Abstract
A preliminary assessment of groundwater quality for drinking and irrigational purposes in the lower Jia Bharali river catchment and adjoining areas of North Brahmaputra Plain, India, was carried out with respect to major cations, anions, and trace elements. Water samples from 50 shallow alluvial dug wells were collected in wet and dry seasons for a period of 3 hydrological years (2008–2011). Seasonal analysis showed major cation and anion contents in a definite trend across the basin as Ca: 4.2–68.9 mg/L > Na 1.9–62.5 mg/L > Mg:1.0–29.2 mg/L > K (1.3–26.1 mg/L) and HCO3 (16–118 mg/L) > Cl (2.8–138.3 mg/L) > SO4 (1.2–99.5 mg/L) > PO4 (BDL—1.50 mg/L) > NO3 (BDL—1.20 mg/L) within WHO acceptable limit for drinking water in all the seasons. Low SAR (< 10) and RSC (< 1.25 meq/L) values of shallow wells showed their suitability as potential source for agricultural purposes, while a few wells were found unsuitable with respect to Na% > 60% and Kelly’s Index > 1. However, majority of the wells were found not suitable for drinking as well as irrigational purposes due to the presence of trace elements Cd (BDL—0.070 mg/L), Cr (BDL—0.24 mg/L), Fe (0.10–11.76 mg/L), Mn (BDL—1.37 mg/L), Ni (BDL—0.18 mg/L), and Pb (BDL—0.22 mg/L) beyond their respective standard limits. Piper trilinear diagram identified the major hydrochemical facies of the groundwater as alkaline earths (Ca, Mg) and weak acids (HCO3) over alkalis (Na, K) and strong acids (SO4, Cl).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater chemistry based on hydrochemical parameters presents preliminary information on water types, classification of water for various purposes, as well as study of different chemical processes (Saxena et al. 2003; Jalali 2007; Sarwade et al. 2007; Mondal and Singh 2011; Mondal et al. 2011). Groundwater quality changes due to geogenic interaction or any type of anthropogenic influence (Kelley 1940; Wilcox 1948). Different hydrogeochemical processes like dissolution, precipitation, ion-exchange processes, etc. control the chemical composition of the ground water in the shallow alluvial aquifers (Apodaca et al. 2002).
Groundwater forms the major source of water supply for irrigation as well as drinking and domestic purposes in most parts of the India (Sridhar et al. 2013). A total of 90% of rural and 30% of urban Indian population still depend completely on untreated surface or groundwater resources (Kumar et al. 2005). Though recent years, microbiological problems in rural India are controlled to a certain extent by changing in usage from surface water to groundwater, but the same has led to sources of newer problems like fluorosis, arsenicosis, and salinity due to overexploitation of groundwater (Singh et al. 2013). However, a number of studies on groundwater quality with respect to drinking and irrigation purposes have been reported to be carried out in different river basins of India (Majumdar and Gupta 2000; Dasgupta and Purohit 2001; Khurshid et al. 2002; Sujatha and Reddy 2003; Pulle et al. 2005; Hussain et al. 2005; Sunitha et al. 2005; Subba Rao 2006; Mondal et al. 2008, 2016; Sundaray et al. 2009; Mondal and Singh 2010; Tiwari et al. 2016; Kaur et al. 2017; Kumar et al. 2017).
The river Jia Bharali, a major north bank tributary of Brahmaputra plain, originates from higher Himalayan ranges and travels through geologically younger Himalayan ranges which contain unconsolidated sedimentary rocks. The north Brahmaputra plain of NE India is an actively subsiding foreland river basin with subtle neotectonic changes and catchment area tectonics. The people in the Jia Bharali river basin widely use the groundwater for drinking as well as for their daily household purposes including irrigation of crops, rearing of poultry and fish, etc. The ground water quality of the basin is thus intimately connected with public health and is very important for sustaining aquatic life in the area. Although the drinking water quality of the aquifers of this area was analysed with respect to a few water quality parameters (Khound et al. 2012), the irrigational and drinking aspects of the groundwater sources are yet to be taken up. In this context, the major objectives of this study were to evaluate the suitability of groundwater regime of Jia Bharali river basin for drinking and agricultural purposes by comparing with WHO and Indian Standards (ISI) guidelines, respectively.
Materials and methods
Study area
The Jia Bharali catchment is bound by longitudes 92°00′–93°25′E and latitudes 26°39′–28°00′N. The river Jia Bharali is one of the major south flowing tributaries of the river Brahmaputra with a major part of its catchment within the Himalayan range of the Arunachal province of India. In the piedmont zone where it debouches from the Himalayas, the river is flowing along a northwest–southeast course and follows a straight braided course to enter the Brahmaputra river plain near Tezpur (92°53′53″E, 26°39′15″N), Assam. The northern part along the foothills of Arunachal Himalaya is made up of Reserve Forests of evergreen deciduous forestry and sparsely populated forest-villages. The study area falls in the subtropical climatic region and enjoys monsoon type of climate (Jain et al. 2007). The southwest monsoon causes widespread rainfall in the basin during June–September. About 65–80% of the average annual rainfall takes place in this period. Rainfall during July and August is the highest and amounts to more than 33% of the annual rainfall (Jain et al. 2007). The quantity of water utilized for irrigation in the studied basin was observed quite large, particularly in comparison to the quantity used for municipal, industrial, and other household purposes.
Geology
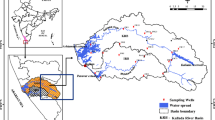
The Jia Bharali river basin is a heart-shaped basin covering an area of 11,300 sq km (Goswami 1985). The river covers 9950 sq km in Arunachal Pradesh and in Assam 1000 sq km. The groundwater potential in this zone is because of fracture system within the crystalline rocks as well as valley fills interspersed with the inselberg (Khound et al. 2012). Older alluvium is found to be composed of partially indurated and oxidized sand, silt, and clay which form higher topographic levels within an overall younger alluvial terrane of the present-day river deposits (Fig. 1). The geomorphic mapping of the alluvial catchment has revealed the presence of a number of river terraces at different topographic levels with extensive tea plantations and paddy fields. The basin is dotted with numerous meander scars, remnant channels, misfit streams, inactive floodplains, and natural levee. The aquifers of the lower Jia Bharali catchment and adjoining areas in central part of North Brahmaputra Plain (NBP), India, are unconfined and shallow alluvial with a general southward flow, which are largely tapped for domestic use through numerous dug wells attached with almost every household. Aquifer materials are generally fine-coarse sand which may be occasionally pebbly.
Water sampling and chemical analysis
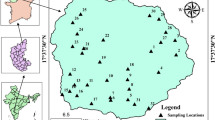
Water samples were collected from 50 shallow hand dug wells spread over the lower Jia Bharali basin and adjoining areas (Fig. 2) for 3 hydrological years 2008–2011. The sampling and analysis programme was conducted for six seasons in these 3 consecutive years as (1) W1: wet season (July 2008), (2) D1: dry season (February 2009), (3) W2: wet season (July 2009), (4) D2: Dry season (February 2010), (5) W3: wet season (July 2010), and (6) D3: dry season (February 2011). The standard methods were followed in collection, storage, and analysis of the water samples (APHA 1998), and represented in Table 1.
Data treatment and classification methods
The parameters such as sodium adsorption ratio (SAR), percent sodium (% Na), residual sodium carbonate (RSC), Kelly’s index (KI), and magnesium ratio were calculated to evaluate the suitability of the groundwater quality for agricultural purposes (Mondal et al. 2016). The standard techniques and methods (APHA 1998; Ayers and Westcot 1994; Tiwari and Manzoor 1988a, b; ISI 1974; Durfer and Backer 1964; USSL 1954; Kelly 1940; Paliwal 1967) were followed for analysis, calculation, and interpretation of the generated database.
Results and discussion
The hydrochemical parameters at 50 wells from shallow alluvial aquifers in the Jia Bharali river basin system with minimum, maximum, mean, and standard deviation values in each wet and dry season from 2008 to 2011 are incorporated in Tables 2, 3, and 4.
The pH of water samples were found in the range of 5.8–8.1 (mean 6.9) in W1, 5.7–8.0 (mean 6.6) in W2, 5.1–7.3 in W3 (mean 6.6), 5.6–7.7 (mean 6.7) in D1, 5.4–7.4 (mean 6.6) in D2, and 5.7–7.2 (mean 6.7) in D3. A number of samples in the wet seasons (16% in W1, 40% in W2, and 32% in W3) as well as in dry seasons (26% in D1and D2, 14% in D3) showed high acidic pH (< 6.5) implying their unsuitability for drinking and irrigation purposes. The pH values in the wet seasons were recorded below 6.0, and in the dry seasons, they were found below 8.0, while average pH values in all the wet and dry seasons were found in between 6.0 and 7.0. The low pH of water samples may be due to (1) eutrophication (Garcia et al. 2001), (2) wide distribution of lateritic soil in the basin or extensive use of acid producing fertilizers like ammonium sulphates and super phosphates of lime (Raghunath et al. 2001) during cultivation. However, the studied ground water regime was found in ‘no problem’ (6.5–8.4) and ‘moderate’ category (5.1–6.4) under Ayers and Westcot (1994) pH classification for irrigation of soils in all the wet and dry seasons.
The EC of the selected shallow wells were found in the range of 80–1010 µS/cm (mean 307 µS/cm) in W1, 60–1070 µS/cm (mean 383 µS/cm) in W2, 60–1350 µS/cm (mean 320 µS/cm) in W3, 50–1130 µS/cm (mean 233 µS/cm) in D1, 50–510 µS/cm (mean 191 µS/cm), and 60–1010 µS/cm (mean 245 µS/cm) in D3, respectively. These values were lower than WHO (1984) permissible limit (1400 µS/cm) for drinking water and indicated their suitability for household purposes. However, mean EC values of the shallow wells were found in the range of 200–400 µS/cm in all the wet and the dry seasons. The low EC values indicated the effect of (1) dilution due to high rainfall, (2) insoluble geologic rock and mineral types, (3) very little solute dissolution, and (4) rapid ion exchange between the soil and water. The higher EC values indicated the input of alkali and alkaline earth metal ions through agricultural and domestic sewage (Bernard et al. 2004). EC variation pattern was not uniform and this may be due to non-uniform influence of various local factors on ionic contents of the water. Human activities and inflows from surrounding agricultural fields are likely to be significant influences on the salt contents of the groundwater. Considering US Salinity Laboratory (1954) classification, it was found that all the aquifers could be categorized under low salinity (< 2500 µS/cm) regime, and therefore, the water was highly suitable for the irrigation of crops. Moreover, the studied water samples were found in ‘Low Class’ (< 1500 µS/cm) category under Indian Standard guidelines (1985) which implied their suitability for irrigation uses. Except a few wells, all the studied shallow wells were found in ‘excellent’ (< 250 µS/cm) to ‘permissible’ (250–500 µS/cm) category for the irrigation of crops (Ayers and Westcot 1994) in all the wet and dry seasons.
Total Hardness (TH) of the groundwater regime varied from 24 to 260 mg/L (mean 109 mg/L) in W1, 59–268 mg/L (mean 132 mg/L) in W2, 46–220 mg/L (mean 114 mg/L) in W3, 20–168 mg/L (mean 71 mg/L) in D1, 30–220 mg/L (mean 79 mg/L) in D2, and 50–154 mg/L (mean 85 mg/L) in D3, respectively. According to WHO (1984) classification (Table 5), the studied shallow wells were found in different categories from ‘Soft’(0–50 mg/L) to ‘Very hard’ (> 200 mg/L) in all the wet and dry seasons with majority of the wells in ‘moderately soft’ category (50–100 mg/L) for drinking uses. Using Durfer and Backer (1964) TH classification, most of the samples were found in the ‘Moderately soft’ category (60–120 mg/L), while a few wells in ‘very hard’ category (> 180 mg/L) found doubtful for irrigation purposes (Table 5). The sources of hardness in the groundwater regime might be due to discharge of sewage, detergents, and large-scale human use (Jain and Sharma 2002). However, all water bodies had hardness (CaCO3/L) well below the WHO (1984) permissible limit of 500 mg/L implying their suitability for household purposes.
Major cations
Calcium (Ca) content of the shallow wells varied in the range 6.4–68.9 mg/L (mean 27.0 mg/L) in W1, 5.8–51.0 mg/L (mean 21.2 mg/L) in W2, 10.2–46.4 mg/L (mean 23.5 mg/L) in W3, 4.2–47.1 mg/L (mean 16.0 mg/L) in D1, 7.5–46.5 mg/L (mean 19.3 mg/L) in D2, and 6.4–38.5 mg/L (mean 18.7 mg/L) in D3, respectively. Corresponding magnesium contents were found in the range of 1.0–29.2 mg/L (mean 9.2 mg/L) in W1, 2.4–27.5 mg/L (mean 9.6 mg/L) in W2, 2.8–28.9 mg/L (mean 9.7 mg/L) in W3, 1.9–19.5 mg/L (mean 6.8 mg/L) in D1, 2.6–26.5 mg/L (mean 9.0 mg/L) in D2, and 2.4–17.8 mg/L (mean 8.1 mg/L) in D3, respectively. The calcium and magnesium concentration of the study area could be attributed to rock weathering. Calcium and magnesium contents of all water samples were found well below the guideline value of WHO (1984) (Ca: 100 mg/L, Mg: 150 mg/L) and ISI (1991) (Ca: 75 mg/L, Mg: 30 mg/L), respectively, and indicated their suitability for drinking purposes in both the rainy and dry seasons. Moreover, Ca and Mg contents of all the wells were found well below the tolerance limits (Ca: 80 mg/L, Mg: 24 mg/L) of irrigation (ISI 1974) for both the wet and dry seasons in the study area.
Magnesium hazard (MH)
Harmful effect of magnesium could be presented with the help of an important ratio called index of MH or magnesium ratio (MR) (Paliwal 1972). Magnesium ratios of more than 50% would adversely affect the crop yield as the soils become more alkaline (Paliwal 1972; Sundaray et al. 2009). MR of the studied shallow wells were observed to be in the range of 9–55% (mean 35%) in W1, 25–63% (mean 43%) in W2, 20–61% (mean 40%) in W3, 24–63% (mean 42%) in D1, 28–65% (mean 43%) in D2, and 29–64% (mean 42%) in D3, respectively, during the sampling periods. Only a few wells (≤ 16%) in all the wet and dry seasons showed MR more than 50% indicating their unsuitability for irrigation purposes. High Mg ratio in these wells may be due to high interaction between surface–subsurface water and passage through the limestone, and kankar and granitic rock formation in the study area (Pandian and Sankar 2007). The published reports also favour this result. Assessing the quality for domestic and agricultural uses in the Tiruvallur District of Tamil Nadu state of India, Kumar and co-workers (2017) found MH values of majority of the groundwater samples below 50% and suggested their suitability for irrigational uses.
Na and K
Sodium (Na) contents of the shallow wells were in the range of 5.6–46.7 mg/L (mean 17.4 mg/L) in W1, 4.2–42.0 mg/L (mean 10.8 mg/L) in W2, 4.2–62.5 mg/L (mean 15.3 mg/L) in W3, 1.9–30.1 mg/L (mean 10.8 mg/L) in D1, 5.1–28.4 mg/L (mean 10.2 mg/L) in D2, and 3.5–32.8 mg/L (mean 11.5 mg/L) in D3, respectively. Similarly, wet season potassium (K) contents varied from 2.7 to 26.1 mg/L (mean 10.2 mg/L) in W1, 2.6–24.5 mg/L (mean 6.6 mg/L) in W2, 2.2–25.6 mg/L (mean 8.4 mg/L) in W3, while dry season values ranged from 1.3 to 16.7 mg/L (mean 5.3 mg/L) in D1, 1.9–18.5 mg/L (mean 5.4 mg/L) in D2, and 1.9–14.9 mg/L (mean 5.5 mg/L) in D3, respectively. However, all the wells were found to have Na content below WHO (1984) permissible limit (200 mg/L), while a few number of wells (< 20% in wet seasons and 4% in the dry seasons) showed K content more than recommended limit (12 mg/L) of WHO (1984) for drinking water. The sodium hazard of irrigation water is usually specified with the help of two sodium-related indices named as (1) sodium adsorption ratio (SAR) and (2) sodium percentage (Na %) (Mondal et al. 2016; Tiwari et al. 2016; Kaur et al. 2017).
Sodium absorption ratio (SAR)
SAR defined irrigation quality of water sources with respect to sodium hazard, since it is more closely related to exchangeable sodium percentages in the soil (Tiwari and Manzoor 1988a). In the present work, wet season SAR values of the shallow aquifers ranged from 0.21 to 2.90 (mean 0.77) in W1, 0.18 to 1.58 (mean 0.51) in W2, and 0.14 to 1.94 (mean 0.68) in W3, while, in case of dry seasons, SAR values varied from 0.11 to 1.99 (mean 0.59) in D1, 0.22 to 1.32 (mean 0.51) in D2, and 0.19 to 1.40 (mean 0.57) in D3 during sampling periods. According to Richards’ (1954) and ISI (1991) classification, all the samples could be classified under excellent (< 10) category for irrigation in all the seasons. Similar SAR values (< 10) have also been reported in the Malwa region of Punjab (Kaur et al. 2017) bounded by river Sutlej indicating the absence of alkali hazards in ground water samples. Tiwari and co-workers (2016) also measured similar groundwater SAR values which range from 0.40 to 1.90 (avg. 1.00) in the West Bokaro coalfield area of Jharkhand State of India.
Sodium percentage (Na %)
The Na% of the studied groundwater samples varied from 12 to 73% (mean 34%) in W1, 9–52% (mean 27%) in W2, 6–59% (mean 31%) in W3, 12–66% (mean 31%) in D1, 8–55% (mean 27%) in D2, and 12–59% (mean 29%) in D3, respectively. Na% values presented that most of the water sources (≥ 78%) under “excellent” (< 20%) to “good” (20–40 Na%) category except a few sources for irrigation of soils. As per ISI (1974) guidelines, the maximum tolerance limit of Na% for inland water used for irrigation is 60%, and hence, except two wells, all the other studied wells of Jia Bharali river basin were found suitable for irrigation purposes. Similar results have also been found in several published research works. In the Sutlej river basin of Punjab, Kaur and co-workers (2017) found most of the groundwater samples in excellent (< 20%) and good (20%–40%) categories which could be safely used for irrigation purposes.
Kelly’s index
Water quality towards irrigation could also be classified based on Kelly’s index (KI). It is the ratio of sodium ion to calcium and magnesium ions in meq/L (Kelly 1940). A Kelly’s index of more than one indicates an excess level of sodium in waters (Kelly 1940; Paliwal 1967). Kelly’s index in the present study varied from 0.15 to 2.05 (mean 0.54) in W1, 0.13 to 1.12 (mean 0.36) in W2, 0.10 to 1.37 (mean 0.48) in W3, 0.08 to 1.41 (mean 0.42) in D1, 0.15 to 0.94 (mean 0.36) in D2, and 0.14 to 0.99 (mean 0.40) in D3, respectively. Waters with a Kelly’s index less than one are considered as suitable for irrigation, and except a few wells (3% wells in W1, 4% wells in W2, 12% wells in W3, and 2% wells in D1), all the wells showed their suitability for agricultural purposes in both the wet and dry seasons. Published reports are in conformity with the results of the present work. The groundwater regime of Markandeya River basin of India (Ravikumar et al. 2010) showed Kelly’s index in the range from 0.057 to 1.12, and all the water samples are suitable for irrigation according to Kelly’s index, except for one sample with a KI value of 1.12.
Major anions
In the present study, the groundwater samples had pH lower than 8.3, and hence, no carbonate (CO3) alkalinity could be found in the studied wells (Narain et al. 2000). The total alkalinity was, therefore, almost entirely due to the presence of bicarbonate, which was found at slightly higher levels in the post-monsoon period, indicating that some contribution might have come from the carbonate weathering process due to heavy downpour in the catchment. Bicarbonate content (as CaCO3) of the groundwater samples varied from 20 to 114 mg/L (mean 75 mg/L) in W1, 27 to 118 mg/L (mean 67 mg/L) in W2, and 34 to 118 mg/L (mean 69 mg/L) in W3 season, while, for dry seasons, its values varied from 16 to 92 mg/L (mean 47 mg/L) in D1, 20 to 70 mg/L (mean 38 mg/L) in D2, and 17 to 88 mg/L (mean 44 mg/L) in D3, respectively. High values of bicarbonate indicated the contribution from silicate and carbonate chemical weathering into the shallow aquifers (Sridhar et al. 2013). All the water samples showed the bicarbonate alkalinity higher in the dry seasons than that of the wet seasons, and the values were well within the WHO permissible limit (120 mg/L) for drinking water. The hazardous effect of carbonate and bicarbonate on the quality of water for agricultural uses could be explained in terms of RSC (residual sodium carbonate) indices (Eaton 1950).
The most common toxicity in the irrigation water originates from chloride (Sundaray et al. 2009). Cl showed a wide range of values as 2.8–116.4 mg/L (mean 37.0 mg/L) in W1, 4.9–112.5 mg/L (mean 29.6 mg/L) in W2, 5.9–129.5 mg/L (mean 50.9 mg/L) in W3, 2.8–99.4 mg/L (mean 31.1 mg/L) in D1, 8.2–110.2 mg/L (mean 30.3 mg/L) in D2, and 8.5–138.3 mg/L (mean 48.9 mg/L) in D3. High concentration of chloride makes water unpalatable and unfit for drinking and livestock watering. However, all the shallow wells showed Cl content below the permissible limit of 250 mg/L (WHO 1984) for drinking water. The Cl content of the ground water sources of the basin was also within the tolerance limit for irrigation (ISI 1974, 600 mg/L). The groundwater sources of Jia Bharali river basin were also free from chloride hazard and they are classified under ‘no problem’ category (< 4 meq/L) for irrigation uses (Sundary et al. 2009). The sulphate contents were much below the permissible limit for drinking water (400 mg/L, WHO 1984) with the wide range of values in different seasons as W1: 5.8–99.5 mg/L (mean 23.9 mg/L), W2: 2.6–80.5 mg/L (mean 19.2 mg/L), W3: 6.9–69.3 mg/L (mean 21.2 mg/L), D1: 1.2–43.0 mg/L (mean 10.9 mg/L), D2: 1.2–30.0 mg/L (mean 11.9 mg/L), and D3: 5.5–71.7 mg/L (mean 31.7 mg/L). However, the ground water sulphate content of the Jia Bharali river basin was within the tolerance limit (1000 mg/L) for irrigation purposes (ISI 1974). The shallow wells of the investigated area were found to contain only small amounts of NO3 in both wet and dry seasons as from BDL to 1.20 mg/L (mean 0.17 mg/L) in W1, BDL to 0.45 mg/L (mean 0.07 mg/L) in W2, BDL to 0.64 mg/L (mean 0.08 mg/L) in W3, BDL to 0.30 mg/L (mean 0.04 mg/L) in D1, BDL to 0.28 mg/L (mean 0.05 mg/L) in D2, and BDL to 0.32 mg/L (mean 0.02 mg/L) in D3, respectively. Smaller values of nitrate in the study area indicate that the nitrifying bacteria are not much active due to the presence of anaerobic conditions (the area having a water cover for most of the time) for the large part of the year. The occurrences of nitrate in the wells are likely to be the application of fertilizers like ammonium sulphate, [(NH4)2SO4] in the agricultural fields of the locality. However, all the groundwater sources in all the seasons showed nitrate content below the WHO (1984) permissible limit (50 mg/L) for drinking water. Ayers and Westcot (1994) classification showed that all the aquifers were fall under ‘no problem’ category (< 5 mg/L) for irrigation water, and hence, they were free from NO3 hazards. The wet and dry season PO4 contents of the shallow wells were recorded as 0.04–1.50 mg/L (mean 0.26 mg/L) in W1, BDL to 1.45 mg/L (mean 0.13 mg/L) in W2, BDL to 0.88 mg/L (mean 0.08 mg/L) in W3, BDL to 1.15 mg/L (mean 0.06 mg/L) in D1, BDL to 1.04 mg/L (mean 0.09 mg/L) in D2, and BDL to 0.83 (mean 0.06 mg/L) in D3, respectively. The presence of vast paddy rice cultivation in the study area obviously points to agricultural runoff being the main source.
The nutrient boron showed its presence in most of the water samples ranging from BDL to 0.20 mg/L (mean 0.08 mg/L) in W1, BDL—0.24 mg/L (mean 0.10 mg/L) in W2, BDL to 0.25 mg/L (mean 0.12 mg/L) in W3, BDL—0.39 mg/L (mean 0.17 mg/L) in D1, BDL to 0.41 mg/L (mean 0.19 mg/L) in D2, and BDL—0.40 mg/L (mean 0.21 mg/L) in D3, respectively. All the sources in all six seasons were found to have B content well below the WHO (1984) permissible limit (0.50 mg/L) for drinking water. Boron affects plant growth and yield, and an overdose or an underdose of boron can result in toxicity or deficiency symptoms in plants. All the wells showed B content below the tolerance level for semi tolerant (< 0.67 mg/L) and tolerant (1.00 mg/L) crops (ISI 1991) in all the wet and dry seasons. However, in dry seasons, a few wells (≤ 12%) showed B content above the tolerance limit (0.33 mg/L) for sensitive crops (ISI 1991) in the studied basin.
Residual sodium carbonate (RSC)
The ground water samples showed RSC values in the range of − 2.47 to 0.23 meq/L (mean − 0.60 meq/L) in W1, − 3.63 to − 0.04 meq/L (mean − 1.09 meq/L) in W2, and − 2.40 to 0.04 meq/L (mean − 0.89 meq/L) in W3, whereas, in dry season, RSC values were observed in the range of − 3.84 to 0.23 meq/L (mean − 0.92 meq/L) in D1, − 4.13 to 0.43 meq/L (mean − 0.80 meq/L) in D2, and − 3.38 to 0.18 meq/L (mean − 0.84 meq/L) in D3, respectively. All the samples had RSC values much less than 1.25 meq/L, and hence, groundwater regime is ‘safe’ (1.25 meq/L) as well as ‘excellent’ quality for irrigation (USSL 1954, ISI 1991) in all the wet and dry seasons. Furthermore, more than 80% wells in all the six seasons showed negative RSC values which implied the absence of complete precipitation of calcium and magnesium (Tiwari and Manzoor 1988b) in the studied basin. Except a few sources, groundwater regime of Tiruvallur District of Tamil Nadu State of India showed RSC values which range from − 12.29 to 2.51 meq/L indicating their suitability for agricultural purposes.
Trace metals
The As concentration of the ground water samples was observed in the range of BDL to 0.009 mg/L (mean 0.002 mg/L) in W1, BDL to 0.007 mg/L (mean 0.001 mg/L) in W2, and BDL to 0.009 mg/L (mean 0.002 mg/L) in W3, while dry season As were observed as BDL to 0.019 mg/L (mean 0.003 mg/L) in D1, BDL to 0.011 mg/L (mean 0.003 mg/L) in D2, and BDL to 0.012 mg/L (mean 0.003 mg/L) in D3, respectively. Dry season arsenic concentration was found higher than that of wet seasons mainly due to reduction in water volume. Except one source in foothills of Arunachal Himalaya, all the groundwater sources were found to have As content well below the recommended maximum permissible limit (0.01 mg/L) of drinking water (WHO 1984) in both wet and dry seasons. Similarly, all the wells showed As content well below tolerance limit (0.10 mg/L) for irrigational uses (ISI 1991). Arsenic poisoning causes cancers, melanosis, hypertension, and heart disease (Guha et al. 2000; Morales et al. 2000; Rahman 2002). Arsenic inhibits plant growth by interfering the plant metabolism and nutrient uptake by competing directly with nutrients or altering metabolic processes (Farnese et al. 2014).
Cadmium is one of the most toxic elements with reported carcinogenic effects in humans (Goering et al. 1994). Cd content of the present samples varied from BDL to 0.025 mg/L (mean 0.002 mg/L) in W1, BDL to 0.040 mg/L (mean 0.003 mg/L) in W2, BDL to 0.013 mg/L (mean 0.002 mg/L) in W3, BDL to 0.038 mg/L (mean 0.004 mg/L) in D1, BDL to 0.070 mg/L (mean 0.008 mg/L) in D2, and BDL to 0.022 mg/L (mean 0.008 mg/L) in D3, respectively. The relatively consistent distribution of Cd throughout the study area (Fig. 3) implied that it could have been derived from non-point sources such as agricultural runoff in the absence of industrial activities. There is, however, Cd concentration in a number of groundwater samples (10% samples in W1, 18% samples in W2 and W3, 30% samples in D1, 50% samples in D3, and 66% samples in D3) which were found to exceed the desirable limit of 0.005 mg/L (WHO 1984) for drinking water. Similarly, in the wet seasons, 4% samples in W1, 10 samples % in W2, 6% samples in W3, and, in the dry seasons, 2% samples in D1, 22% samples in D2, and 40% samples in D3 had cadmium content above the tolerance limit (0.01 mg/L) for irrigational water (ISI 1991). Excess cadmium affects plants growth by disturbing structure and functional activity of the photosynthetic processes (Vassilev et al. 1995).
WHO (1984) has not proposed any drinking water guideline value for Co, but the shallow wells showed Co content in the considerable ranges from 0.017 to 0.055 mg/L (mean 0.039 mg/L) in W1, BDL to 0.064 mg/L (mean 0.035 mg/L) in W2, BDL to 0.054 mg/L (mean 0.023 mg/L) in W3, 0.004 to 0.109 mg/L (mean 0.053 mg/L) in D1, BDL to 0.112 mg/L (mean 0.058 mg/L) in D2, and 0.009 to 0.099 mg/L (mean 0.042 mg/L) in D3, respectively. However, most of the groundwater samples (20% in W1, 22% in W2, 4% in W3, 56% in D1, 62% in D2, and 26% in D3) showed Co content exceeding permissible limit (0.05 mg/L) for irrigational water (ISI 1991) in both the wet and dry seasons. Co is an essential element for humans and plants, but, at higher concentration, it can cause anorexia, nausea, vomiting, and diarrhea in human beings (Bhagure and Migaue 2010) and irreversible damage to plant cell and cell membrane (Jayakumar et al. 2008). Cu is an essential nutrient, but overdoses of Cu cause hypertension, liver, and kidney dysfunctions (USEPA 1999; Stenhammar 1999). In the present study, the Cu concentration [W1: 0.03 to 0.83 mg/L (mean 0.21 mg/L), W2: BDL to 0.31 mg/L (mean 0.06 mg/L), W3: BDL to 0.19 mg/L (mean 0.05 mg/L), D1: 0.03 to 1.10 mg/L (mean 0.26 mg/L), D2: 0.05 to 1.43 mg/L (mean 0.24 mg/L), and D3: 0.01 to 0.21 mg/L (mean 0.07 mg/L)] of the water samples was found far below the desirable value of 2 mg/L (WHO 1984) for drinking water. However, a large number of groundwater samples in some seasons (62% in W1, 46% in D1 and D2) showed Cu content above the tolerance limit (0.20 mg/L) for irrigational water (ISI 1991), and these sources could be considered as unsafe for agriculture use with respect to copper content. Copper may be toxic to plants, affecting mainly the growth of the roots (Owen 1981) of plants like cauliflower, potato, carrot, lettuce seedling, and rice seedling which are widely cultivated in the study area.
Cr (III) is considered as an essential trace element for the maintenance of an effective glucose, lipidd, and protein metabolism in humans, animals, plants, and microorganism (Nielsen 1998), while Cr(VI) is regarded as serious toxic pollutant due to its carcinogenic and mutagenic properties (Mondal and Singh 2010; Cohen et al. 1993; Cieslak-Golonka 1995). In the present work, the total Cr concentration [both Cr(III) and Cr(VI)] was measured, and it varied from low to sufficiently high values, i.e., in the range of BDL − 0.15 mg/L (mean 0.05 mg/L) in W1, BDL − 0.14 mg/L (mean 0.06 mg/L) in W2, BDL − 0.17 mg/L (mean 0.06 mg/L) in W3, BDL − 0.24 mg/L (mean 0.08 mg/L) in D1, BDL − 0.22 mg/L (mean 0.09 mg/L) in D2, and BDL − 0.17 mg/L (mean 0.08 mg/L) in D3, respectively. However, 40% wells in W1, 64% wells in W2, 54% wells in W3, as well as 46% wells in D1, 70% wells in D2, and 72% wells in D3 of the studied basin showed very high Cr concentration exceeding the permissible limit (0.05 mg/L) for drinking water (WHO 1984). Spatial distribution contours in the Fig. 4 showed that the regions having higher Cr content are far away from the river. Cr-rich situation in the basin needs to be investigated further. The Cr enrichment of the water samples could be attributed to the transportation from the Cr-rich rocks and soils of the basin as well as to the human activities like agricultural cultivation and fertilization of the studied area. However, all the studied wells were found to have chromium content far below the maximum permissible limit (1.0 mg/L) for irrigational water (ISI 1991), and hence, they could be used as potential source for agricultural purposes. Consumption of Cr-contaminated plants and their foodstuffs may pose a serious health hazard to human beings through food-chain biomagnifications (Nriagu and Pacyna 1988).
In the present work, iron contents of the groundwater samples varied from 0.10 to 5.64 mg/L (mean 1.03 mg/L) in W1, 0.10 to 3.86 mg/L (mean 0.96 mg/L) in W2, 0.14 to 4.15 mg/L (mean 0.92 mg/L) in W3, 0.14 to 9.15 mg/L (mean 1.09 mg/L) in D1, 0.10 to 11.76 mg/L (mean 1.32 mg/L) in D2, and 0.13 to 3.68 mg/L (mean 0.64 mg/L) in D3, respectively, with majority of the sources exceeding WHO (1984) limit (0.3 mg/L) for drinking water. Fe, along with Mn, can affect the organoleptic quality of water as well as coloration of the water, thus initiating sedimentation in the system. It was observed that, in case of wet seasons, 40% wells in W1, 70% wells in W2, and 92% wells in W2, while, in case of dry season, 54% wells in D1, 78% wells in D2, and 58% wells in D3 had Fe content above the maximum permissible limit (0.30 mg/L) for drinking water (WHO 1984). The enrichment of iron in these shallow wells was mainly due to soil leaching and chemical weathering linking particular geology of the plain (Puttaiah and Kiran 2008; Zorer et al. 2008). The high iron content of the studied wells may also be, plausibly, due to interaction with highly oxidized partially indurate older alluvium with limonitic layers of the study area (Fig. 5). The lowering of iron content in the monsoon season had been attributed to the dilution effect of the rainfall and consequent increased flow of water into the water sources (Sharma and Subramanian 2010). However, iron contents of all the shallow wells were found far below the permissible limit (5.0 mg/L) of ISI (1991) showing their suitability for agricultural purposes in all the seasons.
Mn plays an essential micronutrient in the plant metabolic processes and becomes toxic when it is in excess (Kochian et al. 2004; Pulle et al. 2005). In human beings, overdoses of Mn can cause kidney, pancreas, and liver damages (Underwood 1977). The shallow wells of the study area showed Mn content in the range of BDL − 0.99 mg/L (mean 0.18 mg/L) in W1, BDL − 0.88 mg/L (mean 0.15 mg/L) in W2, and BDL − 0.90 mg/L (mean 0.13 mg/L) in W3, while the dry season manganese contents were observed as 0.10–1.26 mg/L (mean 0.35 mg/L) in D1, BDL − 1.37 mg/L (mean 0.42 mg/L) in D2, and BDL − 1.29 mg/L (mean 0.22 mg/L) in D2 season, respectively. In the wet seasons, 40% wells in W1, 34% wells in W2, and 17% wells, while, for dry seasons, all wells in D1, 90% in D2, and 66% in D3 were found to have Mn content above the permissible limit of 0.10 mg/L (WHO 1984) for drinking water. Similarly, 30% wells in W1, 24% wells in W2, 14% wells in W3, 56% in D1, 68% in D2, and 26% in D3 showed Mn content above the maximum tolerance limit (0.20 mg/L) for irrigational water (ISI 1991).
In this work, Ni contents ranged from BDL to 0.07 mg/L (mean 0.02 mg/L) in W1, BDL to 0.09 mg/L (mean 0.04 mg/L) in W2, BDL to 0.10 mg/L (mean 0.04 mg/L) in W3, 0.01 to 0.18 mg/L (mean 0.04 mg/L) in D1, BDL to 0.16 mg/L (mean 0.03 mg/L) in D2, and BDL to 0.12 mg/L (mean 0.04 mg/L) in D3, respectively. 64% samples in W1, 72% samples in W2, 82% samples in W3, 98% samples in D1, 60% samples in D2, and 88% samples in D3 were found to have Ni content above the permissible limit (0.02 mg/L) for drinking water (WHO 1984). Excess Ni could cause laryngeal and lung cancer in human being (Lessard et al. 1978). The nickel toxicity in plants appears as chlorosis or yellowing of leaves usually followed by necrosis (Rathor et al. 2014). However, all the selected shallow wells showed Ni content below the permissible limit (0.20 mg/L) of irrigational water (ISI 1991) in both the wet and dry seasons.
Long-term exposure to lead or its salts can affect the kidney and nervous system of human beings adversely (Mugica et al. 2002). In this work, the concentration of Pb was in the range of BDL − 0.18 mg/L (mean 0.09 mg/L) in W1, BDL − 0.15 mg/L (mean 0.09 mg/L) in W2, BDL − 0.19 mg/L (mean 0.09 mg/L) in W3, 0.03–0.20 mg/L (mean 0.11 mg/L) in D1, 0.04–0.22 mg/L (mean 0.11 mg/L) in D2, and 0.03–0.21 mg/L (mean 0.11 mg/L) in D3, respectively. In the present study, almost all the wells had higher Pb-content in the dry season than in the wet season. Contours in the Fig. 6 showed that lead was present in all the aquifers in both the seasons, and it was likely that its adsorption on organic and inorganic sediment surfaces (Hem 1976) may have enhanced its concentration in the ground water. Majority of the wells (≥ 88%) in both the seasons had Pb concentration above the permissible limit of 0.05 mg/L (WHO 1984) for drinking water. The low concentration of Pb in natural waters is normally ascribed to less solubility of Pb-containing minerals in water, while high concentration of lead in natural water occurs mainly contributed by geological and anthropogenic sources (Mondal et al. 2010; Singh et al. 2006). Pb concentration of the studied wells was found very low in compared to permissible limit (5 mg/L) for irrigational water (ISI 1991). High level of Pb in irrigational water may reduce the plant growth and other metabolic activities (John et al. 2009).
Zn, an essential trace element, plays an important role in the physiological and metabolic processes of many organisms and plants and becomes toxic in high concentrations (Pillai and Paul 1983). Zinc showed a significant presence in the wells from BDL − 0.11 mg/L (mean 0.03 mg/L) in W1, BDL − 0.12 mg/L (mean 0.04 mg/L) in W2, BDL − 0.14 mg/L (mean 0.03 mg/L) in W3, BDL − 0.19 mg/L (mean 0.05 mg/L) in D1, BDL − 0.20 mg/L (mean 0.04 mg/L) in D2, and BDL − 0.16 mg/L (mean 0.03 mg/L) in D3, respectively. Zn content of the water sam may be attributed to leaching from the overlying soil layers by the runoff. The values were very low compared to WHO (1984) permissible limit (3 mg/L) for drinking water as well as permissible limit (2 mg/L) for irrigational water (ISI 1991). Hence, due to enrichment of the micronutrient Zn, the groundwater sources of the study area are suitable for agricultural purposes.
Piper classification
In the present study, Piper (1944) classification showed that the 50 alluvial shallow wells could be grouped into two categories in all the wet and dry seasons (Table 6). Thus, Piper’s classification revealed that alkaline earths (Ca, Mg) and weak acids (HCO3) are preponderance over alkalis (Na, K) and strong acids (SO4, Cl) in majority of the selected aquifers of the study area, thereby indicating calcium and magnesium bicarbonate type of water. Piper diagrams for the wet season W1 and the dry season D1 are presented in Figs. 7 and 8, respectively.
Correlation analysis
The correlation matrices for 23 variables were prepared for both wet (Table 7) and dry seasons (Table 8) using SPSS 16.0. If the correlation coefficient is nearer to + 1 or − 1, it shows the perfect linear relationship between the two variables (Tiwari et al. 2016). A correlation coefficient of < 0.50 exhibits poor correlation, 0.50 indicates good correlation and > 0.50 represents strong correlation (Kumar et al. 2017). Thus, correlation analysis reveals the nature of the relationship between the water quality parameters. A good correlation between EC–TH [r = 0.61(wet) and 0.51 (dry)], EC–Ca [r = 0.56 (wet) and 0.59 (dry)], EC–Mg [r = 0.66 (wet) and 0.64 (dry)], EC–Na [r = 0.75 (wet) and 0.55 (dry)], EC– Cl [r = 0.65 (wet) and 0.62 (dry)], and EC–SO4 [r = 0.65 (wet) and 0.60 (dry)] was observed in all the groundwater samples. This suggested that the presence of TH, calcium, sodium, chloride, and sulphate greatly influences the EC of the studied wells (Kaur et al. 2017). Besides this, the correlation matrices also showed significant positive correlations between different physicochemical parameters such as TH–Ca [r = 0.70 (wet) and 0.83 (dry)], TH–Mg [r = 0.69 (wet) and 0.67 (dry)], TH–Cl [r = 0.51(wet) and 0.58 (dry)], TH–HCO3 [r = 0.64(wet) and 0.59 (dry)], and TH–SO4 [r = 0.59(wet) and 0.50 (dry)]. This showed that there was great dependence of hardness on calcium, magnesium, bicarbonate, chloride, and sulphate. Chloride content was significantly and positively correlated with Ca [r = 0.62 (wet) and 0.70 (dry)], Mg [r = 0.70 (wet) and 0.68 (dry)], Na [r = 0.67 (wet) and 0.62 (dry)], and sulphate [r = 0.63 (wet) and 0.60 (dry)]. Good correlations were also observed in between HCO3–Ca [0.71 (wet) and 0.55 (dry)], TH – Ca [r = 0.70 (wet) and 0.83 (dry)], and TH – Mg [r = 0.69 (wet) and 0.67(dry)] which indicated similar sources and/or geochemical behaviour during ionic mobilization (Tiwari et al. 2015). The positive correlations between Cl – Na [r = 0.67 (wet) and 0.62 (dry)] indicated that Cl and part of the Na were derived from anthropogenic sources (Tiwari and Singh 2014). The good correlation between Ca – SO4 [0.61 (wet) and 0.62 (dry)] indicated that gypsum dissolution could be the major contributor for the dissolved ions in the water of study area.
However, no such significant relationships were observed in between the other constituents of the ground water samples.
Conclusion
Hydrochemical analysis in and around Jia Bharali river basin showed that majority of the shallow wells had preponderance of alkaline earths (Ca, Mg) and weak acids (HCO3) over alkalis (Na, K) and strong acids (SO4, Cl), thereby indicating calcium and magnesium bicarbonate type of water. Major cations Ca, Mg, Na, and K as well as major anions HCO3, Cl, SO4, and NO3 were found well within maximum permissible limits for drinking and irrigational purposes. Majority of the wells were found to come under the ‘excellent’-to-‘good’ category for irrigation purposes with respect to Electrical Conductivity values. The ground water regime was also free from alkalinity, chloride, and nitrate-nitrogen hazard. Low RSC (1.25 meq/L) and SAR (< 10) implied suitability of shallow wells for irrigation purposes, while a few wells found unfit for agricultural uses due to high Na% (> 60%) and Kelly’s Index (> 1). The results also revealed that a number of water sources are not suitable for drinking and agricultural uses due to the presence of excess trace metals like Cd, Cr, Fe, Mn, Ni, and Pb. Hence, this study suggests some limitations in the use of the shallow alluvial hand dug wells for drinking and irrigational purposes with respect to health hazard in the localities.
References
American Public Health Association, AWWA (1998) Standard methods for the examination of water and wastewater, 20th edn. Washington, DC
Apodaca LE, Jeffrey BB, Michelle CS (2002) Water quality in shallow alluvial aquifers, Upper Colorado River Basin, Colorado. J Am Water Res Assoc V 38(1):133–143
Ayers RS, Westcot DW (1994) Water quality for agriculture: FAO irrigation and drainage paper, 29. Rev. 1 (pp. 1–130)
Bernard P, Antoine L, Bernard L (2004) Principal component analysis: an appropriate tool for water quality evaluation and management—application to a tropical lake system. Ecol Model 178:295–311
Bhagure GR, Mirgane SR (2010) Heavy metal concentrations in ground waters and soils of Thane Region of Maharashtra, India. Environ Monit Assess. https://doi.org/10.1007/s10661-010-1412-9
Cieslak GM (1995) Toxic and mutagenic effects of chromium (VI) a review. Polyhedron 15:3667–3689
Cohen MD, Kargacin B, Klein CB, Costa M (1993) Mechanisms of chromium cancerogenicity and toxicity. Crit Rev Toxicol 23:255–268
Dasgupta AM, Purohit KM (2001) Status of surface and groundwater quality of Mandiakadar-part II: agricultural utilities. Pollut Res 20(2):219–225
Durfer CM, Backer E (1964) Public water supplies of the three largest cities in the U.S. US Geological Survey water supply paper no. 1812,364
Eaton FM (1950) Significance of carbonates in irrigated waters. Soil Sci 69:127–128
Farnese FS, Alves JO, Gusman GS, Leao GA, Silveira NM, Silva PEM, Ribeiro C, Cambraia J (2014) Effects of adding nitroprusside on arsenic stressed response of Pistia stratiotes L, under hydroponic conditions. Int J Phytorem 16:123–137
Garcia MG, Hidalgo Del VM, Blesa MA (2001) Geochemistry of groundwater in the alluvial plain of Tucuman province, Argentina. Hydrogeol J 9:597–610
Goering PL, Waalkes MP, Klaassen CD (1994) Toxicology of metals. In: Goyer RA, Cherian MG (eds) Handbook of Experimental Pharmacology, vol 115. Springer, New York, 189 pp
Goswami DC (1985) Brahmaputra river, Assam, India: physiography, basin denudation and channel aggradation. Water Resour Res 21:959–978
Guha Mazumder DN, Haque R, Ghose N, De BK, Santra A, Chakraborti D (2000) Arsenic in drinking water and the prevalence of respiratory effects in West Bengal, India. Int J Epidemiol 29:1047–1052
Hussain I, Hussain J, Dhinsa SS (2005) Groundwater quality variation in Bhilwara district, Rajasthan. Pollut Res 24(3):723–725
ISI (Indian Standards Institution) (1974) Indian standard tolerance limits for surface waters subject to pollution, (IS: 2296). New Delhi
ISI (Indian Standards Institution) (1991) Indian standard drinking water specifications, (IS: 10500). Bureau of Indian Standards, New Delhi
Jain CK, Sharma MK (2002) Regression analysis of ground water quality data of Sagar district, M. P. Indian J Environ Health 42(4):159–163
Jain SK, Agarwal PK, Singh VP (2007) Hydrology and water resources of India. Springer, Dordrecht (13-978-1-4020-5180-7, e-book)
Jalali M (2007) Salinization of ground water in arid and semiarid zones: An example from Tajarak, western Iran. Environ Geol 52:1133–1149
Jayakumar K, Vijayarengan P, Xing ZC, Jaleel CA (2008) Colloids Surf, 67. Biointerf, B, p 272
John R, Ahmad P, Gadgil K, Sharma S (2009) Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int J Plant Product 3(3):65–76
Kaur T, Bhardwaj R, Arora S (2017) Assessment of groundwater quality for drinking and irrigation purposes using hydrochemical studies in Malwa region, southwestern part of Punjab, India. Appl Water Sci 7:3301–3316. https://doi.org/10.1007/s13201-016-0476-2
Kelly WP (1940) Permissible composition and concentration of irrigated waters. Proceedings of the ASCF 66 (p. 607)
Khound NJ, Phukon P, Bhattacharyya KG (2012) A comparative study of ground water and surface water quality in the Jia—Bharali river basin, Assam, India with reference to physico–chemical characteristics. Int J Appl Sci Eng Res 1(3)
Khurshid SH, Hasan N, Zaheeruddin M (2002) Water quality status and environmental hazards in parts of Yamuna–Karwan sub-basin of Aligarh–Mathura district, Uttar Predesh. India. J Appl Hydrol 14(4):30–37
Kochian L, Hoekenga O, Piñeros M (2004) How do crops plants tolerate acid soils? Mechanisms of aluminium tolerance and phosphorus efficiency. Annu Rev Plant Biol 55:459–493
Kumar R, Singh RD, Sharma KD (2005) Water resources of India. Curr Sci 89:794–811
Kumar KS, Babu SH, Rao PE, Selvakumar S, Thivya C, Muralidharan S, Jeyabal G (2017) Evaluation of water quality and hydrogeochemistry of surface and groundwater, Tiruvallur District, Tamil Nadu, India. Appl Water Sci 7:2533–2544. https://doi.org/10.1007/s13201-016-0447-7
Lessard R, Reed D, Maheux B, Lambert J (1978) Lung cancer in New Caledonia, a nickel smelting island. J Occup Med 20:815–817
Majumdar D, Gupta N (2000) Nitrate pollution of groundwater and associated human health disorders, India. J Environ Health 42(1):28–39
Mondal NC, Singh VP (2010) Need of groundwater management in tannery belt: A scenario about Dindigul town, Tamil Nadu. J Geol Soc India 76(3):303–309
Mondal NC, Singh VP (2011) Hydrochemical analysis of salinization for a tannery belt in Southern India. J Hydrol 405(3):235–247
Mondal NC, Saxena VK, Singh VS (2008) Occurrence of elevated nitrate in groundwaters of Krishna delta, India. Environ Sci Technol 2(9):265–271
Mondal NC, Singh VS, Puranik SC, Singh VP (2010) Trace element concentration in groundwater of Pesarlanka Island, Krishna Delta, India. Environ Monit Assess 163(1–4):215–227
Mondal NC, Singh VP, Singh VS (2011) Hydrochemical characteristic of coastal aquifer from Tuticorin, Tamilnadu, India. Environ Monit Assess 175:531–550
Mondal NC, Tiwari KK, Sharma KC, Ahmed S (2016) A diagnosis of groundwater quality from a semiarid region in Rajasthan, India. Arab J Geosci 9(12):1–22
Morales KH, Ryan L, Kuo TL, Wu MM, Chen CJ (2000) Risk of internal cancers from arsenic in drinking water. Environ Health Perspect 108:655–661
Mugica V, Maubert M, Torres M, Munoz J, Rico E (2002) Temporal and spatial Variations of metal content in TSP and PM10 in Mexico City during 1996–1998. J Aerosol Sci 33:91–102
Narain S, Chauhan R (2000) Water quality status of river complex Yamuna at Panchnada dist. Etawah UP. I: An Integrated management approach. Poll Res 19(3):351–364
Nielsen FH (1998) Ultra trace elements in nutrition: Current knowledge and speculation. J Trace Elements Exp Med 11:251–274
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134–140
Owen CA (1981) Copper deficiency and toxicity: acquired and inherent, in plants, animals, and man. Noyes Publications, New Jersey, p 84
Paliwal KV (1967) Effect of gypsum application on the quality of irrigation waters. Madras Agric J 59:646–647
Paliwal KV (1972) Irrigation with saline water, Monogram no. 2 (New series). IARI, New Delhi, p 198
Pandian K, Sankar K (2007) Hydrogeochemistry and groundwater quality in the Vaippar river basin, Tamil Nadu. J GSI 69:970–982
Pillai KC, Paul P (1983) Trace metals in a tropical river environment: distribution. Water Air Soil Pollution 19:63–73
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–923
Pulle JS, Khan AM, Ambore NE, Kadam DD, Pawar SK (2005) Assessment of groundwater quality of Nanded City. Pollut Res 24(3):657–660
Puttaiah ET, Kiran BR (2008) Heavy metal transport in a sewage fed lake of Karnataka, India. Proceedings of Taal, 2007, the 12th world lake conferences: 347–354
Raghunath R, Sreedhara Murthy TR, Raghavan BR (2001) Spatial distribution of pH, EC and total dissolved solids of Nethravathi river basin, Karnataka state, India. Pollut Res 20(3):413–418
Rahman M (2002) Arsenic and contamination of drinking-water in Bangladesh, a public-health perspective. J Health Popul Nutr 20:193–197
Rathor G, Chopra N, Adhikari T (2014) Effect of variation in nickel concentration on growth of maize plant: a comparative overview for pot and hoagland culture. Res J Chem Sci 4(10):30–32
Ravikumar P, Somashekar RK, Angami M (2010) Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin, Belgaum District, Karnataka State, India. Environ Monit Assess. https://doi.org/10.1007/s10661-010-1399-2
Richards LA (1954) Diagnosis and improvement of saline and alkali soils, Agric. Handbook 60, U.S. Dept. of Agric., Washington, D.C., 160 pp
Sarwade DV, Nandakumar MV, Kesari MP, Mondal NC, Singh VS, Singh B (2007) Evaluation of seawater ingress into an Indian Attoll. Environ Geol 52(2):1475–1483
Saxena VK, Singh VS, Mondal NC, Jain SC (2003) Use of chemical parameters to delineation fresh ground water resources in Potharlanka Island, India. Environ Geol 44(5):516–521
Sharma SK, Subramanian V (2010) Source and distribution of trace metals and nutrients in Narmada and Tapti river basins. India Environ Earth Sci. https://doi.org/10.1007/s12665-010-0452-3
Singh KP, Malik A, Mohan D, Singh KV, Sinha S (2006) Evaluation of groundwater quality in northern Indo-Gangetic alluvium region. Environ Monit Assess 112:211–230
Singh AK, Raj B, Tiwari AK, Mahato MK (2013) Evaluation of hydrogeochemical processes and groundwater quality in the Jhansi district of Bundelkhand region, India. Environ Earth Sci 70(3):1225–1247
Sridhar SGD, Kanagaraj G, Mahalingam S, Amaladas P (2013) Hydro chemical analysis of groundwater between Sadras and Chinnakuppam, Kancheepuram District, Tamilnadu, India. J Acad Ind Res V 2:160–166
Stenhammar L (1999) Diarrhoea following contamination of drinking water with copper. Eur J Med Res 4:217–218
Subba Rao N (2006) Seasonal variation of groundwater quality in a part of Guntur district, Andhra Pradesh, India. Environ Geol 49:413–429
Sujatha D, Reddy RB (2003) Quality characterization of groundwater in the south-eastern part of the Ranja Reddy district, Andhra Pradesh, India. Environ Geol 44(5):579–586
Sundaray SK, Nayak BB, Bhatta D (2009) Environmental studies on river water quality with reference to suitability for agricultural purposes: Mahanadi river estuarine system, India—a case study. Environ Monit Assess 155:227–243
Sunitha V, Sudarsha V, Rajeswara Reddy B (2005) Hydrogeochemistry of groundwater, Gooty area, Anantapur district, Andhra Pradesh, India. Pollut Res 24(1):217–224
Tiwari TN, Manzoor A (1988a) River pollution in Kathmandu valley (Nepal) suitability of river water for irrigation. Indian J Environ Prot 8(4):269–274
Tiwari TN, Manzoor A (1988b) Pollution of Subarnarekha river near Jamshedpur and the suitability of its water for irrigation. Indian J Environ Prot 8(7):494–497
Tiwari AK, Singh AK (2014) Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh District, Uttar Pradesh. J Geol Soc India 83(3):329–343
Tiwari AK, Singh AK, Singh AK, Singh MP (2015) Hydrogeochemical analysis and evaluation of surface water quality of Pratapgarh District, Uttar Pradesh, India. Appl Water Sci. https://doi.org/10.1007/s13201-0150313-z
Tiwari AK, Maio MD, Singh P, Singh AK (2016) Hydrogeochemical characterization and groundwater quality assessment in a coal mining area, India. Arab J Geosci 9:177. https://doi.org/10.1007/s12517-015-2209-5
Underwood EJ (1977) Trace elements in human and animal nutrition. 4th edn. Academic Press Inc., New York
USEPA (1999) National primary drinking water regulation. United States Environmental Protection Agency
USSL (US Salinity Laboratory) (1954) Diagnosis and improvement of Saline and Alkaline soils, US Department of Agriculture. Handbook no. 60, p. 160
Vassilev A, Kerin V, Yordanov I (1995) Photosynthetic activities in Cd-treated barley Plants. Izvestia Timiriazev Acad Agric Sci 1:207–213 (In Russ.)
WHO (World Health Organization) (1984) Guidelines for drinking water quality. vol 2. Health criteria and other supporting information, WHO Publ, Geneva, 335
Wilcox LV (1948) The quality of water for irrigation use. US Department of Agriculture, Washington, DC, Technical Bulletin, p. 19
Zorer OS, Ceylan H, Dogru M (2008) Assessments of some trace heavy metals and radioactivity concentration in water of Bendimahi River Basin (Van, Turkey). Environ Monit Assess 147:183–190
Acknowledgements
The work was financed from the Department of Science and Technology, Govt. of India for the years 2007–2011. Research Grant No. was SR/S4/ES-21/BRAHMAPUTRAII/2005/(P-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khound, N.J., Phukon, P. & Bhattacharyya, K.G. Shallow alluvial aquifers for drinking and agricultural purposes: a case study from Jia Bharali River Basin, North Brahmaputra Plain, India. Sustain. Water Resour. Manag. 5, 989–1007 (2019). https://doi.org/10.1007/s40899-018-0276-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40899-018-0276-y