Abstract
Purpose
Bacteria are known to have a high ability to manufacture many compounds with biological functions in a short time compared with eukaryotic cells due to the fact that bacterial cells possess efficient metabolic mechanisms for the manufacture of these compounds (intracellular or extracellular). Herein, the goal of this study is to use pathogenic Enterococcus aerogenes bacteria strains, namely, S1, S2, and S3, isolated from the mouths of individuals with dental decay to produce silver nanoparticles in an environmentally friendly and cost-effective manner.
Methods
These nanoparticles have been tested for antibacterial activity against Streptococcus mitis, an MDR bacterium, either alone or in combination with antibiotics. These bacteria were identified using morphological characteristics and biochemical tests, in addition to molecular methods such as PCR and DNA sequences. Besides, their identification was done on the basis of their alignment with the reference strains in the NCBI blast to calculate the degree of similarity among these strains (S1, S2, and S3).
Results
The results of the current study showed a clear synergistic effect in the inhibition of Streptococcus mitis bacteria when mixing silver nanoparticles with some antibiotics, and it was found that there is a synergistic effect when mixing those AgNPs with erythromycin, followed by streptomycin and tetracycline. In contrast, the effect was antagonistic in the case of streptomycin and tetracycline antibiotics.
Conclusion
Enterobacter aerogenes AgNPs demonstrated excellent antibacterial efficacy on Streptococcus mitis isolates. Therefore, AgNPs in the dental care area have a wide range of applications.
Lay Summary
The current study attempted to show how AgNPs have a broad range of uses in the dental care field. Therefore, the study employed AgNPs that were created by Enterobacter aerogenes bacterial strains (S1, S2, and S3) for dental caries patients. AgNPs from Enterobacter aerogenes exhibited strong antibacterial activity against Streptococcus mitis isolates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The discovery of penicillin opened the way for humanity to search for new sources of antibiotics due to the action of these antibiotics in killing or inhibiting pathogenic bacteria that cause diseases in humans or animals. Nevertheless, a problem arose after the discovery of antibiotics: the level of bacterial resistance to antibiotics and the resulting risks to humanity. That would be a threat to humans at the level of all countries, whether developed or developing [1, 2]. In this regard, because of the distinct properties of silver nanoparticles, they have been the focus of attention for researchers during their development and have since been used in many fields, for example, in killing or inhibiting pathogenic bacteria [3].
According to the study’s statement which was done by Parthasarathi and Thilagavathi [4], the use of nanoparticles in several disciplines represents the fourth industrial revolution on the scale of inventions that changed the course of human history. One of the uses of nanoparticle science is that it is used in many industries, such as sunscreens, antibiotic substitutes, or enhancers, and in the production of many household appliances, such as refrigerators. Using biological approaches in the manufacture of metal nanoparticles gave them advantages over other manufacturing methods, being less expensive and more environmentally friendly [5].
According to several recent studies, nanotechnology science evolved throughout time and entered many industries, such as the manufacture of dyes or medical ointments. Here, it must be noted that the use of these particles in humans has been approved by the US Food and Drug Administration, such as in the treatment of many diseases resulting from infection with pathogenic bacteria, especially those that cause superficial infections [6].
The bacteria that cause human dental caries are part of a unique ecosystem of bacteria present in the human mouth. One of the most contagious illnesses is dental loss of bacterial origin, and the infection may spread to other tissues surrounding the tooth, including infection of the gingiva or pulp of the tooth [7]. The presence of this bacterium in the human mouth and the development of its infection may lead to tooth loss as a result of damage to the enamel and cementum, and it may extend to the gingiva, especially in most children of school age, and thus, it will represent a major medical problem [8].
Infection can also be transmitted from another person or domestic animal, and the situation has been exacerbated by the transformation of commensal bacteria in the human mouth cavity into bacteria that are pathogenic to him [9]. The bacteria that cause tooth decay can adhere to the surface of the tooth through the formation of biofilm, and they can be transformed from the mobile form to the stable form attached to the surface of the tooth as a result of the formation of biofilm, which is extracellular secretions that allow germs to adhere to the surface of the tooth and cause damage to the teeth and then the occurrence of decay [14]. Furthermore, gingivitis is a disease caused by some types of pathogenic bacteria, such as Fusobacterium nucleatum and Aggregatibacter actinomyces, which have special mechanisms through which they can penetrate deep into the tissues surrounding the tooth or deeper, causing damage to the tissues supporting the tooth and possibly reaching the jawbones [12].
