analysis carried out in 2019 were used (Fig. 1). The database used in this project included the following parameters: temperature (°C), pH, Specific Conductivity (μScm−1), alkalinity (mgL−1 CaCO3), major ions (mgL−1), arsenic (mgL−1) and fluoride (mgL−1).
Hydro-chemical analysis
A hydro-chemical analysis is useful for determine the quality of the water, its interaction with the environment, mixtures of different waters along a flow line and its origin. This type of analysis provides a preliminary assessment of groundwater types and their relationship to lithology (Singhal and Gupta 2010). The charge balance error consider for the samples was less than 5%. Geochemist's Workbench (Student edition) was used to determine the type of water family. It is a software to perform numerical and graphical analyses that are used to interpret water quality data. A Stiff Diagram was used to represent the family type of SR water samples, for it allows to visualize and compare the different families’ water with the spatial variation. A correlation analysis was performed to evaluate the strength and direction of the relationship of iAs and F− with other physical–chemical parameters analyzed. A high correlation value indicates that the elements measure the same characteristic. If the elements are not highly correlated, it indicates that they could measure different characteristics or are not clearly defined.
Spatial distribution analysis
A spatial distribution analysis was carried out with the use of ArcMap 10.5, to determine the distribution of the concentrations of iAs and F− in the groundwater, and to locate the areas with the highest concentrations. Special attention was given to those areas in which the statistical model predicted a concentration greater than the MPL established by the Mexican regulations (modification to NOM-127-SSA1-1994, 2000) and the MPL established by the WHO (WHO 2017). The Mexican regulations indicate that the MPL for iAs in water for human consumption is 25 µL−1 and for F− is 1.5 mgL−1, and the WHO indicates that the MPL of these elements is 10 µL−1 and 1.5 mgL−1, respectively. For this analysis, the Symbology (Graduated Symbols) visualization tool from ArcMap 10.5 was used.
Results
Hydro-chemical analysis
The temperature of the water samples showed a range between 22 and 36 °C, with an average of 26.20 °C. Of the total samples analyzed, 28% showed a temperature greater than or equal to 29 °C and were in the center and south of SR. The pH corresponded to neutral to alkaline values. The pH values varied between 7.1 and 8.1, with an average of 7.57. The Specific Conductivity values varied between 314 µScm−1 and 3512 µScm−1, with an average of 792.58 µScm−1. The highest values of Specific Conductivity were in the valley of SR. The order of predominance for cations in the water samples was sodium > calcium > magnesium > potassium. While the order of predominance for anions was bicarbonate > sulfate > chloride. Table 1 shows the minimum, maximum and average values of the main physicochemical parameters measured in the wells studied and compares these with the MPLs established by the Mexican regulations and the WHO.
Water family
An analysis was carried out to determine the type of water family of the collected samples and to determine their geochemical processes. The Stiff diagram was used to visualize the different types of water depending on spatial variation (Fig. 2). According with the concentration of major ions 52% of the samples analyzed showed a bicarbonate-calcium water type, 20% of the samples showed a bicarbonate-calcium-sodium water type, 16% of the samples showed a bicarbonate-sodium water type and 12% of the samples showed a bicarbonate-chloride-sodium water type. Bicarbonate-calcium water type samples were in the northern and east zone of the SR, where the main recharge zone (Sierra de Guanajuato) is located. In addition, this area is influenced by rocks of the basalt and andesite type. Bicarbonate-calcium-sodium water type samples were in the center and south of SR. This type of water represents a mixture of different waters, but with a predominance of calcium over sodium. These samples may be influenced by the andesitic-basaltic composition of Cerro El Veinte, located south of SR. In the SR valley, bicarbonate-sodium water type samples were predominant. Water samples of the bicarbonate-chloride-sodium type were in the south-east of SR.

Source: The Digital Model of Elevation ranges taken from INEGI (2011), and the rests are own data
Water samples from SR classified according to Stiff diagram. The white diagrams present an overview of the characteristics of the water. Other details shown include geological faults, the main bodies of water, streams, municipal capitals, equipotential lines, flow directions, mines and the Digital Model of Elevation ranges.
Distribution of the concentrations of iAs and F− in the groundwater of SR
Figure 3 shows the distribution of the concentration of iAs. The colors and size of the markers are based on the MPLs by the aforementioned regulations. Green markers indicate those samples whose concentration was 10 µgL−1 or less, yellow markers indicate those samples whose concentration was greater than 10 µgL−1 but less than 25 µgL−1, and red markers indicate those samples whose concentration exceeds 25 µgL−1. Samples with concentrations greater than 25 µgL−1 were located mainly in the south of SR. The sample with a concentration of 1077.60 µgL−1 was taken from P14 located in the south-east of SR. Of the 25 well water samples analyzed, approximately 44% of them had iAs concentrations above the MPL established by the WHO, and 28% of them above the MPL established by the Mexican regulations.

Source: The Digital Model of Elevation ranges taken from INEGI (2011), and the rests are own data.
Distribution of the concentration of iAs in the groundwater of SR in 2019. High concentrations are distinguished in red, medium in yellow and low in green, according to the MPLs established by the Mexican regulations and the WHO for drinking water consumption. Also shown are geological faults, the main bodies of water, streams, the municipal capitals, equipotential lines, flow directions, mines and the Digital Elevation Model ranges.
Figure 4 shows the distribution of the concentration of F−. Similarly, the colors and size of the markers are based on the MPLs by the aforementioned regulations for F−. Green and yellow markers indicate those samples whose concentration was 1.5 mgL−1 or less. Green markers indicate those samples whose concentration was 1.0 mgL−1 or less, and yellow markers indicate those samples whose concentration could be at risk of exceeding the MPL. Red markers indicate those samples whose concentration exceeds 1.5 mgL−1. Sites with sample concentrations greater than 1.5 mgL−1 were mainly located in the south of SR. In the case of F−, of the 25 well water samples analyzed, approximately 8% of samples exceeded the MPL established by the Mexican regulations and the WHO.

Source: The Digital Model of Elevation ranges taken from INEGI (2011), and the rests are own data
Distribution of the concentration of F− in the groundwater of SR in 2019. High concentrations are distinguished in red, and low in yellow and green, according to the MPLs established by the Mexican regulations and the WHO for drinking water consumption. Also shown are geological faults, the main bodies of water, streams, the municipal capitals, equipotential lines, flow directions, mines and the Digital Elevation Model ranges.
Relationship of iAs and F− with other physicochemical parameters
Correlation analysis
To explain the relationship between different elements, a correlation matrix was produced, shown in Table 2. A high correlation coefficient (> 0.5) indicates a strong correlation between two variables, and a low correlation coefficient (< 0.5) indicates a lower correlation. Concentrations of iAs showed a low negative correlation with HCO3− (R2 = − 0.02), a high positive correlation with Na+ (R2 = 0.73), a moderate negative correlation with Ca2+ (R2 = − 0.54), and a low positive correlation with SO42− (R2 = 0.24), a moderate positive with pH (R2 = 0.54) and low positive with temperature (R2 = 0.29). Concentrations of F− showed a low negative correlation with HCO3− (R2 = − 0.03), a high positive correlation with Na+ (R2 = 0.68), a moderate positive correlation with Ca2+ (R2 = 0.43), a low positive correlation with SO42− (R2 = 0.22), a high positive with pH (R2 = 0.66) and moderate positive with temperature (R2 = 0.44). The correlation between both elements was high positive (R2 = 0.68). Figures 5 and 6 show the relationship between the concentrations of iAs and F− with the concentration between the main cations and anions, respectively. The P14 well located south-east of SR was analyzed separately from the other wells, because its concentration was considered as an outlier of the sample set, the concentration of its major ions is shown in Fig. 7.
Temperature and pH are parameters that are closely related to the presence and mobility of iAs and F− in groundwater. Therefore, an analysis was performed to determine the correlation between these. Figure 8a shows the relationship between of the concentration of iAs with temperature and pH. The iAs concentration showed a low positive correlation with temperature and moderate positive correlation with pH: R2 = 0.29 and R2 = 0.54, respectively. Samples with concentrations of iAs above the MPL of the Mexican regulations, showed a temperature range of 23–30.7 °C. However, samples were taken with recorded temperatures of approximately 25–34 °C that showed concentrations within the MPL of the aforementioned regulations. The sample with the highest temperature recorded (36 °C) had a concentration of iAs of 23.60 µgL−1, which is below the MPL of the Mexican regulations, but above the limit established by the WHO. The highest concentration of iAs had the most alkaline pH recorded.
Figure 8b shows the relationship between of the concentration of F− with temperature and pH. The F− concentration showed a high positive correlation with pH and moderate positive with temperature: R2 = 0.66 and R2 = 0.44, respectively. Samples with high concentrations of F− showed a temperature range of 25–30 °C. However, samples were taken with recorded temperatures of approximately 25–36 °C that showed concentrations within the MPL of the aforementioned regulations. The concentration of F- was higher with pH greater than 7.7.
A spatial distribution analysis was performed to locate the areas in which the highest recorded water temperature and pH were found. Figure 9 shows the distribution of the temperature recorded in the groundwater for the 25 samples analyzed. Samples whose maximum recorded temperature was 25 °C (room temperature) are shown in green. According to the Geological Mining Institute of Spain (IGME, by its acronym in Spanish), to be considered thermal springs, wells must have an annual average water temperature of at least 4 °C above the temperature at the place of emergence. Under this definition, wells considered thermal are distinguished in orange and red. The high temperatures predominated in the south and center of SR.

Source: The Digital Model of Elevation ranges taken from INEGI (2011), and the rests are own data
Distribution of the groundwater temperature in SR in samples taken in 2019. Samples whose maximum recorded temperature was 25 °C (room temperature) are shown in green, and samples with temperatures considered thermal according to IGME are distinguished in orange and red, with the colour indicating the increase in temperature from orange to red. Also shown are geological faults, the main bodies of water, streams, municipal capitals, equipotential lines, flow directions, mines and the Digital Elevation Model ranges.
Figure 10 shows the distribution of the pH recorded in the groundwater of the 25 samples analyzed. Neutral samples in yellow, slightly alkaline samples in light green and alkaline samples in dark green. The most alkaline values were recorded in the south and center of SR, which spatially coincide the highest concentrations of F− and iAs.

Source: The Digital Model of Elevation ranges taken from INEGI (2011), and the rests are own data
Distribution of groundwater pH in SR for samples taken in 2019. Neutral samples are shown in yellow, slightly alkaline samples in light green and alkaline samples in dark green. Also shown are geological faults, the main bodies of water, streams, municipal capitals, equipotential lines, flow directions, mines and the Digital Elevation Model ranges.
Discussion
The hydrogeological behavior of the SR plays an important role in the hydrochemistry of the groundwater. In SR, the prevailing rocks are basic rocks like basalts, intermediate compositions like andesitic rocks, and acidic volcanic rocks like rhyolites and ignimbrites. Andesitic rocks are characterized by their Ca and Mg content due to their intermediate composition, while acidic volcanic rocks such as rhyolites and ignimbrites, due to their felsic composition, have a higher content of Na and K (Tarbuck and Lutgens 2005). The influence of this type of rock is reflected in the water chemistry, and as shown in Fig. 2, where the bicarbonate-calcium and bicarbonate-sodium water type prevails. As explained in Singhal and Gupta (2010), when the minerals present in these rocks dissolve in the water, they cause the release of cations such as Ca, Mg, Na, and K. This situation is also observed in Fig. 5, as the concentration of Na increases, the concentration of Ca and Mg decreases. This is also demonstrated in the results presented in Table 1, where Na, Ca, Mg, and K are found in order of predominance. In order of predominance, the anions are HCO3, SO4, and Cl, and this also gives an indication that the composition of the water comes from the interaction of water with rock. This information provides an idea of the geochemical evolution of groundwater—as Chebotarev explained in 1955, natural groundwater tends to evolve towards the composition of seawater, and chemical evolution in terms of the dominant anions follows the following sequence:
Changes occur as water moves from recharge zones, to discharge zones, and the water is therefore older in geologic time. Water dissolves the minerals that it finds until it reaches the limit set by the corresponding equilibrium constant. When that happens, it will no longer dissolve that mineral, and it will instead continue to dissolve other minerals that have a higher constant until it reaches equilibrium again and so on (Chebotarev 1955). According to this information, it could be concluded that the water being extracted in SR is a mixture of recently infiltrated water and deeper water. The bicarbonate-sodium water type samples and bicarbonate-chloride-sodium type were in the south and south-east of SR, where the higher concentration of iAs and F− were recorded. This hypothesis is supported by the results obtained by Horst et al. (2008), where residence time in groundwater was calculated using chlorofluorocarbon, radiocarbon and tritium. The most reliable estimation of the residence time was obtained by interpreting the tritium activities. The results showed mean residence times of 70 to over 300 years, applying the exponential flow model. Although it is important to mention that, in at least one case, the existence of groundwaters with residence times of more than 10,000 years was found, and some other groundwaters were found with residence times between 400 and 1700 years, using radiocarbon. The increase in Specific Conductivity is also related to this situation due to the dissolved ions during the water flow path, in the deeper aquifers the salinity is generally higher. The concentration of nitrates in general was low in the samples analyzed, its origin is attributed to agriculture, which occupies the main land use in SR. Fertilizers used in agriculture and urban septic leachates are the main sources of nitrate concentrations in both surface and groundwater (Singhal and Gupta 2010).
The highest concentrations of iAs and F− are found mainly towards the south, east and west of SR and their presence is mainly related to local geology. The rocks that predominate in the valleys and hills of the north, west and south correspond to alluvial deposits, basalts, and volcanic rocks with age of Tertiary to Quaternary (Nieto-Samaniego et al. 2016). According to data obtained through geophysical and geological analysis by Lesser (1998), in the south of the valley of SR, the basement of the aquifer corresponds to altered rhyolites, which were mainly characterized by hydrothermalism. Quaternary material is found on the rhyolites with thicknesses ranging from 150 to 450 m. Volcanic rocks can contain As in their structure, and As concentrations in rhyolites can range from 3.2 to 5.4 mg kg−1 (Smedley and Kinniburgh 2002). There are very few studies related to the water quality of the SR aquifer. Most of them are focused on sediments and surface waters, especially in the north of SR due to the presence of the Guanajuato Mining District. The gangue mineralogy reported is mostly quartz, feldspar, pyrite, calcite, and clays (kaolinite, smectite, and chlorite). The ore is disseminated in sulfides and sulfosalts, such as pyrite (FeS2), galena (PbS), chalcopyrite (CuFeS2), sphalerite (ZnS), polybasite (Ag, Cu) (16Sb2S11), native silver, a solid solution of acanthite-aguilarite-neumanite (Ag2SAg4SeS-Ag2Se), and electrum (Miranda-Avilés et al. 2012). These sulfide minerals can contain As and it is known that the chemistry of As and S is very similar. Therefore, it is common to find As in sulfurous environments (Smedley and Kinniburgh 2002). When these minerals contact with the atmosphere, they oxidize, and As is released. The following reaction (1) is an example of this mechanism (arsenopyrite oxidation):
As mentioned previously, most of the water quality studies in SR focus on surface waters such as river water or dams, in addition to soils and sediments, and few have addressed the water in wells. High concentrations of As, Cu, Pb, Cs, Sb and Zn have been reported in sediments and it has been determined that the greatest contribution of heavy metals occurs in areas near mines and mineral deposits in the Guanajuato Mining District (Miranda-Avilés et al. 2012; Solis-Reyes et al. 2018). The results of sampling from various reservoirs and tailings dams determined that the most of them are not releasing potentially toxic elements into the environment. Only in the case of one tailings dam did the concentrations of As, Pb and Cr exceed the corresponding maximum permissible limits (Esparza-Claudio and Córdova 2016). The presence of As has been also determined in the first soil horizons near the south-east of SR, and these soils are irrigated with groundwater rich in As. Horizons richer in clay-sized particles inhibit the downward migration of As, while Fe oxides form as a product of mineral weathering during the soil formation and organic matter likely scavenge any As by adsorption (Zanor et al. 2019). Cano-Rodríguez et al. (2000) evaluated the water quality of some wells in Puentecillas and in the La Purísima dam. No metals were determined in the well water, only in sediments, rivers and in the dam. Concentrations above the MPL of As, Pb, Hg, and Se were found in the dam. High concentrations of phosphorus were found only in the well water.
As for F−, it is known that there are also non-metallic mineral deposits in the area, such as deposits of kaolin, which was formed from strong hydrothermal alterations. These deposits are also associated with the sites of rhyolitic domes. The presence of fluorine in topaz and biotite, which are found in the rhyolite rocks that are part of MC, has been corroborated (SGM 1999; Orozco-Esquivel et al. 2002), as that has the presence of topaz in rhyolites in San Luis Potosí (Aguillón-Robles et al. 1994). Their temporal and spatial relationship suggests that they come from the same magmatic chamber as rhyolites and the rhyolites which are from the basin north-east of SR (Parga 2003).
As mentioned in Singhal and Gupta (2010), the presence of As, Fe, Pb, Zn, Hg, low pH, and high SO4 are related to mining and related activities. The results obtained show very low correlation of the iAs concentration with Fe2+ (R2 = − 3.94E−17) and low correlation with SO42− (R2 = 0.24). For this reason, the presence of iAs is not attributed to the oxidation of sulfide minerals. In addition, in the wells nearest to the Guanajuato Mining District, the concentration of iAs tended to be below the MPL of the Mexican regulation.
On the other hand, the hydro-chemical results showed a high positive correlation of the concentration of iAs with Na+ (R2 = 0.73) and a low negative correlation with HCO3− (R2 = − 0.02), a moderate negative correlation with Ca2+ (R2 = − 0.54). The results showed a low negative correlation of the concentration of F− with HCO3− (R2 = − 0.03), a high positive correlation with Na+ (R2 = 0.68), a moderate positive correlation with Ca2+ (R2 = 0.43) and a low positive correlation with SO42− (R2 = 0.22). A high positive correlation was found between iAs and F− (R2 = 0.68), which could indicate that they may have same sources of origin. Additionally, the type of water that prevailed in samples with high concentrations of these elements was bicarbonate- sodium. Therefore, it is suggested that the presence of iAs and F− is due to the dissolution of volcanic rocks, especially rhyolites.
It is hypothesized that iAs and F− are adsorbed on the volcanic rock, which then begins to dissolve when it meets water at high temperature and pH conditions. The following reaction (2) is an example of this mechanism (Biotite dissolution according to Pettenati et al. 2013):
At very alkaline pH (greater than 8.1), As and F− desorption of the geological material occurs (Dzombak and Morfel 1990; Carrillo-Rivera et al. 2002; Smedley and Kinniburgh 2002). The results obtained show a high positive correlation of the concentration of iAs with pH (R2 = 0.54), and a moderate positive correlation of the concentration of F− with temperature (R2 = 0.44) and a high positive correlation with pH (R2 = 0.66), respectively.
The following reaction shows the process of dissolution of albite, which was recorded as present in the volcanic rocks from the basin north-east of SR (Mahlknecht et al. 2004b). Kaolinite has been shown to be present in SR (SGM 1999; Miranda-Avilés et al. 2012). Na and OH− ions, silicic acid (H4SiO4) and kaolinite (Al2Si2O5(OH)4) are integrated into the solution as a result of the dissolution of sodium feldspar, contributing to the increase in alkalinity and pH (Stumm and Morgan 1981). The following reactions (3 and 4) shows this process:
The presence of geology seemed to influence the concentration of iAs and F−. Therefore, it could be considered that the groundwater contains high concentrations of these elements because of deep thermal waters, which have had a long interaction time with volcanic rocks, and then mix with more recent infiltration waters and are transported to the surface through pumping. Due to the volcanism generated in the Oligocene, it is possible that there are underground geological faults that facilitate the transport of these elements in the extracted groundwater. Further analysis in this area is recommended to confirm this hypothesis. Possibly, some faults or geological structures exist near to the wells with the highest concentrations of these elements. Lesser (1998) also mentioned three connected aquifers: a shallow one, an intermediate one, and a deep one. The shallow and intermediate layers were overexploited in recent years and are depleted, so the main current source of water is the deep aquifer. The border between these layers is made up of numerous clay lenses, and this could influence the concentration of these elements in the water. As mentioned by Smedley and Kinniburgh (2002), As could be adsorbed to oxides or oxyhydroxides of the quaternary material. It is very important to continue with different analyzes of the water and the geology of the SR. Especially in areas where the concentration of these elements exceeded the MPL by the aforementioned regulations. Primarily in the south-east of SR in well P14, which showed the highest concentrations of Na, HCO3, SO4, Cl, iAs and F−. Figure 7 shows the concentrations of the largest ions in the water from this well. While in the other SR wells, the maximum concentration of Na, HCO3, SO4, and Cl was 152 mgL−1, 487 mgL−1, 95 mgL−1 and 86 mgL−1, respectively, and in P14 the concentration of the same ions was 689 mgL−1, 1245 mgL−1, 295 mgL−1 and 251 mgL−1, respectively. Furthermore, this well is characterized by its thermal temperature (30 °C) and alkaline pH (7.6). Probably in this area, there is some geological fault that allows the extraction of deeper groundwater. With the results presented here, it has not been attributed to anthropogenic contamination, because nitrate concentrations were relatively low (0.2 mgL−1). For this reason, it is extremely important to continue with the corresponding studies to determine the origin of these elements and reduce the risk to the health of the population.
Conclusions
The Silao-Romita aquifer is an important source of water supply since it supports more than one million inhabitants and auto industry of the state of Guanajuato. Unfortunately, there are no water quality studies for this aquifer.
Hydro-chemical characterization is carry out to evaluate the quality of the water and its interaction with the subsurface environment, to identify water–rock reactions, groundwater flow that occur in aquifer systems.
The results obtained in this study made it possible to determine the areas where there is a presence of hydrothermal flows, mainly in the center and south of the SR, and where there is an intensive pumping action. The alkaline pH values and the high Specific Conductivity values coincided spatially with the temperature, which made it possible to determine that the water extracted in this area is that more mineralized, that is, from deeper aquifers.
The analysis of the type of water family allowed to corroborate that there is a marked influence of the geological units present in the study area. The basalts, andesites, rhyolites and ignimbrites, cause the release of cations such as Ca, Mg, Na, and K. It also allowed the delimitation of the groundwater recharge zones, which were found near the Sierra de Guanajuato and Cerro El Veinte, and the discharge zones, which occurred in the SR valley.
Of the samples analyzed, approximately 44% showed concentrations of iAs above the MPL established by the WHO, and 28% showed concentrations of iAs above the MPL given in the Mexican regulations. Concentrations of F− in approximately 8% of the samples exceeded the MPL for both regulations. Sites with the highest sampled concentrations of iAs and F− were located mainly in the south, west and east of SR. These samples were taken from sites that tended to be located on altered rhyolitic rocks with presence of hydrothermalism. The type of water that predominated in samples with a high content of these elements was bicarbonate-chloride-sodium, therefore, according to the Cheboratev anion sequence, the water comes from deeper and older flows.
Due to the results obtained from the correlations between iAs, F− and the physicochemical parameters, it is suggested that the presence of iAs and F− is due to the dissolution of acidic volcanic rocks (rhyolites). Groundwater contains high concentrations of these elements, because deep thermal waters, which have had a long interaction time with volcanic rocks, mix with more recent infiltration waters and are carried upwards through pumping. The results obtained showed a low correlation between iAs with Fe and SO4, therefore, mining activities were dismissed as a source of As.
The difference in concentrations of iAs and F− in groundwater could also be related to the adsorption of these elements in oxides or oxyhydroxides present in the quaternary material, in addition to possible geological faults or underground fractures that facilitate the transport of these elements to the surface.
This project serves as the basis for future studies on the water quality of the SR aquifer. With the spatial distribution of the concentration of iAs and F−, it is possible to focus on areas with higher risk of water quality (high concentrations) for future hydrogeological studies. It also encourages further studies on the aquifer system in general, since there is a large lack of knowledge on the subject and of available information. To corroborate the hypotheses raised in this study, further work is necessary on additional hydrogeochemical analyzes and on the reactive transport model, which will offer very useful tool to resolve the water-related problems in the area.
Data availability
The data that support the findings of this study are available from the State Water Commission of the state of Guanajuato (CEAG), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the State Water Commission of the state of Guanajuato (CEAG).
References
Aguillón-Robles A, Aranda-Gómez JJ, Solorio-Munguía JG (1994) Geología y tectónica de un conjunto de domos riolíticos del Oligoceno Medio en el sur del Estado de San Luis Potosí, México. Revista Mexicana De Ciencias Geológicas 11:29–42
Alanis-Ruiz E (2002) Evolución geológica de la CI y sus alrededores, Estado De Guanajuato, México. Universidad Nacional Autónoma de México, México, México
Armienta M, Rodríguez R (1996) Arsénico en el Valle de Zimapán, México: Problemática ambiental. Revista Mapfre Seguridad 63:33–43
Birkle P, Merkel B (2002) Mineralogical-chemical composition and environmental risk potential of pond sediments at the geothermal field Of Los Azufres, Mexico. Env Geol 41:583–592. https://doi.org/10.1007/s002540100360
Chebotarev II (1955) Metamorphism of natural water in the crust of weathering. Geochim Cosmochim Acta 8:22–48 (137–170, 198–212)
Cano-Rodríguez I, Gómez-Vallejo F, Ramírez-Méndez V, Martínez-Barbosa P, Rodríguez-Rodríguez E, Aguilera-Alvarado A (2000) Determinación de contaminantes en la presa la Purísima y su efecto en el sistema de pozos puentecillas de Guanajuato. International Water Management Institute, Serie Latinoamericana De México 20:123–133
Carrillo-Chávez A, Drever JI, Martínez M (2000) Arsenic content and groundwater geochemistry of The San Antonio-El Triunfo, Carrizal and Los Planes Aquifers in Southernmost Baja California, Mexico. Env Geol 39:1295–1303. https://doi.org/10.1007/S002540000153
Carrillo-Rivera JJ, Cardona A, Edmunds WM (2002) Use of abstraction regime and knowledge of hydrogeological conditions to control high-fluoride concentration in abstracted groundwater: San Luis Potosí Basin, Mexico. J Hydrol 261:24–47. https://doi.org/10.1016/S0022-1694(01)00566-2
CEAG (2016) Agua subterránea. Comisión Estatal del Agua del Estado de Guanajuato
CEAG (2018) Compendio del agua subterránea en Guanajuato. Comisión Estatal del Agua de Guanajuato
CONAGUA (2018a) Estadísticas del agua en México, edición 2018. Comisión Nacional del Agua. México
CONAGUA (2018b) Acuerdo por el que se actualiza la disponibilidad media anual de agua subterránea de los 653 acuíferos de los Estados Unidos Mexicanos, mismos que forman parte de las regiones hidrológico-administrativas que se indican. Diario Oficial de la Federación. http://www.dof.gob.mx/nota_detalle.php?codigo=5510042&fecha=04/01/2018. Acceded 12 Aug 2019
CONAGUA (2018c) Actualización de la disponibilidad media anual de agua en el acuífero Silao-Romita (1110), Estado de Guanajuato. Comisión Nacional del Agua. México.
Dzombak D, Morfel F (1990) Surface complexation modeling-hydrous ferric oxide. Wiley-Interscience, New York
Esparza-Claudio JJ, Córdova SE (2016) Generación de escenario de potencial de contaminación en el acuífero Silao-Romita, en Guanajuato. Revista Internacional De Estadística 7:40–56
Gutiérrez-Ojeda C (2008) Arsenic origin determination by geochemical modeling: Region Lagunera Aquifer System, Northern Mexico. In: Bundschuh A, Birkle B, Matschullat M (eds) Natural Arsenic in Grounndwaters of Latin America, 1st edn. CRC Press, London, p 741
Horst A, Mahlknecht J, Merkel BJ (2007) Estimating groundwater mixing and origin in an overexploited aquifer in Guanajuato, Mexico, using stable isotopes (stronium-87, carbon-13, deuterium and oxygen-18). Isot Environ Health Stud 43:323–338. https://doi.org/10.1080/10256010701701756
Horst A, Mahlknecht J, Merkel BJ, Aravena R, Ramos-Arroyo YR (2008) Evaluation of the recharge processes and impacts of irrigation on groundwater using CFCs and radiogenic isotopes in the Silao-Romita basin, Mexico. Hydrol J 16:1601–1614. https://doi.org/10.1007/s10040-008-0318-x
Huízar ARJJ, Carrillo R, Juárez F (2016) Fluoruro en el agua subterránea: Niveles, origen y control natural en la región de Tenextepango, Morelos, México. Investigaciones Geográficas 90:40–58. https://doi.org/10.14350/rig.4734
INEGI (2004) Guía para la interpretación de la Cartografía Edafológica. Instituto Nacional de Estadística y Geografía. México
INEGI (2005) Guía para la interpretación de la Cartografía Climatológica. Instituto Nacional de Estadística y Geografía. México
INEGI (2011) Modelos Digitales de Elevación de Alta Resolución LiDAR, con resolución de 5m. Instituto Nacional de Estadística y Geografía. México
INEGI (2017) Guía para la interpretación de la Cartografía: Uso del Suelo y Vegetación escala 1:250,000: serie V. Instituto Nacional de Estadística y Geografía. México
INEGI (2020) Censo de Población y Vivienda 2020. Instituto de Estadística y Geografía. México
Knappett PSK, Li Y, Hernández H, Rodríguez R, Aviles M, Deng C, Piña V, Giardino JR, Mahlknecht J, Datta S (2018) Changing recharge pathways within in intensively pumped aquifer with high fluoride concentrations in Central Mexico. Sci Total Environ 622–623:1029–1045. https://doi.org/10.1016/j.scitotenv.2017.12.031
Knappett PSK, Li Y, Loza I, Hernandez H, Avilés M, Haaf D, Majumder S, Huang Y, Lynch B, Piña V, Wang J, Winkel L, Mahlknecht J, Datta S, Thurston W, Terrel D, Nordstrom K (2020) Rising arsenic concentration from dewatering a geothermally influenced aquifer in central Mexico. Water Res 185:116257
Lesser (1998) Estudio hidrogeológico y modelo matemático del acuífero Valle de Silao-Romita, Guanajuato. Lesser y Asociados S.A. de C. V
Li Y, Hernandez H, Aviles M, Knappett PSK, Giardino JR, Miranda R, Puy MJ, Padilla F, Morales J (2020) Empirical Bayesian Kriging method to evaluate inter-annual water table evolution in the Cuenca Alta del Río Laja aquifer, Guanajuato, México. J Hydrol 582:124517
Mahlknecht J, Steinich B, Navarro de León I (2004a) Groundwater chemistry and mass transfer in the Independence Aquifer, Central Mexico, by using multivariate statistics and mass balance models. Env Geol 45:781–795. https://doi.org/10.1007/s00254-003-0938-3
Mahlknecht J, Schneider J, Merkel BJ, Navarro de León I, Bernasconi SM (2004b) Groundwater recharge in a sedimentary basin in semi-arid Mexico. Hydrogeol J 12:511–530. https://doi.org/10.1007/s10040-004-00332-6
Miranda-Avilés R, Puy-Alquiza MJ, Pérez Arvizu O (2012) Anthropogenic metal content and natural background of overbank sediments from the mining district of Guanajuato, Mexico. Soil Sediment Contam Int J 21:604–624
Morales-Arredondo I, Rodríguez R, Armienta MA, Villanueva-Estrada RE (2016) The origin of groundwater arsenic and fluorine in a volcanic sedimentary basin in central Mexico: a hydrochemistry hypothesis. Hydrogeol J 24:1029–1044. https://doi.org/10.1007/s10040-015-1357-8
Navarro de León I, Gárfias-Soliz J, Mahlknecht J (2005) Groundwater flow regime under natural conditions as inferred from past evidence and contemporary field observations in a semi-arid basin: Cuenca de la Independencia, Guanajuato, México. J Arid Environ 63:756–771
Nieto-Samaniego AF, Alaniz-Álvarez SA, Camprubí-Cano A (2005) La Mesa Central de México: estratigrafía, estructura y evolución tectónica cenozoica. Boletín De La Sociedad Geológica Mexicana 3:285–318
Nieto-Samanieto AF, Báez-López JA, Levresse G, Alaniz-Alvarez SA, Ortega-Obregón C, López-Martínez M, Noguez-Alcántara B, Solé-Viñas J (2016) New stratigraphic, geochronological, and structural data from the southern Guanajuato Mining Distric, México: implications for the caldera hypotesis. Int Geol Rev 58:246–262. https://doi.org/10.1080/00206814.2015.1072745
NOM-127-SSA1–1994 (2000) Modificación a la Norma Oficial Mexicana NOM-127-SSA1–1994, Salud ambiental. Agua para uso y consumo humano. Límites permisibles de calidad y tratamientos a que debe someterse el agua para su potabilización. Diario Oficial De La Federación. http://www.salud.gob.mx/unidades/cdi/nom/m127ssa14.html
Orozco-Esquivel MT, Nieto-Samaniego AF, Alaniz-Álvarez SA (2002) Origin of rhyolitic lavas in the Mesa Central, Mexico, by crustal melting related to extension. J Volcanol Geoth Res 118:37–56. https://doi.org/10.1016/s0377-0273(02)00249-4
Ortega-Guerrero A (2009) Presencia, distribución, hidrogeoquímica y origen de arsénico, fluoruro y otros elementos traza disueltos en agua subterránea, a escala de cuenca hidrológica tributaria de Lerma-Chapala, México. Revista Mexicana De Ciencias Geológicas 26:143–161
Panagopoulos A, Haralambous KJ (2020) Environmental impacts of desalination and brine treatment-Challenges and mitigation measures. Mar Pollut Bull 161:111773
Panagopoulos A (2021) Water-energy nexus: desalination technologies and renewable energy sources. Environ Sci Pollut Res 1–14
Panagopoulos A, Haralambous KJ, Loizidou M (2019) Desalination brine disposal methods and treatment technologies—a review. Sci Total Environ 693:133545
Parga J (2003) Inventario físico de los recursos minerales del municipio de San Luis De La Paz, Gto. Consejo de Recursos Minerales:Dirección de minas de Guanajuato. México
Pettenati M, Perrin J, Pauwels H, Ahmed S (2013) Simulating fluoride evolution in groundwater using a reactive multicomponent transient transport model: application to a crystalline aquifer of Southern India. Appl Geochem 29:102–116. https://doi.org/10.1016/j.apgeochem.2012.11.001
Reyes-Cortés IA, Vázquez-Balderas JF, Ledesma-Ruiz R, Reyes-Cotés M, Barrera-Prieto Y (2012) As en el sistema hidrogeológico del Valle de Delicias, Chihuahua, México. Biblioteca Virtual del Estado de Chihuahua. México, pp 40.
Sandoval MA, Fuentes R, Nava JL, Coreño O, Li Y, Hernández JH (2019) Simultaneous removal of fluoride and arsenic from groundwater by electrocoagulation using a filter-press flow reactor with a three-cell stack. Sep Purif Technol 208:208–216
SGM (1997) Carta geológico-minera Guanajuato F14–7 escala 1:250,000. Servicio Geológico Mexicano.
SGM (1999) Carta geológico-minera Querétaro F14–10 escala 1:250,000. Servicio Geológico Mexicano.
Singhal BBS, Gupta RP (2010) Applied hydrogeology of fractured rocks. Springer, New York
Smedley PL, Kinniburgh DG (2002) a review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
SMN (1981 – 2010) Normales Climatológicas. Servicio Meteorológico Nacional. México
Solis-Reyes KF, Zanor GA, Martínez Jaime OA (2018) Geoquímica de sedimentos de la presa La Purísima (Guanajuato). Jóvenes En La Ciencia 4:978–984
Stumm W, Morgan JJ (1981) Aquatic Chemistry: An Introduction Emphasizing Equilibria in Natural Waters. John Wiley & Sons Ltd., New York
Tarbuck E J, Lutgens F K (2005). Ciencias de la Tierra. Una introducción a la geología física. P.P. Hall. España
Wang Q, Qin Y, Jia F, Li Y, Song S (2021) Magnetic MoS2 nanosheets as recyclable solar-absorbers for high-performance solar steam generation. Renew Energy 163:146–153
Wang Q, Jia F, Huang A, Qin Y, Song S, Li Y, Corona MA (2020) MoS2@sponge with double layer structure for high-efficiency solar desalination. Desalination 481:114359
Wang Q, Peng L, Gong Y, Jia F, Song S, Li Y (2019) Mussel-Inspired Fe3O4@Polydopamine(PDA)-MoS2 Core−shell Nanosphere as a Promising Adsorbent for Removal of Pb2+ from water. Journal of Molecular Liquids. J Mol Liq 282:598–605
Wester P, Sandoval-Minero R, Hoogesteger J (2011) Assessment of the development of aquifer management councils (COTAS) for sustainable groundwater management in Guanajuato. Mexico Hydrol J 19:889–899
WHO (2017) Guidelines for drinking-water quality: fourth edition incorporating first addendum. World Health Organization, Geneva
Zanor GA, García MG, Venegas-Aguilera LE, Saldaña-Robles A, Saldaña-Robles N, Martínez-Jaime O, Segoviano-Garfias JJ, Ramírez-Santoyo LF (2019) Sources and distribution of arsenic in agricultural soils of Central Mexico. J Soils Sediments. https://doi.org/10.1007/s11368-019-02269-8
Acknowledgements
The authors would like to thank to the State Water Commission of the state of Guanajuato (CEAG) for the information provided.
Funding
This work is supported by several projects as TAMU-CONACyT 2017-034 s, TAMU-CONACyT 2014-001, Convocatoria Institucional para Fortalecer la Excelencia Académica¨ UG 2015-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Piña González, V., Fuentes Hernández, V., Bian, J. et al. The presence of total inorganic arsenic (iAs) and fluoride (F−) in the groundwater of the center-west of the state of Guanajuato: a hydro-chemical and spatial distribution analysis. Sustain. Water Resour. Manag. 8, 54 (2022). https://doi.org/10.1007/s40899-022-00631-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-022-00631-2