Abstract
Field trials where fungicides have been applied for Fusarium head blight (FHB) control were established and monitored in 17 municipalities in the Guarapuava region, Southern Paraná, Brazil, during four years (2011 to 2014). A total of 227 isolates belonging to the Fusarium graminearum species complex were obtained from symptomatic wheat heads. The majority of the 227 isolates (64%) were of the 15-(A)cetyl-deoxynivalenol (DON), 29% of the nivalenol (NIV) and 4% of the 3-ADON genotype. A random sample of 35 isolates was selected to determine the effective concentration of tebuconazole and carbendazim that reduces 50% of the mycelial growth (EC50). The estimated means of the EC50 ranged from 0.0004 to 3.0 and from 0.91 to 2.65 μg/ml for tebuconazole and carbendazim, respectively. Isolates of the 15-ADON genotype were less sensitive to tebuconazole compared to NIV + 3ADON. Evidence of lower sensitivity for both fungicides were detected. Discriminatory doses (carbendazim = 1.4 μg/ml and tebuconazole = 2.0 μg/ml) were used to screen the entire population (n = 227) for less-sensitive isolates. In the 2011–2014 population, four tebuconazole-less sensitive (2%) and nine carbendazim-less sensitive (4%) isolates were found, all originating from Guarapuava. A contemporary sample of isolates from 2018 to 2020 (n = 42) obtained from the same region were screened using the discriminatory doses. The frequency of the less-sensitive isolates increased over time from 2 and 4% to 24 and 95% for tebuconazole and carbendazim, respectively. Control efficacy was generally lower in plants inoculated with less-sensitive isolates. Greater number of less-sensitive individuals were recovered from plants inoculated with a mixture with a sensitive isolate and sprayed with either fungicide. The presence of less sensitive isolates suggest that selection may be associated with the frequent reports of decline in control efficacy for both fungicides tested in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fusarium head blight (FHB) is one of the most destructive diseases of wheat and small grains worldwide (McMullen et al. 2012). FHB epidemics may lead to significant losses in crop yield (Duffeck et al. 2020) but also affect the quality of the grain due to mycotoxins that may accumulate at unsafe levels in the grains. The disease is caused by members of the Fusarium graminearum species complex (FGSC). At least five species have been reported associated with FHB in winter cereals in Brazil (Astolfi et al. 2011; Del Ponte et al. 2015). However, the dominant species (> 85%) in wheat in southern Brazil is F. graminearum 15-acetyl-deoxynivalenol (15ADON) genotype followed by F. meridionale nivalenol (NIV) genotype, which has a higher prevalence (around 30%) in the state of Paraná (Del Ponte et al. 2015). F. graminearum is a major DON producer, although some isolates are able to produce small amounts of its acetylated derivatives (3ADON, 15ADON), NIV and also zearalenone (Ward et al. 2008; Schmale et al. 2011; Del Ponte et al. 2012; Nicolli et al. 2015; Machado et al. 2022).
Despite the worldwide importance of the disease, FHB management is not an easy task. Although the disease is better managed integrating cultural practices, such as crop rotation to ensure residue management, and the use of moderately resistant cultivars, fungicide sprays play a major role to suppress disease development and mycotoxin accumulation (Wegulo et al. 2011).
Fusarium head blight (FHB) management heavily relies on the strategic use of fungicides, specifically when applied during peak flowering and alongside the cultivation of less vulnerable varieties, this strategy enhances the disease control (Mesterházy et al. 2003; Wegulo et al. 2011, 2015; Willyerd et al. 2012). Particularly, demethylation inhibitors (DMIs) have been persistently identified as superior in suppressing FHB and DON (Paul et al. 2008; Mesterházy et al. 2011, 2018; Machado et al. 2017). In the U.S. for example, metconazole or prothioconazole, either individually or combined, have exhibited superior efficacy, reducing FHB and DON more effectively than standalone uses of tebuconazole and propiconazole (Paul et al. 2008, 2018). In the context of Brazil, a meta-analysis demonstrated that tebuconazole outperforms propiconazole and carbendazim in terms of wheat yield, with an average increase of + 100 kg/ha (Machado et al. 2017) (Machado et al. 2017).
Quinone outside inhibitor (QoI) fungicides are typically not advised for Fusarium head blight (FHB) and deoxynivalenol (DON) management due to their reduced effectiveness relative to triazoles (Pirgozliev et al. 2003; Magan et al. 2011; Feksa et al. 2019; Bolanos-Carriel et al. 2020). In China, carbendazim, a benzimidazole chemical class, have been vastly applied but in recent years efficacy has decreased resulting in a failure of control (Liu et al. 2019). In fact, carbendazim-resistant isolates have been reported in China (Chen et al. 2007; Qiu and Shi 2014). In Brazil, carbendazim has also long use, prior to DMIs, for managing FHB (Deuner et al. 2011; Machado et al. 2017) which still remains as a cost-effective option for managing wheat diseases, including FHB, and little is known about fungicide resistance.