Currently, there has been a large trend towards using nanotechnology as one of the alternatives to antibiotics or as one of the factors that contribute to improving the performance of these antibiotics against pathogenic bacteria that infect humans or animals. Nanoparticles were biosynthesized using live creatures including bacteria, algae, fungus, and plants [5]. Silver nanoparticles possess a broad spectrum of antibacterial, antifungal, and antiviral properties. Silver nanoparticles have the ability to penetrate bacterial cell walls, changing the structure of cell membranes and even resulting in cell death. Their efficacy is due not only to their nanoscale size but also to their large ratio of surface area to volume. They can increase the permeability of cell membranes, produce reactive oxygen species, and interrupt replication of deoxyribonucleic acid by releasing silver ions ([22].
This study belongs to a series of studies that employ the use of nanotechnology as one of the alternatives to antibiotics or as one of the factors that contribute to improving the performance of these antibiotics against bacteria that cause diseases that affect humans and animals alike. Therefore, in the current study, extracellular extract from Enterobacter aerogenes S2 strain bacteria that cause dental diseases was used in the biogenic synthesis of silver nanoparticles (AgNPs). The role of these AgNPs synthesized in this study in inhibiting dental disease bacteria and in enhancing the action of some antibiotics against the bacteria collected in the current study
Materials and Procedures
Samples Collection
Bacterial samples were collected from some medical dentists in the center of Misan Governorate. Swabs were obtained from the mouths of patients suffering from dental caries. It was then transferred to the laboratory by means of a special container prepared for this purpose. It was cultured on special media for these bacteria. Pure cultures were purified and diagnosed based on morphological characteristics, biochemical tests, and molecular methods.
Biomass Production
The biomass of the producing bacteria was obtained using the bacteria obtained in the previous step. Then, it transferred sufficient colonies to make the bacterial suspension. Inoculate 100 mL of nutrient broth medium with the bacterial suspension in a conical flask. Forty-eight-hour incubation period at 37 °C in a shaking incubator.
Extracellular Extract Collection
After incubation for 48 h, the biomass was centrifuged. To ensure that there were no bacterial cells in the supernatant, it was filtered through 0.2-m-pore filter paper, collected in a sterile flask, and incubated at 40 C. The composition of AgNPs was tested on intracellular extract.
Biogenic AgNP Synthesis
In the current study, AgNPs were bio-manufactured by combining 250 ml of bacterial extract and 250 ml of silver nitrate solution (1 mM). For 5 days, the mixture was incubated at 37 °C in a shaking incubator. The creation of silver nanoparticles was observed on a regular basis until the synthesis of silver nanoparticles was completed.
Characterization of Synthesized AgNPs
The change in hue of the reaction mixture indicated the composition of silver nanoparticles from light yellow to dark brown; this indicates the creation of these particles. The mixture was centrifuged, the filtrate was left behind, and the precipitate was collected, which represented silver nanoparticles. To remove the bacterial extract that remains, wash it multiple times with distilled water. It should be mentioned here that it was examined with a UV-Vis spectrophotometer with a wavelength of 330–800 nm to make sure that these particles were synthesized. The AgNPs that were synthesized in this study were characterized by using FTIR, SEM, and XRD methods.
Antibacterial Activity of AgNPs
Biogenically synthesized silver nanoparticles were used in the current study to evaluate their antibacterial activity against multi-drug-resistant (MDR) Streptococcus mitis pathogenic bacteria by the agar well diffusion method. These particles were also tested to find out their synergistic effect with some antibiotics, and that was done by adding 10 ml of AgNP solution to the antibiotic disks. To test the synergetic impact of AgNPs with antibiotics, the diameters of the inhibitory zones surrounding the antibiotic disks were measured in millimeters. The synergetic effect of AgNPs combined with antibiotics was calculated using two of the following methods [11]:
-
1.
$$\mathrm{Percentage\;fold\;increase\;area =}\left[{~}^{\left(b-a\right)}\!\left/ \!{~}_{a}\right.\right]\times 100$$(1)
where
a: antibiotic inhibition zone
b: antibiotic + AgNPs [20]
-
2.
$$FIC=\frac{MIC\;of\;drug\;A\;in\;the\;combination}{MIC\;of\;drug\;A\;alone}+\frac{MIC\;of\;drug\;B\;in\;the\;combination}{MIC\;of\;drug\;B\;alone}$$(2)
where
FIC index: fractional inhibitory concentration
(identify the interaction between two antibacterial combinations)
MIC: minimum inhibitory concentration
Results and Discussion
Characterization Morphologically and Biochemically
One hundred bacterial strains were obtained from 76 patients diagnosed using morphological characters, biochemical tests, and molecular methods. The bacterium Enterococcus aerogenes S2 strain was selected in the manufacture of nanoparticles (Fig. 1).
Physical Characterization
The conformation of silver nanoparticles composed of extracellular extract of E. aerogenes S2 bacteria used in this study was determined using a UV-visible spectrophotometer. It is recorded that the highest peak was above 400 nm, as seen in Fig. 2, so this indicates that the metal nanoparticles synthesized were composed of silver [13, 15]. That happens owing to the excitation of the surface plasmons existed on the outer surface of the AgNPs that get excited owing to the applied electromagnetic field [16]. This result agreed with the result obtained by Roy et al. [17].
The bioactive compounds present in the extracellular extract produced by E. aerogenes S2 bacteria, which is responsible for the formation of AgNPs, were identified using FTIR analysis. According to the results, the highest peak above 3000 cm−1 corresponds to O–H of alcohol or phenolic compounds. There were also peaks recorded around 1600 cm−1, which represent primary and secondary amines in the extracellular extract (Fig. 3). These compounds are known to be the primary constituents of proteins and to play an important role in the formation of silver nanoparticles. These results were in agreement with Castro et al. [10], who found that the proteins can interact with nanoparticles through cysteine or free amine group residues in the proteins.
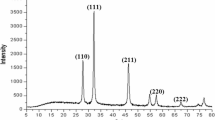
XRD analysis was used to find out the metallic nature of the nanoparticles. In the current study, the values of 2Ɵ ranged from 30 to 80, and the FIC values ranged from 100 to 300 (Fig. 4; Table 1), confirming the crystalline nature of the nanoparticles [21].
A scanning electron microscope (SEM) was also used in this study to determine the shapes and sizes of the AgNPs synthesized by extracellular E. aerogenes S2 strain. It was recorded that the AgNPs had spherical shapes, and their sizes were lower than 70 nm, as shown in Fig. 5. This is consistent with what was recorded by many studies: that the size of silver nanoparticles is less than 100 nm, such as in a study by Castro et al. [10].
Molecular Identification
Three strains belonging to Enterococcus aerogenes bacteria were obtained, named S1, S2, and S3, and identified based on their morphological characters, biochemical tests, and the diagnostic gene 16SrRNA. Genomic DNA was extracted from the bacteria included in this study (Fig. 6). The 16SrRNA gene was amplified by the PCR technique. The PCR products were shipped to Macrogen Company/Korea for sequencing. The results of 16SrRNA sequencing were aligned with the database found in the NCBI blast GenBank.
Enterobacter aerogenes Strain S2 was selected for producing silver nanoparticles. The bacterial filtrate (extracellular extract) of strain S2 was used in the manufacture of silver nanoparticles, where the creation of silver nanoparticles was indicated by a shift in solution color from bright yellow to dark brown.
Centrifugation was used to extract the broth culture pellet cells’ supernatant. The supernatant is then collected in a sterile flask and utilized to generate silver nanoparticles in a subsequent step [19]. Silver nanoparticles were created by mixing 250 ml of cell-free supernatant from an Enterobacter aerogenes 48-h liquid culture with 250 ml of a 1 mM silver nitrate (AgNo3) solution. The supernatant combination was then cultivated for 5 days at 37 °C in the dark on an orbital shaker (200 rpm). The experimenter’s control was a flask containing cell-free supernatant free of AgNO3 [18]. As shown in Figs. 7 and 8, AgNPs from the Enterobacter aerogenes strain (S2) were effective against the multidrug-resistant bacterial isolate Streptococcus mitis.
Antibacterial Activity
Disk diffusion was used to assess the antimicrobial activity of the antibiotics optochin OP, penicillin P, tetracycline TE, cefalexin CN, and streptomycin S alone and in combination with biogenic AgNPs synthesized by Enterobacter aerogenes. It has been evaluated for their antimicrobial activity against some pathogenic bacteria isolated from dental patients using an agar well and the disk diffusion method (Fig. 9).
According to the CLSI Standard, it was determined that, at a concentration of 0.009 (mg/ml), the mixed formulation of AgNPs exhibited antibacterial activity via a variety of antibiotic discs, including optochin, penicillin, tetracycline, erythromycin, cephalexin, and streptomycin as shown in Fig. 10. Based on the assay of the inhibition zone, biosynthesized NPs combined with the antibiotic erythromycin showed great synergistic antibacterial activity against the bacterial strain Streptococcus mitis, as shown in Fig. 11.
As mentioned earlier, the antibacterial activity of the manufactured silver nanoparticles in this study was determined by using an extracellular extract of the bacterium E. aerogenes S2. In the first method that AgNPs were used to inhibit the antibiotic-resistant Streptococcus mitis MDR by the agar well diffusion method, it was found that these particles contributed to the inhibition of the growth of MDR S. mitis bacteria with an 8-mm diameter inhibition zone.
The second method involved combining silver nanoparticles prepared in the current study with antibiotics used to treat dental caries bacteria in order to determine the synergetic effect when these silver nanoparticles were combined. To determine the synergy in the current study, two methods were used: knowing the increase in inhibition or calculating the fractional inhibitory concentration (FIC).The current study found that when silver nanoparticles were added to the antibiotic erythromycin, the percentage of inhibition increased, followed by the antibiotic penicillin, and a slight increase with the antibiotic optochin (Fig. 11).
The same is the case when calculating the FIC, where the results were similar to what was previously recorded in the percentage, where the best values of synergy were with the antibiotic erythromycin, followed by penicillin, and the lowest with the antibiotic optochin, as shown in Fig. 12. These results are the same as those of Birla et al. (2009), where the green synthesized AgNPs were estimated with antibiotics against gram-positive and gram-negative bacteria.
Lastly, the results of this study show that the silver nanoparticles made in this study have helped some of the antibiotics used in this study to work better. Researchers like Smekalova et al. [20] and Fayaz et al. [11] found similar results when they added AgNPs to antibiotics made from fungi or by the chemical makeup of silver nanoparticles, respectively. The inhibitory action of the silver nanoparticles is still not known accurately, but the most acceptable explanation is that the silver nanoparticles, due to their small size, will penetrate the cell wall of bacteria that are resistant to antibiotics. So, either go inside the bacterial cell by yourself or let antibiotics go inside the cell. This may explain the synergistic effect of silver nanoparticles when mixed with antibiotics.
After AgNPs break through the cell wall and enter a bacterial cell, they produce reactive oxygen species (ROS), bind to DNA, or interact with messenger RNA (mRNA). This may stop the cell from making proteins, which kills the cell (Skvitek et al., 2008).
Conclusion
The findings of the current study suggest that the silver nanoparticles manufactured during this study have helped to improve the efficacy of some of the antibiotics that were investigated in this study. Similar results have been reported by some researchers, such as Smekalova et al. [20] and Fayaz et al. [11], when they added AgNPs to antibiotics produced from fungi or by the chemical composition of silver nanoparticles, respectively. In light of this, the previous findings were validated by the current findings.
The results of this study also show that Enterobacter aerogenes nanoparticles may help treat oral infections caused by MDR Streptococcus mitis bacterial isolates, either on their own or with antibiotics.
The inhibitory action of the silver nanoparticles is still not known accurately, but the most acceptable explanation is that the silver nanoparticles, due to their small size, will penetrate the cell wall of bacteria that are resistant to antibiotics. Therefore, enter inside the bacterial cell alone or allow the entry of antibiotics inside the bacterial cell. This may explain the synergistic effect of silver nanoparticles when mixed with antibiotics. After AgNPs enter the bacterial cell after penetrating the cell wall, they produce reactive oxygen species (ROS), bind to DNA, or interact with mRNA, which may cause the inhibition of the cell replication process or stop the protein synthesis process, leading to cell death.
References
Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol. 2007;3(9):541–8.
Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metabol Synd Related Dis. 2015;13(10):423–44.
Deshmukh SP, Patil SM, Mullani SB, Delekar SD. Silver nanoparticles as an effective disinfectant: a review. Mater Sci Eng: C. 2019;97:954–65.
Parthasarathi V, Thilagavathi G. Synthesis and characterization of zinc oxide nanopartilce and its application on fabrics for microbe resistant defence clothing. Int J Pharm Pharmaceu Sci. 2011;3(4):392–8.
Abdeen S, Geo S, Praseetha PK, Dhanya RP. Biosynthesis of silver nanoparticles from Actinomycetes for therapeutic applications. International Journal of Nano Dimension. 2014;5(2):155–62.
Morones JR, Frey W. Room temperature synthesis of an optically and thermally responsive hybrid PNIPAM–gold nanoparticle. J Nanopart Res. 2010;12(4):1401–14.
Sabella FM, de Feiria SNB, Ribeiro ADA, Theodoro LH, Höfling JF, Parisotto TM, Duque C. Exploring the interplay between oral diseases, microbiome, and chronic diseases driven by metabolic dysfunction in childhood. Front Dental Med. 2021;2:718441.
Shay K. Infectious complications of dental and periodontal diseases in the elderly population. Clin Infect Dis. 2002;34(9):1215–23.
Shanmuganathan R, MubarakAli D, Prabakar D, Muthukumar H, Thajuddin N, Kumar SS, Pugazhendhi A. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environ Sci Poll Res. 2018;25(11):10362–70.
Castro L, Blázquez ML, Muñoz JA, González F, Ballester A. Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol. 2013;7(3):109–16.
Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomed: Nanotechnol, Biol Med. 2010;6(1):103–9.
Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44.
Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B: Biointerf. 2008;65(1):150–3.
Loesche W. Dental caries and periodontitis: contrasting two infections that have medical implications. Infect Dis Clin North Am. 2007;21(2):471–502.
Luo K, Jung S, Park KH, Kim YR. Microbial biosynthesis of silver nanoparticles in different culture media. J Agricult Food Chem. 2018;66(4):957–62.
Naheed A, Seema S, Singh V. Biosynthesis of silver nanoparticles from desmodium triflorum: a novel approach towards weed utilization. Biotechnol Res Int. 2011;8(1):1.
Roy A, Khanra K, Mishra A, d Bhttacharyya N. Highly cytotoxic (PA-1), less cytotoxic (A549) and antimicrobial activity of a green synthesized silver nanoparticle using Mikania cordata L. International. J Adv Res. 2013;1(5):193–8.
Saifuddin N, Wong CW, Yasumira AA. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. E-J Chem. 2009;6(1):61–70.
Singh H, Du J, Singh P, Yi TH. Extracellular synthesis of silver nanoparticles by Pseudomonas sp. THG-LS1. 4 and their antimicrobial application. J Pharma Anal. 2018;8(4):258–64.
Smekalova M, Aragon V, Panacek A, Prucek R, Zboril R, Kvitek L. Enhanced antibacterial effect of antibiotics in combination with silver nanoparticles against animal pathogens. Vet J. 2016;209:174–9.
Vanaja M, Paulkumar K, Baburaja M, Rajeshkumar S, Gnanajobitha G, Malarkodi C, et al. Degradation of methylene blue using biologically synthesized silver nanoparticles. Bioinorg Chem Appl. 2014;2014. https://doi.org/10.1155/2014/742346.
Yin IX, Zhang J, Zhao IS, Mei ML, Li Q, Chu CH. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int J Nanomedicine. 2020;15:2555–62. https://doi.org/10.2147/IJN.S246764.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abd Ali, M.A., Shareef, A.A. Antibacterial Activity of Silver Nanoparticles Derived from Extracellular Extract of Enterococcus aerogenes Against Dental Disease Bacteria Isolated. Regen. Eng. Transl. Med. 10, 68–77 (2024). https://doi.org/10.1007/s40883-023-00304-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-023-00304-2