Abstract
Hydrochemical evaluation of groundwater sampled hand-dug wells around Kankara was undertaken with the aim of determining its geochemical facies and suitability of water for drinking. A total of ten (10) hand-dug wells were sampled randomly within Kankara and its environs during the peak of dry season with two set of samples collected for major cations and anions. The water samples were analyzed for major cations (magnesium, calcium, sodium and potassium) and anions (bicarbonate, sulfate, nitrate and chloride) using atomic absorption spectrometry, flame photometry emission and titration method at the Department of Soil Science, Faculty Agriculture, Ahmadu Bello University, Zaria. Results obtained were subjected to qualitative evaluation and multivariate statistical analysis to delineate the hydrochemical facies of groundwater within the study area. Descriptive statistical analysis and comparison with World Health Organisation (WHO), Gutenberg, 468–475, (2011) standards were employed to assess water drinking suitability. Water–rock interaction was observed to be the dominant influence of groundwater chemistry. Ca–Mg, Na–K–HCO3 were found to be the dominant water facies in the study area reflecting the biotite granite, granite gneiss and migmatite geology of the study area. The assessed hand-dug wells were found to be chemically in line with WHO standard for safe drinking water except for iron II in which local remediation by aeration for oxidation to iron III was recommended. Water in the area is thus suitable for drinking.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surface water has been the major source of water for domestic and industrial uses. Recently, however, due to the high population growth, the interest in the exploitation of groundwater over the years has increased and so is the need for water quality assessment for enhanced socio-economic growth and development (Ishaku 2011).Groundwater is believed to be less contaminated (Frederick 1990) having unique features, which renders it suitable for use as public water supply with excellent natural quality, usually free from pathogens, color and turbidity, and can be consumed directly without treatment (Jain et al. 1996). Nevertheless, there are various ways groundwater may suffer pollution for instance, land disposal of solid wastes, sewage disposal on land, agricultural activities, urban runoff and polluted surface water (Jain et al. 1995). Thus the quality of water is just as important as its quantity (Abimbola et al. 2002; Adelana and Olasehinde 2003; Adeyemi et al. 2003) and this quality depends on its purpose (Todd 1980; Hamill and Bell 1986; Ezeigbo 1998; Anudu et al. 2008). Water quality has direct implication on health of human beings, animals, and yield of crops. Good water quality depends on a large number of physicochemical parameters, and the magnitude and source of any pollution load which require monitoring assess (Raddi et al. 1993). Batagarawa and Idris (2017), Danhalihu et al. (2018) and Sani et al. (2018) are among workers who have studied groundwater quality around Katsina state, northwestern Nigeria. Jidauna et al. (2017) in a study conducted in the same state (though a different local government area) recommended periodic monitoring of water quality. The importance of regional overview of groundwater resources availability, quantitatively and qualitatively for the enrichment of the hydrological information system (HIS) is important for planning purposes has also been emphasized (NIHSA 2012). However, little has been done on groundwater quality studies in some parts of Nigeria including Kankara despite an estimated population of over 122,870 (NPC 2006). It is on this premise that the hydrochemistry of groundwater of Funtua NE Sheet 78 located in semi-arid zone of Nigeria was carried out, with the aim to establish the quality of groundwater as well as groundwater facies association.
The study area
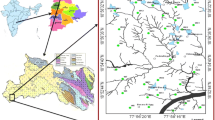
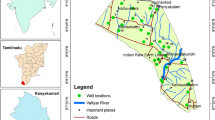
The area lies between latitudes 11°45′N–12°00′N and longitudes 7°15′E–7°30′E. It is located in northwestern Nigeria and is contained in the topographical map of Funtua NE Sheet 78 areal coverage of about 770 km2 (Fig. 1). Easy accessibility is provided mostly by a network of footpaths linking villages and the river banks. Seasonal motorable roads form important paths as well as two well tarred roads of trunk ‘A’ level. The topography of the area is that of gentle undulating landscape which reflects the underlying geology with the most prominent physical features being clusters of Older Granite hills in the west ranging in height from 300 to 700 m above the mean sea level. All rivers in the area are seasonal and contain water only during the rainy season. River Gagara and River Petsa are the largest rivers in the area. The climate is the tropical continental type with mark wet and dry season. It has an average annual rainfall ranging from 800 to 1000 mm (Schoeneich 2003).

Modified after Federal Survey of Nigeria (1967)
Location map of the study area.
Geology and hydrogeology of the area
Regionally, the study area lies within the Basement Complex and is underlain by all rocks older than the late Proterozoic metasediments of high metamorphic grade such as paragneiss, basic and calcareous schist, marble and quartzite, as well as orthogneiss and possible early (Eburnian) granite (Rahaman 2006; Dada 2006). The whole Basement Complex has been through at least two tectono-metamorphic cycles as consequent metamorphism, migmatisation and granitisation have extensively modified the original rocks which generally occur as relict raft and xenoliths, in migmatites and granites (Oyawoye 1965; Cooray 1974; Rahaman 1976, 1988; Rahaman and Ocan 1978).
Locally, three major rock lithologies were identified in Kankara and its environs. These include the migmatite, granite gneiss and biotite granite (Fig. 2) (Abusu 2014). Texturally the migmatites are medium to coarse grained having light-colored bands alternating with the paleosome (dark-colored component). Granite gneiss rocks outcrop in the northern to southern parts of the study area occurring as ridges and dykes which are often fractured and faulted. Biotite granite occurs in the area in the north–west part of the study area often occurring as massive inselberg. They are highly jointed and fractured with abundance of veins.
Hydrogeologically, two types of groundwater aquifers occur in the area viz: weathered overburden aquifer and fractured basement aquifer (Abusu 2014). Because of the semi-arid nature of the area characterized by less rainfall and thick overburden, hand-dug wells are deep (along with water table from the ground surface). The average depth to water table at the peak of dry season is 12.26 m below the ground surface while that of raining season was 8.68 m. The groundwater that constitutes recharge, flows laterally towards streams and river channels as indicated by arrows drawn perpendicular to equipotential lines and tends to diverge from the recharge areas and coverage towards the drainage channels (Fig. 3).
Methodology
Hydrochemical studies were carried out in three stages: field work, laboratory work, and evaluation of both the field and the laboratory data. Water was collected from ten selected hand-dug wells for chemical analysis using standard procedure (EPA 2013). Twenty-two containers of 1 leach were washed and taken to the field. Two separate samples were collected from each water point for determination of anions and cations, respectively. Physical parameters such as temperature, conductivity, PH and the total dissolved solids were obtained in situ using four-in-one meter (PH/EC/TDS/Temperature-model Mi 806 combined meter). The sample for the cations was acidified with concentrated nitric acid (HNO3) to a pH of approximately two and was firmly capped. This is done in other to reduce the absorption of ions from the solution and the formation of bonds between the containers and the ions. Thereafter the samples were preserved in the deep freezer before taken to the laboratory for analysis.
Laboratory analyses were done for both major cations and anions in the Department of Soil Science Laboratory Ahmadu Bello University, Zaria. Concentrations of major cations (magnesium, calcium, sodium and potassium) and major anions (bicarbonate, sulfate, nitrate and chloride) were measured using atomic absorption spectrophotometer, flame photometer and titration. Flame photometer was used for sodium, calcium and potassium; titrimetric method was used for bicarbonate, sulfate and chloride; and atomic absorption spectrophotometer was used for magnesium and iron.
Groundwater hydrochemical facies (GHF) analysis was done using Piper and Schoeller Diagrams (Piper 1944; Schoeller 1967). Piper diagrams plot the percentage abundance of the major ions together for comparison of waters and interpretation of chemical evolution. These diagrams are useful for displaying large amounts of data and determining major trends visually. Piper (1944) recognized the fact that many waters differ only slightly in chemical composition and, as such, divided the diamond field into distinct categories depending on chemical composition. Additionally, because multivariate statistical methods allow interpretation of multiple mixing trends while providing greater accuracy in delineating GHF (Amadi et al. 2010; Davis 2002), hierarchical cluster analysis was also employed.
Results and discussion
Physical characteristics of water
Table 1 displays a summary of the physio-chemical characteristics of water in the study area. Temperatures of the water measured in situ vary from 27.5 to 30.1 °C with an average of 28.69 °C.
The exponent of hydrogen-ion concentration (pH) which is a measure of the acid and base concentration of water samples of the study area ranges from 6.10 to 7.24 with an average of 7.01. This falls within the recommended limit of WHO (2011) of drinking water quality. The pH of water is a measure of its acid–base equilibrium and is usually controlled by the carbon dioxide picked up from atmosphere as rain falls, the presence of acids of peaty origin, decaying vegetation, and the presence of sulfate which causes low pH of water (Morgan and Winner 1962). On the other hand, high pH results from the presence of magnesium, potassium and sodium in high concentration (Schoeneich, 2001). In pure water, a decrease in pH of about 0.4 leads to temperature rise by about 25 °C (Schoeneich, 2001). Most groundwater has pH values between 6.0 and 8.0 (Davis and De Wiest, 1966).
Total dissolved solids (TDS) of groundwater in the hand-dug wells of the study area ranges from 67 to 361.8 mg/l with an average of 232.49 mg/l (Table 1). Electrical conductivity (EC) was measured at the spot of sample collection in micro-Siemens/cm in water at the temperature of 20 °C. It ranges from 100 to 540 µS/cm, with an average of 347 µS/cm (Table 1). Based on the conductivity of the water samples, seven of the samples are excellent for irrigation (conductivity < 250 µS/cm); the remaining four of the samples are good for irrigation (conductivity between 250 and 750 µS/cm) (Wilcox 1962).
Chemical quality of groundwater
For the cations (Table 1), sodium ion (Na+) concentration ranges from 9.10 to 38.00 mg/l, on average 22.65 mg/l (Table 1) which is typical of the water in igneous and metamorphic terrain with moderate to high rainfall as groundwater characteristics in the area are principally rock dominated (Fig. 4) (Gibbs 1970). The main source of sodium in groundwater is from the release of soluble products during the weathering of plagioclase feldspar (Davis and Dewest 1960). Its occasional predominance over the other cations depends on the nature of the feldspar found at the location. Some parts of it could have been derived from clay minerals as exchangeable sodium. Calcium ion (Ca2+) concentration in the water sampled within the area of study is found to range from the values of 4.28–44.56 mg/l, on average 21.76 mg/l (Table 1). It is the second most dominant cations in the water samples analyzed and may have been released from weathering of calcium-rich minerals in migmatites, e.g., plagioclase feldspar, amphibole and pyroxene. Other good sources of Ca2+ may be fertilizers and atmospheric precipitation.

After Gibbs (1970)
Controlling mechanisms for groundwater quality in the study area.
Magnesium ion (Mg2+) in the hand-dug wells of the study area ranges from 13.09 to 46.95 mg/l, on average 26.81 mg/l (Table 1). It falls within the recommended limit of WHO (2011) standard for drinking water. Magnesium can be obtained in the solution by the weathering of igneous and metamorphic rocks, containing feldspars, mica (biotite) and pyroxene. The magnesium concentration in the water analyzed is safe for all purposes. Potassium ion (K+) is released to water by weathering of orthoclase and microcline (Schoeneich 2001). Potassium ranges from 2.0 to 4.6 mg/l, on average 3.13 mg/l (Table 1). It is unimportant in drinking water (WHO 2011).
Iron ion (Fe2+) in groundwater from the study area ranges between 0.23 and 2.66 mg/l, on average 0.59 mg/l (Table 1). The principal minerals containing iron as an essential component include the pyroxenes, amphiboles, biotite and magnetite. Iron concentration is higher than the permissible limit of WHO (2011) standard of the drinking water especially in location 2 (Kakumi) (Fig. 2) of the study area. The high concentration of iron in the weathered overburden/fractured aquifer of Kankara may be due to a large amount of iron released from the silicates which are completely hydrolyzed (Millot 1970). Iron infiltrates into the groundwater as a result of chemical weathering of rock/lateralization which is responsible for the reddish-brown color in laterites (Juang et al. 2009). The iron is transported by rivers in the form of ferric oxide, hydroxide stabilized by colloidal organic substances and absorbed in clay minerals. The possible source of iron in the study area is from weathered biotite. The impact of iron on both plants and animals include brownish colouration on laundry clothes, brownish deposit in water, growth of iron bacteria, and effects on taste of drinking water as well as beverages (Schoeneich 2001). Locally, the solution to high iron content in water is to expose the water to oxygen by fetching water in an open container to allow for precipitation of divalent iron by oxidation to less soluble trivalent iron. This is in congruence with popular practice in water treatment. If the groundwater is oxygen-poor, iron (and manganese) will dissolve more readily, particularly if the pH of the water is on the low side (slightly more acidic) as in this case. Decomposition of the organic matter depletes the oxygen in the water and the iron dissolves as Fe2+. When this water is pumped to the surface, the dissolved iron reacts with the oxygen in the atmosphere, changes to Fe3+ (i.e., is oxidized) (Public Health 2018).
In the case of the anions, chloride ion (Cl−) concentration of Cl− in groundwater in the study area ranged from 1.00 to 3.50 mg/l, on average 1.62 mg/l (Table 1). In the Basements Complex generally, some of the chloride in crystalline areas, are from minerals which contains chlorine as essential constituents. The values fall below the permissible limit of WHO (2011) standard of drinking water quality. Sulfate (SO4) in the study area ranges from 0.12 to 3.02 mg/l, on average 0.62 mg/l (Table 1). Sulfate is probably derived from oxidation of sulfides in igneous rocks. The values fall within desirable concentration limit of WHO (2011) standard of drinking water. Bicarbonate ion (\({\text{HCO}}_{3}^{ - }\)) concentration on its part varies from 160 to 830 mg/l, on average 447.27 mg/l (Table 1). Most bicarbonate ions in groundwater are derived from the carbon dioxide in the atmosphere, carbon dioxide in the soil and respiration of plants in the zone (Adanu, 1989). Nitrates ion (\({\text{NO}}_{3}^{ - }\)) concentration ranges from 0.18 to 0.46, 0.26 mg/l (Table 1). Most nitrates in natural water come from organic sources or from industrial and agricultural chemicals. These values are below the recommended limited by WHO (2011). In general, comparison of major ions (both cations and anions) to WHO standard (2011) show water from hand-dug wells is potable (Figs. 5, 6).
Comparison of mean values of major cations with WHO (2011) standard
Comparison of mean values of major anions with WHO (2011) standard
Water facies
Based on the results of the chemical analysis (Table 1), the water facies in the area were determined using Piper (1944) trilinear diagram.Ca–Mg, Na–K–HCO3 facies were found to be most dominant while Ca–Mg–\({\text{HCO}}_{3}^{ - }\) occurred marginally at Yargoje and Tudunwada 1 (Fig. 7). Variability was observed within the ions in the study area. Schoeller plot was used to show dominance of ions. Among cations, Mg > Na > Ca > K while \({\text{HCO}}_{3}^{ - } > > > {\text{Cl}}^{ - } > {\text{NO}}_{3}^{ = } > {\text{SO}}_{4}^{ - }\) among the anions (Fig. 8). This trend was corroborated by R-mode hierarchical cluster analysis (HCA) which displayed the dominance of all the ions as done by Dibal (2015) (Fig. 9).
Though HCA is done to place objects in homogeneous groups thus showing similarities and vice versa (Meng and Maynard, 2001; Yakubo et al. 2009; Agoubi et al. 2013; Zhang et al. 2014), the water facies were not discriminated senso stricto. However, Ochsenkühn et al (1997) performed a cluster analysis on groundwater geochemical data to identify major trend axes representing dominant groundwater flow pathways. Ions in a R-mode HCA dendogram are usually arranged based on magnitude of distance from each other. Consequently, the first ion in a HCA R-mode dendogram is the least in terms of magnitude and the last ion has the highest magnitude. It can thus be observed that \({\text{HCO}}_{3}^{ - }\) was the most dominant among all the major ions while \({\text{SO}}_{4}^{ - }\) was least dominant (Figs. 8, 9).The chemical composition of groundwater depends on geology, degree of chemical weathering of various rocks, quality of recharge and anthropogenic inputs (Afolabi et al. 2017). High values of nitrate are often used as index for measuring anthropogenic pollution (Schoeneich, 2001). The water facies is thus a reflection of the geology even as earlier established for this area using the method of Gibbs (1970) (Fig. 4). Biotite granite, granite gneiss and migmatites which underlie the study area (Fig. 2) differ only structurally as mineralogical and chemical similarities occur within rocks. The migmatites of the Nigerian basement complex (NBC) are described as being of tonalitic–granodioritic composition (Cooray 1974,). The granite gneiss forms part of the felsic components (Adekoya et al. 2003; Rahaman 2006; Dada 2006; Obaje 2009), while the biotite granites are classed with the Pan-African granitoids of the NBC. This similar mineralogical composition accounts for the ubiquitous Ca–Mg, Na–K–HCO3 character displayed by waters in the study area.
Correlation analysis
Pearson correlation analysis was carried out on the results (Table 2).
Very strong correlation (0.939) was observed between Fe2+ and \({\text{SO}}_{4}^{ - }\) indicating a close association. The presence of sulfate may have affected the dissolution of iron as Cravotta (2006) observed the solubility control of Fe by sulfates. A close association was observed between Ca2+ and HCO3− as strong correlation (0.832) was seen. This may have occurred as surface water dissolved CO2 forming \({\text{HCO}}_{3}^{ - }\)-rich water which interacted with the rock. It can be recalled that water–rock interaction was observed to have predominantly influenced the water chemistry in the study area (Fig. 4). Moderate correlation existed between \({\text{NO}}_{3}^{ - }\) and Cl− which may be related to fertilizer use and thus anthropogenic sources. Amadi et al. (2010) while working on hydrochemistry of water around Aba, northeastern Nigeria made a similar assertion. The negative correlation observed between \({\text{NO}}_{3}^{ - }\) and Mg2+ (− 0.553) is a further pointer to non-geogenic source for \({\text{NO}}_{3}^{ - } .\) Other metal associations observed were low (0.484) to negative (− 0.385). This may be attributed to the fact that dissolution of metals in aqueous solution is controlled by chemical character and solubility of respective metals, as well as other chemo-thermodynamic conditions as stated by Afolabi et al. (2017).
Conclusion
The drinking quality of groundwater in the area as sampled from hand-dug wells with reference to standards set by the World Health Organizations (WHO 2011) indicates the suitability for drinking. Though high iron II content was observed in some locations, remediation by exposure to air for oxidation of iron II to iron III is recommended. The predominant water facies of the area was also established to be Ca–Mg, Na–K–HCO3 reflecting the geology of the area. The dominant influence on the hydrochemistry of the study area was confirmed to be the underlying geology as plots of evolution of groundwater from the area occurred in the field of rock-dominance. In terms of ionic dominance, cations were in the order: Mg > Na > Ca > K while anions were in the order \({\text{HCO}}_{3}^{ - } > > > {\text{Cl}}^{ - } > {\text{NO}}_{3}^{ = } > {\text{SO}}_{4}^{ - } .\) Slight anthropogenic influence on groundwater was noticed on the basis of moderate negative correlation between Mg+2 and \({\text{NO}}_{3}^{ - } .\) (0.553) and positive correlation between Cl− and \({\text{NO}}_{3}^{ - }\) (0.627).
Since the provision of water quality information for the enrichment of the hydrological information system database of developing countries such as Nigeria is important for water resources management. It is thus anticipated that this study will benefit the water authorities of the area in managing water supply projects while providing an overview of the water quality condition in typical agrarian settlements of northwestern Nigeria.
References
Abimbola AF, Odukoya AM, Olatunji AZ (2002) Influence of basement on hydrochemical characteristics of ground water in northern part of Ibadan metropolo SW Nigeria. J Nig Assoc Hydrogeol 13:1–6
Abusu CO (2014) Hydrogeology of sheet 78 NE, Northwestern Nigeria. Unpublished MSc Thesis, Department of Geology, Ahmadu Bello University, Zaria, p 76
Adanu EA (1989) Hydrogeology of the Basement Complex Terrain in the Zaria – Kaduna Area (Nigeria). Ph.D. Thesis, FU TFH TU, Berlin, p 75
Adekoya JA, Kehinde-Phillips OO, Odukoya AM (2003) Geological distribution of mineral resources in Southwest Nigeria. In: Elueze AA (ed) Prospects for investment in mineral resources of southwestern Nigeria. Elsevier, New York, pp 1–13
Adelana SMA, Olasehinde PI (2003) High nitrate in water supply in Nigeria: implications for human health. Water Resour J Nigeria Assoc Hydrogeol 14:1–11
Adeyemi GO, Adesile AO, Obayomi OB (2003) Chemical characteristics of some wells in Ikire southwestern Nigeria. Water Resour J Nigeria Assoc Hydrogeol 14:12–18
Afolabi OO, Olatunji SO, Wategire ED (2017) Hydrogeochemical characterization of groundwater in Afe Babalola University, Ado-Ekiti Southwestern Nigeria. Int J Water Resour Environ Eng 9(7):133–141. https://doi.org/10.5897/IJWREE2016.0677
Agoubi B, Kharroubi A, Abichou T, Abida H (2013) Hydrochemical and geoelectrical investigation of Marine Jaffara Aquifer, southeastern Tunisia. Appl Water Sci 3:415–429
Amadi AN, Yisa J, Okoye NO, Okunlola IA (2010) Multivariate statistical evaluation of hydrochemical Facies in Aba, southeastern Nigeria. Sci Focus 15(2):326–337
Anudu GK, Obrike SE, Onuba LN (2008) Physic-chemical quality of groundwater in Abagama and its environs, Anambra Basin, southeastern Nigeria. Int J Chem Sci 1:296–301
Batagarawa SM, Idris S (2017) Assessment of groundwater quality of Mai’adua local government, Katsina State, Nigeria. Int J Environ Sci Toxicol Res 5(2):31–35
Cooray PG (1974) Some aspects of the Precambrian of Nigeria. A review. J Min Geol. 8:17–43
Cravotta CA (2006) Relations among pH, sulphate, and metals concentrations in anthracite and bituminous coal-mine discharges, Pennsylvania. In: 23rd Proceedings of American Society of Mining and Reclamation conference, March 26–30, 2006, St. Louis, Missouri, pp 378–404
Dada SS (2006) Proterozoic evolution of Nigeria. In: Oshin O (ed) The basement complex of Nigeria and its mineral resources. Akin Jinad and Company, Ibadan, pp 24–44
Danhalihu RL, Mustapha SM, Aliyu AI (2018) Groundwater quality in basement formation of Musawa LGA of katsina Dtate, northwestern Nigeria. Int J Adv Acad Res Sci Technol Eng 4(4):95–105
Davis JC (2002) Statistics and data analysis in geology. Willey, New York
Davis SN, De Wiest RMJ (1966) Hydrogeology. Wiley, New York, p 463
Davis SN, Dewest RJM (1960) Hydrology. Wiley, New York London Sydney, p 60
Dibal HU (2015) Concentration of fluoride in groundwater and incidence of fluorosis in parts of Northern Nigeria. Unpublished Ph.D Dissertation, Department of Geology, Ahmadu Bello University, Zaria, Nigeria, p 217
Ezeigbo HI (1998) Geological and hydrogeological influences on the Nigerian Environment. J Assoc Hydrogeol 1:38–39
Federal Survey of Nigeria (1967) Topographical map of Funtua Sheet 78 NE. Office of the Surveyor General of the Federation, Abuja
Frederick WP (1990) Water quality and treatment. A Handbook on community Water Supply, 4th edn. RR Donnelly, Chicago
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Hamill L, Bell FG (1986) Groundwater resource development. Butterworth Publishers, Stoneham, p 344
Ishaku JM (2011) Hydrochemical evolution of groundwater in Jimeta Yola area, northeastern Nigeria. Global J Geol Sci 9(1):99–121
Jain C, Omkar K, Sharma MK (1996) Groundwater Technical Report, CS (AR) 196. National Institute of Hydrogeology, Roorkee, pp 1995–1996
Jain CK, Bhatia KKS, Vijay T (1995) Groundwater quality monitoring and evaluation in around Kakinada, Andhra Praesh, Technical Report, CS (AR) 172. National Institute of Hydrogeology, Roorkee, pp 1994–1995
Jidauna GG, Barde SR, Ndabula C, Oche CY, Dabi DD (2017) Water quality assessment of selected domestic water sources in Dutsin Ma Town, Katsina State. Sci World J 12(4):43–50
Juang DF, Lee CH, Hsueh SC (2009) Chlorinated volatile organic compounds found near the water surface of heavily polluted rivers. Int J Environ Sci Technol 6(4):545–556
Meng SX, Maynard JB (2001) Use of statistical analysis to formulate conceptual models of geochemical behavior: water chemical data from the Botucatu aquifer in Sao Paulo State, Brazil. J Hydrol 250:78–97
Millot G (1970) Geology of clays. Springer, New York, pp 127–139
Morgan CO, Winner MD (1962) Hydrochemical facies in the ‘400-foot’ and ‘600-foot’ sands of Baton Rouge area, Louisiana. USGeol Surv Prof Pap 450-B, pp B120–B121
NIHSA (Nigerian Hydrological Services Agency) (2012) Hydrological mapping project. http://www.nihydro.gov.ng/hydrogeological-mapping-of-nigeria/. Accessed 30 June 2015
NPC (National Population Commission) (2006) 2006 PHC PriorityTables. http://population.gov.ng/core-activities/surveys/dataset/2006-phc-priority-tables/>. Assessed 19 Jun 2018
Obaje NG (2009) Geology and mineral resources of Nigeria. Springer, Berlin Heidelberg, Lecture Notes, p 120. https://doi.org/10.1007/978-3-540-92685-613
Ochsenkühn KM, Kontoyannakos J, Ochsenkühn-Petroulu M (1997) A new approach to a hydrochemical study of groundwater flow. J Hydrol 194:64–75
Oyawoye MO (1965) Review of Nigerian Pre-Creteceous. In: Reyment RA (ed) Aspects of the geology of Nigeria. Univ. Of Ibadan Press, Ibadan, pp 16–21
Piper AM (1944) A geographic procedure in the geochemical interpretation of water analysis. Transaction American Geophysical Union, Washington, D.C., pp 914–925
Public Health (2018) Fe and Mn in groundwater. Healthy environments drinking water sources. https://www.oregon.gov/oha/PH/HEALTHYENVIRONMENTS/DRINKINGWATER/SOURCEWATER/Documents/gw/FeMnInGroundwater.pdf. Accessed 2 Aug 2018
Raddi KR, Jayaraju N, Suriyakumar I, Sreenivas K (1993) Tidal fluctuation in relation to certain physico-chemical parameters in Swamamukkhi River Estuary, East Coast of Indian. Ind J Mar Sci 22:223–234
Rahaman MA (1976) Review of the basement geology of southwestern Nigeria. In: Kogbe CA (ed) Geology of Nigeria. Elizaberthan Publ. Co., Lagos, pp 41–48
Rahaman MA (1988) Recent advances in the study of the basement complex of Nigeria. In: Precambrian Geology of Nigeria. Nigeria Geological Survey, p 11–43
Rahaman MA (2006) Nigeria’s solid mineral endowment and sustainable development. In: Oshin O (ed) The basement complex of Nigeria and its mineral resources. Akin Jinad and Co., Ibadan, pp 139–168
Rahaman MA, Ocan OO (1978) On relationship in precambrian migmatitic gneisses of Nigeria. J Min Geol 15(1):23–32
Sani MM, Danhalihu RL, Idris AK (2018) Comparative analysis of groundwater quality in basement and sedimentary formations of Katsina State, Northwestern Nigeria. Curr J Appl Sci Technol 27(5):1–8
Schoeller H (1967) Geochemistry of groundwater. An international guide for research and practice. UNESCO, Paris, pp 1–18 (chapter 15)
Schoeneich K (2001) Water quality. Unpublished postgraduate lecture notes, Ahmadu Bello University, Zaria
Schoeneich K, Garba ML (2010) Hydrogeology (Geol 405). Unpublished lecture notes, Ahmadu Bello University, Zaria
Schoeneich K (2003) Water budget for Basin Development Authorities (abbreviated version) pre-prints of the 29th WEDC Conference towards the millennium development goals-action for water and environmental sanitation held at the Conference Centre of Sheraton Hotels and Towers in Abuja from 22nd to 26th September 2003, pp 1–4
Todd DK (1980) Groundwater hydrology. Wiley, New York, p 535
Vail J, France D, Lewis B (2013) Groundwater Sampling: SESD Operating Procedure. United State Environmental protection Agency (EPA), Washington, D.C., p 31
WHO (2011) Guideline for drinking water quality, 4th edn. World Health Organisation, Gutenberg, pp 468–475
Wilcox LV (1962) The quality of water for irrigation use. US Department of Agriculture Bulletin, 40
Yakubo B, Yidana S, Emmanuel N, Akabzaa T, Asiedu D (2009) Analysis of groundwater quality using water quality index and conventional graphical methods: the Volta region, Ghana. Environ Earth Sci 59:867–879
Zhang X, Quian H, Chen J, Qiao L (2014) Assessment of groundwater chemistry and status in a heavily used Semi-Arid Region with multivariate statistical analysis. Water 6:2212–2232. https://doi.org/10.3390/w6082212
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abusu, C.O. Hydrogeochemical characterization of groundwater in Kankara, northwestern Nigeria. Sustain. Water Resour. Manag. 5, 1615–1625 (2019). https://doi.org/10.1007/s40899-019-00316-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40899-019-00316-3