Information on sensitivity to triazole and even to benzimidazole fungicides among head blight pathogens infecting wheat in Brazil is scarce (Spolti et al. 2012a, b; Spolti and Ponte 2013; Avozani et al. 2014a, b) and it is still unclear whether there are any less sensitive isolates within the population. The present study is based on the hypothesis that there is resistance to carbendazim and/or to tebuconazole in FGSC populations in Brazil, especially in populations from regions with intensive application of fungicides. The objective of this study was to investigate the sensitivity to carbendazim and tebuconazole in a collection of F. graminearum associated with symptomatic wheat spikes from a high-fungicide-input region in south-central Paraná during 2011–2014. Additionally, we investigate the frequency of less-sensitive isolates in a sample from contemporary collection (2018–2020) obtained in the same growing region.
Material and methods
Study area and collection of isolates
Yearly survey for FHB symptoms was conducted on commercial fields of spring wheat at the Guarapuava region, southern Paraná, Brazil from 2011 to 2014. Symptomatic wheat spikes were collected at the field prior to kernel maturity. Grains were hand threshed, surface disinfected and placed on Petri dishes containing Nash-Snyder selective media (Leslie and Summerell 2006). After 7–10 days, isolates were obtained from colonies resembling F. graminearum sensu lato. Only one isolate was obtained from a single spike. Each isolate was purified from hyphae tips sub-culture and long-term preserved in SNA (Spezieller Nahrstoffarmeragar) (Astolfi et al. 2012; Gomes et al. 2015). Isolates were selected randomly from each year, and at least 21 or more isolates were obtained yearly, totaling 227 isolates.
A sub collection of F. graminearum 15ADON isolates obtained from symptomatic wheat heads in the same region in 2018 (n = 28), 2019 (n = 3) and 2020 (n = 11) were also used in the discriminatory dose screening assay (Andrade et al. 2022).
Trichothecene genotypes identification
The potential to produce B-trichothecenes was assessed based on a multiplex PCR assay. Fungal mycelia was collected from 7-day-old F. graminearum cultures growing on PDA medium. Genomic DNA was extracted using CTAB protocol with some modifications (Astolfi et al. 2012; Gomes et al. 2015). Three B-trichothecene genotypes, 3-ADON, 15-ADON, or NIV, were determined based on the amplification of portions of Tri3 and Tri12 genes (Ward et al. 2002).
EC50 and discriminatory doses determination
The EC50 (effective concentration of the fungicide able to reduce 50% of the mycelial growth) of the fungicides tebuconazole and carbendazim were determined for a subsample of around 20% of the isolates (n = 35), randomly chosen from the full collection. Aqueous stock solutions (100 µg a.i./ml) were obtained from commercially formulated tebuconazole (Folicur 200 EC; 20% of active ingredient [a.i.]; Bayer CropScience) and carbendazim (Carbomax 500 SC; 50% of a.i.; Nufarm Indústria química e farmacêutica S/A). Stock solutions of each fungicide were added to molten PDA medium (45 to 55 °C). The tested concentrations for both fungicides were: 0 (non-amended agar—PDA), 0.5, 1.0, 2.0, and 4.0 µg a.i./ml. One mycelial agar plug (6 mm in diameter) from the edge of a 7-day-old-culture was placed in the center of a Petri dish (90 mm in diameter) containing 15 ml of PDA amended with fungicides at each concentration. Two mycelial plugs were placed equidistantly on plates at concentrations above 1.0 µg a.i./ml. Preliminary tests did not show any difference whenever one or two plugs were used (data not shown). Each plug was considered an observational unit, and two replicates were used for each concentration. After incubation at 25 °C for 4 days in the dark, radial growth was measured in two perpendicular directions using a digital caliper.
Screening based on discriminatory doses
The discriminatory dose for each fungicide was established as a single dose which allowed the separation of isolates into ‘sensitive’ or ‘less sensitive’ categories (Russell 2004). The entire collection of isolates (n = 227) were tested for both fungicides, tebuconazole and carbendazim, including the 35 isolates previously characterized. For such, one mycelial agar plug (6 mm in diameter) from the edge of a 7-day-old-culture was placed in the center of a Petri dish (60 mm in diameter) containing 7 ml of PDA amended with the discriminatory dose of 2.0 µg a.i./ml for tebuconazole and 1.4 µg a.i./ml for carbendazim. For each isolate, a non-amended agar plate was used as an untreated check. After 3 days of incubation at 25 °C in the dark, colony diameter was measured in two perpendicular directions using a digital caliper. Isolate was considered ‘sensitive’ whether its growth was lower than 50% at the discriminatory dose compared to growth on non-amended agar, and ‘less-sensitive’ otherwise (Russell 2004). The EC50 assays, with the same range of concentrations as described previously, were performed for the less-sensitive isolates to confirm results using the discriminatory dose.
Molecular characterization of fungicide resistance
Partial sequences of CYP51 and β2-tub genes of the two less sensitive (UEM 2536 and UEM 2657) and two sensitive (UEM 3587 and UEM 2795) isolates, were analyzed to detect mutations related to resistance to each fungicide. For such, isolates were grown in YES liquid medium (2 g of yeast extract, 15 g of sucrose for 100 ml of water) for 5 days at 25 °C. Mycelia were removed, dried in filter paper, and macerated in liquid nitrogen. Genomic DNA was extracted using cetyltrimethylammonium bromide (CTAB) protocol as previously described (Del Ponte et al. 2015).
PCR was performed in a final volume of 25 μL with 1 μL of DNA (~ 25 ng/μL), 1 μL of each primer at 10 μM, 16.9 μL of water e 2.5 μL Taq buffer, 0.5 μL of dNTP (0.4 mM each), 2.0 μL of MgCl2 e 0.1 μL of Taq Polymerase (Kapa Biosystems). The CYP51 primer sets F51A-F/R, F51B-F/R and F51C-F1/R1 were used to amplify the full sequence of cyp51A, cyp51B and cyp51C respectively (Yin et al. 2009). The primer Tub2seq-F/R was used to amplify the part of β2-tub gene (Liu et al. 2010). For CYP51 primers, the amplification reaction consisted of an initial denaturation at 95 °C for 3 min, followed by 40 cycles at 94 °C for 1 min, 56 °C for 30 s, 72 °C for 1 min, and a final extension at 72 °C for 5 min. The amplified fragment obtained has around 1200-pb. For β2-tub primers, the amplification reaction consisted an initial denaturation of 95 °C for 3 min, followed by 35 cycles at 94 °C for 40 s, 57 °C for 40 s, 72 °C for 1.5 min, and a final extension at 72 °C for 5 min. The amplified fragment obtained has around 1200-pb. Amplification was confirmed by using 3 μL of PCR product separated by electrophoresis on a 1% agarose gel. PCR products were purified using ExoSAP-IT (USB, Cleveland, OH) and sequenced by Macrogen Services (Kumchun-ku, Seoul, Korea) using each primer set used in PCR. Sequences were edited with DNA Baser Sequence Assembler v4 (Heracle BioSoft, 2013) and aligned using MEGA 7.0 (Tamura et al. 2013).
Fungicide efficacy and competitiveness assay under greenhouse conditions
Seed of ‘BR 18’, a spring wheat variety classified as highly susceptible to FHB, were sown in pots (10 seeds per 1-L pot) filled with substrate (Tropstrato®HT vegetables) fertilized with macronutrients (N-P-K at ratio 4–14-8). After the seedling emergence, plants were thinned to keep the 6 main tillers in each pot. Seedlings were fertilized weekly with 50 ml of nutrient solution containing 1.0 mM KNO3, 0.25 mM NH4H2PO4, 0.1 mM NH4Cl, 0.5 mM MgSO4.7H2O, 1.0 mM Ca(NO3)0.4H2O, 0.30 μM CuSO4.5H2O, 0.33 μM ZnSO4.7H2O, 11.5 μM H3BO3, 3.5 μM MnCl2.4H2O, 0.1 μM (NH4)6Mo7O2.4H2O, 25 μM FeSO4.7H2O and 25 μM EDTA bisodium. Plants were kept in the greenhouse from planting to maturity.
Two factorial experiments were conducted spaced at 15 days between sowing dates. In the first experiment (3 × 5 factorial experiment), three treatments were tested (non-sprayed check, TEBU-sprayed, CARB-sprayed, and metconazole-sprayed) and five inoculation treatments consisting of isolates sprayed singly (S, R1 and R2) or in mixtures (S + R1, S + R2) were used. At early-milk stage, the plants were sprayed with tebuconazole (150 g a.i./ha, Folicur 200 EC; BAYER Corporation), carbendazim (250 g a.i./ha, Carbomax 500 SC, Nufarm Indústria química e farmacêutica S/A) or metconazole (81 g a.i./ha, Caramba 90 SL; BASF Corporation). Both fungicide-treated and nontreated plants (check treatment) were kept in the greenhouse for 48 h and spray inoculated with three distinct inoculum compositions: the R1 (UEM 2536 isolate, less-sensitive to CARB and TEBU), R2 (UEM 2657 isolate less-sensitive to CARB and TEBU) and S (CML 3066, sensitive to CARB and TEBU, and highly aggressive on wheat) isolates inoculated individually or in mixture (1:1 ratio of S:R1 or S:R2). A macroconidia suspension (1 × 104 conidia/ml) was applied uniformly on wheat spikes using a household handheld sprayer at a rate of approximately 1 ml/head. Immediately after inoculation, plants were covered with a plastic bag and kept for 36 h in a growth chamber at 22 °C and a light–dark cycle of 12-12 h, respectively. The bags were removed and the plants remained in the greenhouse until harvest. Assessments of disease incidence and severity were made at 5, 10, and 15 days after inoculation in 6 spikes/pot in four pots (replicates) per treatment. The treatments were arranged in a completely randomized design with four replicates.
Before inoculation of wheat plants, less-sensitive isolates were evaluated for their ability to be distinguished from the sensitive isolate based on a vegetative compatibility assay (McCallum et al. 2004). For such, the absence or presence of barrage zones, when paired with their clonal cultures, were used to identify the recovered isolate from a dual inoculum treatment. At kernel maturity, a random sample of forty kernels, ten from each replicate of the S + R1 and S + R2 treatments, were obtained from each fungicide-sprayed and non-sprayed treatments. Kernels were incubated under wet conditions inside a plastic box containing a wet blotter paper. After incubating for three days at 25 °C with 12 h of light and darkness, F. graminearum-like colony developing on kernels provided a single isolate. For such, hyphae tips were subcultured on SNA plates and long-term preserved as described above. Four mycelial plugs of each recovered isolate were placed equidistantly around the outer edge of a Petri dish containing V8-wheat medium (150 ml of V8 juice, 20 g of ground wheat seed, and 20 g of agar per 1 L of medium) (McCallum et al. 2004). A mycelial plug of either inoculated isolate was placed at the center of the plates. After 10 days of incubation at 22 °C and continuous light, the presence of barrage zones was indicative of an incompatible reaction at the junctions of growing colonies, which allowed us to identify the recovered isolates.
Data analysis
The EC50 was calculated based on the linear regression between the relative mycelial growth inhibition (%) relative to unamend-check and the log-transformed fungicide concentrations (Becher et al. 2010; Liu et al. 2010; Spolti et al. 2014; Lehner et al. 2015). Plug diameter was previously subtracted from mean colony diameter. Multiple resistance was assessed based on the Spearman’s rank correlation analysis (α = 0.05) (because normality could not be assumed) for the relationship between EC50 values of tebuconazole and carbendazim. The distributions of EC50 values of each fungicide, for each genotype, were compared using a Kolmogorov–Smirnov non-parametric test (α = 0.05).
The area under disease severity progress curve (AUDPC) was calculated for each fungicide efficacy assay using function ‘audpc()’ from package `agricolae` (De Mendiburu and Simon 2015). AUDPC data from each trial were tested individually for normality and homoscedasticity. Then, data were subjected to two-way analysis of variance (ANOVA). Fungicide spray, inoculum composition and trial (for the greenhouse experiment replications) were treated as fixed effects, and blocks (for the field trial) were included as a factor in our model to account for the effect of the blocking variable. When differences by the F test were significant (P < 0.05), the averages were compared by the Tukey test at 5% significance with the add-on package ‘easyanova’ (Arnhold 2013).
In the competitiveness assay, the χ2 test and Fisher’s exact test (for small sample size) (P > 0.05) were used to evaluate the differences in the frequencies of recovered isolates from wheat heads inoculated with a mixture of sensitive and less-sensitive isolate. The frequency of the isolates was compared between carbendazim- or tebuconazole-sprayed and non-sprayed plants. All analyses and graphical work were performed using the R statistical computing environment (R Core Team 2021).
Results
Trichothecene genotypes frequency
In total, 227 isolates were obtained in 17 municipalities (Fig. 1A). The number of isolates ranged from 21 to 91 isolates among the years (Fig. 1B). The molecular assay based on Tri3 and Tri12 gene portions showed that the 15-ADON genotype was dominant overall (64%, 145/227), followed by the NIV genotype (29%, 65/227) and 3-ADON genotype (4%, 8/227). The molecular genotyping failed for eight isolates (4%, Fig. 1B).
A) Geographical location and number of Fusarium graminearum isolates (n = 227) obtained from symptomatic spikes on high fungicide-input wheat fields across Paraná state in southern Brazil. B) Frequency of isolates per year characterized as 15-acetyl-deoxynivalenol (15ADON), 3ADON or nivalenol (NIV) trichothecene genotypes. C) Density plots for the distribution of the effective concentration of tebuconazole (TEBU) and carbendazim (CARB) that reduces 50% of mycelial growth (EC50) of a sample of 35 F. graminearum isolates, of 15ADON (n = 22, blue density distribution) and 3ADON + NIV isolates (n = 11, blue density distribution)
EC50 determination
The EC50 values for tebuconazole ranged from 0.0004 to 3.15 µg/ml and the overall mean was 0.39 µg/ml. For carbendazim, the EC50 values were 0.91 to 2.74 µg/ml and the overall mean was 1.26 µg/ml (Fig. 1C). Overall, the estimated EC50 values were lowest for tebuconazole and highest for carbendazim (P < 0.005, Table 1). There were three isolates with higher EC50 values for tebuconazole and one for carbendazim, all of the 15ADON genotype (Fig. 1C). The EC50 for tebuconazole and carbendazim varied among isolates of each trichothecene genotype group. However, the K-S test indicated that the sensitivity to tebuconazole of the 15ADON and NIV + 3ADON isolates were sampled from populations with different distributions, but not for carbendazim (Table 1).
Lower sensitivity between the two fungicides was detected based on the significance of the Spearman’s correlation coefficient (R = 0.30, P < 0.05). In fact, the same isolate (UEM 2536) showed the highest EC50 for tebuconazole and carbendazim (Fig. 1C, Table 1).
Screening based on discriminatory doses
For tebuconazole, discriminatory dose was chosen based on outliers from the EC50 distribution of the 35 isolates (Fig. 2A). For carbendazim, the dose of 1.4 µg a.i./ml used in China (Liu et al. 2010) was initially taken as reference value, which was confirmed by the distribution of the EC50 values distribution of the subsample (Fig. 2B).
Distribution of effective concentration of (A) tebuconazole (TEBU) and (B) carbendazim (CARB) that reduces 50% of mycelial growth (EC50) for a sample of 33 F. graminearum isolates, of 15ADON (n = 22) and 3ADON + NIV isolates (n = 11).The horizontal line inside the box represents the median, the lines at the top and bottom of the boxes represent the 75% and 25% percentiles of the data, respectively. The individual dots represent the mean value for each isolate. The points outside the 25% and 75% percentiles range represent outliers. Dashed red lines represent the defined discriminatory doses for tebuconazole (2 µg/ml) and for carbendazim (1.4 µg/ml)
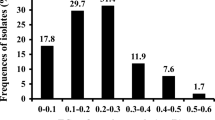
In the 2011–2014 population, four isolates were classified as less-sensitive to tebuconazole (2%, Fig. 3B), but only two were confirmed by the EC50: UEM 2657 (EC50 = 2.44 μg/ml) and UEM 2536 (EC50 = 3.00 μg/ml). Nine isolates were considered less-sensitive to carbendazim (4%, Fig. 3B), and six were confirmed with the EC50: UEM 2657 (EC50 = 1.56 μg/ml), UEM 2655 (EC50 = 1.50 μg/ml), UEM 3325 (EC50 = 1.61 μg/ml), UEM 2519 (EC50 = 1.48 μg/ml), UEM 2536 (EC50 = 2.65 μg/ml) and UEM 3248 (EC50 = 1.56 μg/ml). All of the less-sensitive isolates were obtained from plots in the municipality of Guarapuava. All less-sensitive isolates had the 15ADON trichothecene genotype, except UEM 2519 and UEM 3248 that did not have the genotype characterized (Fig. 3).
Frequency of less sensitive isolates of Fusarium graminearum determined by the discriminatory doses of 2 µg/ml for (A) tebuconazole and 1.4 µg/ml for (B) carbendazim, across the 2011–2014 population (n = 227 isolates, n2011 = 55, n2012 = 60, n2013 = 91 and n2013 = 21) and 2018–2020 population (n = 42, n2018 = 28, n2019 = 3 and n2020 = 11). Isolate was considered ‘sensitive’ whether its growth was less than 50% at the discriminatory dose compared to growth on non-amended agar, and ‘less sensitive’ otherwise
All but two isolates were considered less sensitive to carbendazim (95%) and ten isolates were classified as less-sensitive to tebuconazole (24%) in the 2018–2020 sample of isolates. An increase in frequency of less-sensitive isolates were observed for tebuconazole but more concerning for carbendazim.
Molecular characterization of fungicide resistance
Two selected isolates based on the response to carbendazim and tebuconazole discriminatory doses and confirmed by EC50 were sequenced to check for any mutation in target genes. Analysis of the partial sequences of β2-tub and CYP51 genes for the less sensitive isolates to carbendazim and tebuconazole did not identify any mutations compared with the reference sensitive isolate.
Fungicide efficacy
All the isolates tested caused FHB on wheat heads, but there was a large degree of variation in disease severity under greenhouse or field conditions. All data from the two greenhouse trials were pooled once there was no significant interaction between the data from the two trials and the other fixed effects (P = 0.401). The interaction tested (fungicide spray vs isolates) did not affect the development of the disease (P = 0.843). All the fungicides were able to reduce the AUDPC regardless of inoculum composition. Control efficacy, based on the AUDPC, was 25, 45 and 57% when plants were sprayed with carbendazim, metconazole and tebuconazole, respectively (Fig. 4A). Mean AUDPC did not differ between isolates and their mixtures, except for R1 causing the lowest amount of FHB (Figs. 4B).
Adjusted means and the respective standard error by the linear model fitted to the area under the disease severity progress curves (AUDPC) data pooled from the two greenhouse trials for Factor 1 (fungicide sprays) (A) and for Factor 2 (isolates) (B). Inoculum compositions: R1 (2536 isolate, less-sensitive to CARB and TEBU), R2 (2657 isolate less-sensitive to CARB and TEBU) and S (CML 3066, sensitive to CARB and TEBU, and highly aggressive on wheat) isolates inoculated individually or in mixture (1:1, S:R1 or S:R2). Means with different colors within each factor differed from each other based on Tukey test (α = 0.05)
Competitiveness assay
In total, 677 single-spored isolates were obtained from kernels inoculated with the mixture of isolates (69% the first trial and 31% from second trial). In total, 84, 14 and 2% of the recovered isolates were clonal to the sensitive highly-aggressive isolate (S) and the less-sensitive ones (R2 and R1), respectively. In non-fungicide treated plants, 82 and 99% of the recovered isolates were clonal to the sensitive isolate rather than R2 and R1, respectively. Grains from inoculated plants with the mixture of S + R1, an increase in frequency of the less sensitive isolate were observed when sprayed with tebuconazole (P = 0.027) but not with carbendazim (P = 0.309, Fig. 5A). Similarly, frequency of the less sensitive isolate R2 was higher in tebuconazole-sprayed plants (P = 0.041) but not in carbendazim-sprayed plants (P = 0.130) when compared with untreated plants (Fig. 5B). When metconazole was sprayed, there was no increase in the frequency of recovered less-sensitive isolates.
Mosaic plots for the proportion of less-sensitive (r1, blue and r2, orange fill) and sensitive (s, yellow fill) isolates recovered from grains of wheat plants inoculated with an equal mixture of these isolates at early milk stage of grain development and sprayed with carbendazim (carb), metconazole (metc), tebuconazole (tebu) and water (untreated). Recovered isolates were identified based on the presence of barrage zones in incompatible pair matches (nonclonal)
Discussion
This study provides the first characterization of the sensitivity to a DMI (tebuconazole) and a BMC (carbendazim) in isolates representing the most dominant FGSC species causing head blight in the south of Paraná State, Brazil. Although DMIs are most recommended for FHB control worldwide (Blandino et al. 2006; Willyerd et al. 2012; Chen et al. 2012), carbendazim has found use for FHB control in China for more than thirty years (Chen et al. 2007, 2012). There, several reports of carbendazim-resistant isolates have been made (Chen et al. 2007; Qiu et al. 2014). In Brazil, carbendazim has also long use, prior to DMIs, for managing FHB (Deuner et al. 2011) which has been a cost-effective option for managing wheat diseases, including FHB, and little is known about fungicide resistance. Very recently, carbendazim-based products were banished to be commercialized in Brazil (ANVISA 2022), although growers can still use their own stocks. While previous study reported levels of sensitivity to two DMIs for FHB pathogen populations from southern Brazil (Spolti et al. 2012a, b), data on resistance to carbendazim are very limited. For instance, Avozani et al. (2014b) determined EC50 for carbendazim in only five FGSC isolates obtained from wheat, maize and barley from south of Brazil. The values ranged from 0.02 a 0.14 µg/mL. In our study, the mean EC50 for carbendazim was much greater (1.25 µg/ml), with six isolates being less sensitive to carbendazim (2.64%) based on a discriminatory dose determined for FGSC isolates from China.
Isolates exhibiting relatively high EC50 values for tebuconazole were reported in the United States (Spolti et al. 2014) and in China (Yin et al. 2009). In the state of New York, two least sensitive isolates showed EC50 of 8.09 µg/ml and 2.46 µg/ml in a collection of 50 isolates. In our study, only two isolates (0.8%) exhibited EC50 higher than 2.0 µg/ml. In Uruguay, the mean tebuconazole EC50 was 0.29 µg/ml for F. graminearum (n = 42) and 0.61 µg/ml for F. asiaticum and F. cortaderiae (n = 14) (Umpiérrez-Failache et al. 2013). The mean EC50 for the same fungicide in Rio Grande do Sul in a collection of 50 isolates was 0.0136 µg/ml (Spolti et al. 2012b), much lower than the mean determine in the present study (0.39 µg/ml).
The increased mean EC50 and presence of less sensitive isolates in Paraná state compared with Rio Grande do Sul state may be due to the more intensive use of DMIs for managing wheat diseases including FHB which may be selecting for fungicide resistance. Monitoring the sensitivity to fungicides as well as levels of efficacy in the field is critical, mainly for DMIs and MBCs. Thus far, declines in fungicide efficacy for FHB control over the years has not been reported, especially associated with fungicide resistance. In China, declines in the efficacy of carbendazim in the field has been linked to reduced sensitiveness of the pathogen to this fungicide (Chen et al. 2007).
Overall, 15-ADON isolates were less sensitive than NIV and 3-ADON isolates combined. In a previous study, F. graminearum isolates from Rio Grande do Sul, Brazil, were 20x less sensitive than F. meridionale isolates (Spolti et al. 2012b). In Uruguay, the mean EC50 for F. asiaticum and F. cortaderiae (0.61) were greater to F. graminearum (0.29 µg/ml, Umpiérrez-Failache et al. 2013). Although we did not identify the species, the 15-ADON isolates are probably F. graminearum according to a large survey conducted across years, locations and hosts (Del Ponte et al. 2015; Gomes et al. 2015; Kuhnem et al. 2016). However, the NIV has been assigned to more than one species, but mainly F. meridionale. Our results confirm the lower sensitivity of the dominant species causing FHB in Brazil (Spolti et al. 2012a, b; Nicolli et al. 2018), a fact that may help to explain its dominance over other species.
For the first time in Brazil, we report two isolates that were less sensitive to both tebuconazole and carbendazim, a fact that combined with the significant correlation between the EC50 values of both fungicides, suggest multiple resistance. The literature reports isolates resistant to tebuconazole and procloraz, an imidazole (Yin et al. 2009) as well as cross resistance between tebuconazole and metconazole (Spolti et al. 2012b).
We demonstrated that the frequency of less sensitive isolates to either carbendazim or tebuconazole increased over time specially in Guarapuava. This region is considered a high-fungicide input wheat producing region with at least three sprays per season to control FHB imposing a high selection pressure towards less sensitive isolates. Although the frequency of TEBU less-sensitive increases to 24%, a more concerning scenario is faced with CARB, where almost all isolates (95%) screened in a contemporary sample obtained on the same region were considered to have lower sensitivity.
Moreover, we demonstrated that the FHB control efficacy was generally lower in plants inoculated with less sensitive isolates under greenhouse and field conditions. In fact, a greater number of less-sensitive isolates were recovered from plants inoculated with a mixture of a sensitive highly-aggressive isolate and sprayed with either fungicide. These findings suggest that the divergence from the initial frequency of inoculated isolates may provide evidence of a selection pressure towards less-sensitive isolates in the presence of either fungicide (Kretschmer et al. 2009; Spolti et al. 2014). This evidence corroborates with the increase of less-sensitive isolates obtained in a more recent sample of isolates. Conversely, the ratio of recovered isolates in the absence of either fungicide deviated from the expected 50:50% proportion, suggesting that it might have a pathogenic cost associated with fungicide less-sensitiveness trait of the tested isolates. Future studies should explore the adaptive cost for these isolates with low sensitivity in the absence of the fungicide, as well as whether a decline in control of Fusarium head blight might be associated with the increase of the frequency of less sensitive isolates under field conditions.
Data availability
The data and R codes used for the analysis of the data are available at: https://doi.org/10.17605/OSF.IO/6AZSN
References
Andrade SMP, Augusti GR, Paiva GF, Feksa HR, Tessmann DJ, Machado FJ, Mizubuti ESG, Del Ponte E (2022) Phenotypic and molecular characterization of the resistance to azoxystrobin and pyraclostrobin in Fusarium graminearum populations from Brazil. Plant Pathology 71:1152–1163
Arnhold E (2013) Package in the R environment for analysis of variance and complementary analyses. Brazilian Journal of Veterinary Research and Animal Science 50:488–492
Astolfi P, Reynoso MM, Ramirez ML, Chulze SN, Alves TCA, Tessmann DJ, Del Ponte EM (2012) Genetic population structure and trichothecene genotypes of Fusarium graminearum isolated from wheat in southern Brazil. Plant Pathology 61:289–295
Astolfi P, dos Santos J, Schneider L, Gomes LB, Silva CN, Tessmann DJ, Del Ponte EM (2011) Molecular survey of trichothecene genotypes of Fusarium graminearum species complex from barley in Southern Brazil. International Journal of Food Microbiology 148:197–201
Avozani A, Reis EM, Tonin RB (2014a) In vitro sensitivity reduction of Fusarium graminearum to DMI and QoI fungicides. Summa Phytopathologica 40:358–364
Avozani A, Tonin RB, Reis EM, Camera J, Ranzi C (2014b) In vitro sensitivity of Fusarium graminearum isolates to fungicides. Summa Phytopathologica 40:231–247
Becher R, Hettwer U, Karlovsky P, Deising HB, Wirsel SGR (2010) Adaptation of Fusarium graminearum to tebuconazole yielded descendants diverging for levels of fitness, fungicide resistance, virulence, and mycotoxin production. Phytopathology 100:444–453
Blandino M, Minelli L, Reyneri A (2006) Strategies for the chemical control of Fusarium head blight: Effect on yield, alveographic parameters and deoxynivalenol contamination in winter wheat grain. European Journal of Agronomy 25:193–201
Bolanos-Carriel C, Wegulo SN, Baenziger PS, Funnell-Harris D, Hallen-Adams HE, Eskridge KM (2020) Effects of fungicide chemical class, fungicide application timing, and environment on Fusarium head blight in winter wheat. European Journal of Plant Pathology 158:667–679
Chen C, Wang J, Luo Q, Yuan S, Zhou M (2007) Characterization and fitness of carbendazim-resistant strains of Fusarium graminearum (wheat scab). Pest Management Science 63:1201–1207
Chen Y, Zhang A-F, Gao T-C, Zhang Y, Wang W-X, Ding K-J, Chen L, Sun Z, Fang X-Z, Zhou M-G (2012) Integrated Use of pyraclostrobin and epoxiconazole for the control of Fusarium head blight of wheat in Anhui province of China. Plant Disease 96:1495–1500
De Mendiburu F, Simon R (2015) Agricolae-Ten years of an open source statistical tool for experiments in breeding, agriculture and biology. PeerJ PrePrints 3:e1404v1. https://doi.org/10.7287/peerj.preprints.1404v1
Del Ponte EM, Garda-Buffon J, Badiale-Furlong E (2012) Deoxynivalenol and nivalenol in commercial wheat grain related to Fusarium head blight epidemics in southern Brazil. Food Chemistry 132:1087–1091
Del Ponte EM, Spolti P, Ward TJ, Gomes LB, Nicolli CP, Kuhnem PR, Silva CN, Tessmann DJ (2015) Regional and field-specific factors affect the composition of Fusarium head blight pathogens in subtropical no-till wheat agroecosystem of Brazil. Phytopathology 105:246–254
Duffeck MR, Alves KS, Machado FJ, Esker PD, Del Ponte EM (2020) Modeling yield losses and fungicide profitability for managing Fusarium head blight in Brazilian spring wheat. Phytopathology 110:370–378
Feksa HR, Do Couto HTZ, Garozi R, De Almeida JL, Gardiano CG, Tessmann DJ (2019) Pre- and post-infection application of strobilurin-triazole premixes and single fungicides for control of Fusarium head blight and deoxynivalenol mycotoxin in wheat. Crop Protection 117:128–134
Gomes LB, Ward TJ, Badiale-Furlong E, Del Ponte EM (2015) Species composition, toxigenic potential and pathogenicity of Fusarium graminearum species complex isolates from southern Brazilian rice. Plant Pathology 64:980–987
Kretschmer M, Leroch M, Mosbach A, Walker A-S, Fillinger S, Mernke D, Schoonbeek H-J, Pradier J-M, Leroux P, De Waard MA, Hahn M (2009) Fungicide-driven evolution and molecular basis of multidrug resistance in field populations of the grey mould fungus Botrytis cinerea. PLoS Pathogens 5:e1000696
Kuhnem PR, Ward TJ, Silva CN, Spolti P, Ciliato ML, Tessmann DJ, Del Ponte EM (2016) Composition and toxigenic potential of the Fusarium graminearum species complex from maize ears, stalks and stubble in Brazil. Plant Pathology 65:1185–1191
Lehner MS, Paula Júnior TJ, Silva RA, Vieira RF, Carneiro JES, Schnabel G, Mizubuti ESG (2015) Fungicide sensitivity of Sclerotinia sclerotiorum : A thorough assessment using discriminatory dose, EC 50, high-resolution melting analysis, and description of new point mutation associated with thiophanate-methyl resistance. Plant Disease 99:1537–1543
Liu S, Fu L, Wang S, Chen J, Jiang J, Che Z, Tian Y, Chen G (2019) Carbendazim Resistance of Fusarium graminearum from Henan wheat. Plant Disease 103:2536–2540
Liu X, Yin Y, Wu J, Jiang J, Ma Z (2010) Identification and characterization of carbendazim-resistant isolates of Gibberella zeae. Plant Disease 94:1137–1142
Machado FJ, de Barros AV, McMaster N, Schmale DG, Vaillancourt LJ, Del Ponte EM (2022) Aggressiveness and mycotoxin production by Fusarium meridionale compared with F. graminearum on maize ears and stalks in the field. Phytopathology 112:271–277
Machado FJ, Santana FM, Lau D, Del Ponte EM (2017) Quantitative review of the effects of triazole and benzimidazole fungicides on Fusarium head blight and wheat yield in Brazil. Plant Disease 101:1633–1641
Magan N, Medina A, Aldred D (2011) Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest: Mycotoxins and climate change. Plant Pathology 60:150–163
McCallum BD, Tekauz A, Gilbert J (2004) Barrage Zone Formation Between Vegetatively Incompatible Fusarium graminearum (Gibberella zeae) Isolates. Phytopathology 94:432–437
McMullen M, Bergstrom G, De Wolf E, Dill-Macky R, Hershman D, Shaner G, Van Sanford D (2012) A unified effort to fight an enemy of wheat and barley: Fusarium Head Blight. Plant Disease 96:1712–1728
Mesterházy Á, Bartók T, Lamper C (2003) Influence of wheat cultivar, species of Fusarium, and isolate aggressiveness on the efficacy of fungicides for control of Fusarium head blight. Plant Disease 87:1107–1115
Mesterházy Á, Tóth B, Varga M, Bartók T, Szabó-Hevér Á, Farády L, Lehoczki-Krsjak S (2011) Role of fungicides, application of nozzle types, and the resistance level of wheat varieties in the control of Fusarium head blight and deoxynivalenol. Toxins 3:1453–1483
Mesterházy Á, Varga M, