Abstract
Introduction
Synthesis of multifunctional oxide nanomaterials using a cost-effective and eco-friendly route is being extensively carried out in recent times. NiO is one among the many metal oxides that has various technological applications such as gas sensing and catalysis.
Methodology
In the present study, NiO nanoparticles were successfully synthesized from an aqueous extract of Rhamnus prinoides leaf which acts as reducing and stabilizing agents. The formation of NiO nanoparticles was confirmed by different techniques such as powder X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM). In addition, Rietveld refinement analysis was also used to confirm the formation of face-centered cubic NiO nanoparticles.
Results
XRD pattern analysis revealed that the synthesized NiO nanoparticles have face-centered cubic structure in the bunsenite phase with an average crystallite size of 25.72 nm. FTIR spectral analysis of NiO exhibited the presence of a functional group responsible for the synthesis of NiO nanoparticles. Antibacterial activities of the synthesized nanoparticles were evaluated against Gram-positive (Staphylococcus aureus, listeria Mnocytogenes), and Gram-negative bacteria (Escherichia coli, Salmonella typhimurium) and the synthesized NiO nanoparticles exhibited effective growth inhibition property of Gram-negative bacteria.
Conclusion
In summary, nano-sized NiO nanoparticles were successfully synthesized through a cost-effective and environmentally friendly method, and its effectiveness for Gram-negative antibacterial growth inhibition was tested.
Lay Summary
The green synthesis of nanoparticles using plant leaf extract is a good alternative to conventional synthesis methods because of the simple synthesis procedure and eco-friendly property. Moreover, the synthesized nanoparticles are stable compared to those synthesized by conventional methods. In this work, NiO nanoparticles were successfully synthesized and their antibacterial activity was tested to be effective for Gram-negative bacteria (E. coli).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, nanostructured materials received the attention of several researchers due to their improved performances that arise from increased surface-area-to-volume ratio and the quantum confinement effect compared to their bulk counterpart [1]. Metal oxide nanomaterials, in particular, preserve the lion’s share as a result of their unique optical, electrical, magnetic, and catalytic properties, and their chemical reactivity, and thermal stability [2]. Among these, nickel oxide (NiO) nanostructures, exhibiting wide bandgap (3.6 to 4.0 eV) semiconducting property, receive paramount importance due to its multifunctional applications, inexpensiveness, and non-hazardous behavior [3, 4]. The prominent technological applications of NiO nanostructures include gas sensing [5], supercapacitor electrodes [6], photocatalysis [2, 7,8,9,10,11,12,13], and antibacterial activities [14,15,16].
So far, different synthesis methods have been used to produce NiO nanostructures such as hydrothermal [5], combustion synthesis [17], sol–gel [18], co-precipitation [19], and biosynthesis [20,21,22,23,24]. However, the biosynthesis technique which uses fungi, bacteria, and plant materials is an alternative to the conventional one due to its cost-effectiveness, environmental friendliness, and scalability to large-scale production [21,22,23,24].
Rhamnus prinoides L’Herit (Rhamnaceae) (R. prinoides), Gesho in Amharic, is an ever-green medicinal plant that has been used traditionally for the treatment of different infectious diseases [25,26,27]. In Ethiopia, the leaves, fruits, or roots of R. prinoides are used to treat scabies, hepatitis, tinea capitis, “chiffea” (eczema), ringworm, and dandruff, and for the management of waterborne and related diseases [28]. It has been shown that R. prinoides is an effective reducing and stabilizing agent for the successful synthesis of nanoparticles [29]. In this research work, we make use of the aqueous extract of freshly collected R. prinoides leaf as reducing and stabilizing agents for the successful synthesis of NiO nanoparticles.
Materials and Methods
Materials
All chemicals used in this research work were of analytical reagent and used without further purification. Nickel chloride hexahydrate (NiCl2·6H2O) with purity 97% was supplied by FINKEM Industry of India; sodium hydroxide (NaOH) with purity 98% was from Titan biotech Ltd.; and distilled water 99% purity was purchased from Addis Ababa, Ethiopia. Leafs of R. prinoides were collected from Shashemene Zone, Oromia region, Ethiopia.
Preparation of Plant Extract and Precursor
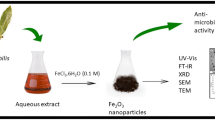
Freshly collected, healthy leaves of R. prinoides were taxonomically authenticated and cleaned with running water followed by distilled water. The leaves were shade dried at room temperature until all moisture was lost (2–5 days). Weighed 30 g of R. prinoides leaves was boiled using 600 ml of distilled water at 50 °C for 60 min. The aqueous extract was then cooled down to room temperature, filtered using Whatman No.1 filter paper, checked pH of 5.9, and stored at 4 °C for further use (Fig. 1). On the other hand, 0.1 M solution of nickel chloride hexahydrate (NiCl2.6H2O) was prepared by dissolving 11.8845 g of NiCi2.6H2O with 500 ml of distilled water while heating the solution at room temperature for about 30 min (Fig. 1). The mixture was stirred continuously until it forms a uniform solution and cooled down to room temperature.
Synthesis of NiO Nanoparticles
In a typical reaction, 250 ml of the precursor solution was added into 250 ml of the aqueous extract of R. prinoides drop-wise. The mixture was then boiled at a temperature of 80 °C while stirring continuously until the mixture forms a uniform solution for 1 h. After the addition of R. prinoides leaves extract, the color of the precursor salt changes from light green (Fig. 1) to brownish (Fig. 1) which is a clear indication of the reducing effect of the extract [30]. Finally, 5 ml of 0.5 M sodium hydroxide was added to complete the reaction (Fig. 1) and the mixture was cooled down to room temperature. The mixture is centrifuged at 4000 rpm for 5 min and washed using distilled water repeatedly and the paste (Fig. 1) was collected and dried in a drying oven at a temperature of 80 °C for 6 h [7] (Fig. 1). The dried powder is then transferred to a ceramic crucible and calcined at 500 °C for 2 h [7] and the resulting powder is carefully packed in a plastic sample holder for later characterization and application (Fig. 1).
Characterization
FTIR spectrometer (Nexus 470, wavelength range from 4000 to 500 cm−1 and 4 cm−1 resolution) was used to confirm the functional biomolecules associated with the R. prinoides extract and the synthesized NiO nanoparticles [24]. The purity and crystal structure of the synthesized NiO nanoparticles were examined with powder X-ray diffraction (XRD) and the pattern was recorded using an X-ray diffractometer (Phillips X Pert Model) equipped with a detector voltage of 40 kV in the 2θ range of 5–80° and scanning speed of 5/min.
The inter-planar spacing, d, for face-centered cubic crystal structure is calculated from the powder XRD pattern using Eq. (1):
where a is the lattice parameter constant, and h, k, and l are Miller’s indices.
The average crystallite size, D, is determined from the XRD peaks using Scherrer’s Eq. (2):
where \(k\) is the Scherrer’s shape factor with a value of 0.9, λ is the wavelength of radiation, \(\beta\) is the full width at half maximum (FWHM), and \(\theta\) is the diffraction angle.
Results and Discussion
Phytochemical Test of R. prinoides
First, the presence of bioactive compounds in the leaf extract of R. prinoides was examined and the result is shown in Table 1 below.
Fourier Transform Infrared (FTIR) Spectroscopy
The FTIR spectrum of R. prinoides leaf extract (Fig. 2) which shows absorption spectrum at 3669.7 cm−1 is assigned to O–H group stretching vibration and bands at 2984.6 cm−1 and 2899.5 cm−1 are attributed to H-OH bending vibration modes [24]. Bands from 1722 cm−1 to 1056 cm−1 are attributed to the C-O stretching and vibration modes. Figure 2 is the FTIR spectrum of the biosynthesized NiO nanoparticles. The broadband at 3361 cm−1 is characteristic of the hydroxyl group (O–H); this is due to the adsorptions of water molecules onto the NiO. Peaks observed at 1576.9 cm−1, 1393.7 cm−1, and 1034.6 cm−1 correspond to the symmetric and asymmetric stretching vibration and C-O. A peak of 452 cm−1 is assigned to the stretching mode of NiO [15, 24].
XRD Patterns of the As-Synthesized Nanoparticles
The synthesized NiO powder sample was calcined at 500 °C [2, 7] and the crystal structure analysis was carried out using powder X-ray diffraction, and the obtained patterns are presented in Fig. 3. XRD analysis revealed that face-centered cubic NiO nanoparticles were synthesized. The series of 2θ diffraction peaks at 37.35°, 43.33°, 62.96°, 74.55°, and 78.59° can be indexed to (111), (200), (220), (311), and (222) planes, respectively (JCPDS card no. 00–047-1049) which is confirmed by the Rietveld refinement analysis (Fig. 3). The lattice constant of the synthesized NiO is calculated as a = 4.18 Å. Furthermore, the sharp diffraction peaks confirm the high crystallinity of the synthesized NiO nanoparticles.
Thus, the average crystallite size is calculated from the existing five peaks at (111), (200), (220), (311), and (222) using Scherer’s Eq. (2), to be 25.72 nm (Table 2). Applying Eq. (1), the average inter-planar spacing is also found to be 16.9 nm.
Scanning Electron Microscope (SEM)
The formation of bunsenite phase NiO nanoparticles was revealed from SEM image (Fig. 4). The synthesized NiO nanoparticles have cubic structure as is observed from the SEM image.
Antibacterial Activities of NiO Nanoparticles
The antibacterial activity of the synthesized NiO nanoparticles was evaluated against the standard and clinical strain of Gram-positive and Gram-negative bacteria using the agar well diffusion method (Fig. 5). S. aureus and Listeria manoytogenes were chosen for the Gram-positive bacteria and S. typhimurium, E. coli, and Candida albicans were used for the Gram-negative bacteria. Initial concentration was prepared by dissolving 0.25 gm of synthesized NiO nanoparticle powder in 10 ml of dimethyl sulfoxide (DMSO) solvent and two successive serial dilutions were made by taking 2 ml and 5 ml from the resulting mixture. Chloramphenicol and DMSO drug solutions were used as the positive and negative control, respectively.
The inhibition diameter which is taken as the mean perpendicular diameter of the inhibition zone for the E coli spot was measured with a ruler (Table 3). The activity index is observed by dividing the inhibition diameter of the sample by that of the reference chloramphenicol drag solution which comes out to be 8 mm for 250 mg/ml, and 7 mm for 125 mg/ml, resulting in an activity index of 0.727 and 0.636, respectively. The synthesized NiO nanoparticle, thus, inhibited the growth of Gram-negative bacteria which is in agreement with earlier reports [15].
Conclusion
In conclusion, NiO nanoparticles with an average crystallite size of 25.72 nm were successfully synthesized from the leaf extract of R. prinoides as a reducing and stabilizing agent and nickel chloride hexahydrate precursor, and investigation of its antibacterial activity was conducted. The Rietveld refinement analysis also confirmed the formation of face-centered cubic structure. The stretching vibration mode observed at 1034.6 cm−1 and 452 cm−1 also corroborates the successful synthesis of NiO nanoparticles. The synthesized NiO inhibits the growth of Gram-positive bacteria, E. coli.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–31.
Sabouri Z, et al. Plant-based synthesis of NiO nanoparticles using salvia macrosiphon Boiss extract and examination of their water treatment. Rare Metals. 2019:1–11.
Haider A, et al. Green synthesized phytochemically (Zingiber officinale and Allium sativum) reduced nickel oxide nanoparticles confirmed bactericidal and catalytic potential. Nanoscale Res Lett. 2020;15(1):1–11.
Ezhilarasi AA, et al. Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. J Photochem Photobiol, B. 2018;180:39–50.
Cao S, et al. Hydrothermal synthesis of nanoparticles-assembled NiO microspheres and their sensing properties. Physica E Low Dimens Syst Nanostruct. 2020;118:113655.
Kundu M, Karunakaran G, Kuznetsov D. Green synthesis of NiO nanostructured materials using Hydrangea paniculata flower extracts and their efficient application as supercapacitor electrodes. Powder Technol. 2017;311:132–6.
Bashir A, et al. Biosynthesis of NiO nanoparticles for photodegradation of free cyanide solutions under ultraviolet light. J Phys Chem Solids. 2019;134:133–40.
Barzinjy AA, et al. Green and eco-friendly synthesis of nickel oxide nanoparticles and its photocatalytic activity for methyl orange degradation. J Mater Sci: Mater Electron. 2020;31:11303–16.
Aminuzzaman M, et al. Biosynthesis of NiO nanoparticles using soursop (Annona muricata L.) fruit peel green waste and their photocatalytic performance on crystal violet dye. J Clust Sci. 2020:1–10.
Sabouri Z, et al. Bio-based synthesized NiO nanoparticles and evaluation of their cellular toxicity and wastewater treatment effects. J Mol Struct. 2019;1191:101–9.
Akbari A, et al. Effect of nickel oxide nanoparticles as a photocatalyst in dyes degradation and evaluation of effective parameters in their removal from aqueous environments. Bioinorg Chem Appl. 2020;115:107867.
Sabouri Z, et al. Egg white-mediated green synthesis of NiO nanoparticles and study of their cytotoxicity and photocatalytic activity. Polyhedron. 2020;178:114351.
Sabouri Z, et al. Tragacanth-mediate synthesis of NiO nanosheets for cytotoxicity and photocatalytic degradation of organic dyes. Bioprocess Biosyst Eng. 2020;43(7):1209–18.
Ramalingam R, et al. Green synthesis, characterization and antibacterial evaluation of electrospun nickel oxide nanofibers. Mater Lett. 2019;256:126616.
Lingaraju K, et al. Biosynthesis of nickel oxide nanoparticles from Euphorbia heterophylla (L.) and their biological application. Arab J Chem. 2020;13(3):4712–9.
Helan V, et al. Neem leaves mediated preparation of NiO nanoparticles and its magnetization, coercivity and antibacterial analysis. Results Phys. 2016;6:712–8.
Ramasami AK, Reddy M, Balakrishna GR. Combustion synthesis and characterization of NiO nanoparticles. Mater Sci Semicond Process. 2015;40:194–202.
Zorkipli NNM, Kaus NHM, Mohamad AA. Synthesis of NiO nanoparticles through sol-gel method. Procedia Chem. 2016;19:626–31.
Sabouri Z, et al. Facile green synthesis of NiO nanoparticles and investigation of dye degradation and cytotoxicity effects. J Mol Struct. 2018;1173:931–6.
Habtemariam AB, Oumer M. Plant extract mediated synthesis of nickel oxide nanoparticles. Mater Int. 2020;2(2):205–9.
Noukelag S, et al. Structural and optical investigations of biosynthesized bunsenite NiO nanoparticles (NPs) via an aqueous extract of Rosmarinus officinalis (rosemary) leaves. Mater Today Proc. 2021;36:245–50.
Chaudhary J, et al. Synthesis and biological function of nickel and copper nanoparticles. Heliyon. 2019;5(6):e01878.
Ezhilarasi AA, et al. Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: cytotoxicity effect of nanoparticles against HT-29 cancer cells. J Photochem Photobiol B. 2016;164:352–60.
Din MI, et al. Single step green synthesis of stable nickel and nickel oxide nanoparticles from Calotropis gigantea: catalytic and antimicrobial potentials. Environ Nanotechnol Monit Manag. 2018;9:29–36.
Campbell M, et al. Rhamnus prinoides (gesho): a source of diverse anti-biofilm activity. J Ethnopharmacol. 2019;241:111955.
Lee M, Regu M, Seleshe S. Uniqueness of Ethiopian traditional alcoholic beverage of plant origin, tella. J Ethn Foods. 2015;2(3):110–4.
Molla Y, et al. Evaluation of the in vitro antibacterial activity of the solvent fractions of the leaves of Rhamnus prinoides L’Herit (Rhamnaceae) against pathogenic bacteria. BMC Complement Altern Med. 2016;16(1):1–9.
Chen G-L, et al. Antioxidant, anti-inflammatory activities and polyphenol profile of Rhamnus prinoides. Pharmaceuticals. 2020;13(4):55.
Habtemariam AB, Alemu Y. Synthesis of WO3 nanoparticles using Rhamnus prinoides leaf extract and evaluation of its antibacterial activities. Biointerface Res Appl Chem. 2022;12(1):529–36.
Al-Zahrani S, et al. Role of synthetic plant extracts on the production of silver-derived nanoparticles. Plants. 2021;10(8):1671.
Acknowledgements
The researchers would like to express their heartfelt gratitude to the Materials Science and Engineering Department at Addis Ababa Science and Technology University, for running SEM characterization, and Kadila Pharmaceutical Plc., Institute of Leather Technology, Addis Ababa, for FTIR characterization. The researchers are also thankful to the Materials Science and Engineering Department at Adama Science and Technology University for running XRD characterization.
Author information
Authors and Affiliations
Contributions
All authors contributed to the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals by any of the authors.
Consent to Participate
Not applicable.
Consent for Publication
Here we declare that the work described has not been published previously and it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out. The submission also implies that, if accepted, it will not be published elsewhere in the same form, in English, or any other language, without the written consent of the publisher.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Habtemariam, A.B., Bekele, E. Facile Synthesis of Nickel Oxide Nanoparticles Using Rhamnus prinoides Leaf Extract and Evaluation of its Antibacterial Activities. Regen. Eng. Transl. Med. 8, 482–488 (2022). https://doi.org/10.1007/s40883-022-00251-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-022-00251-4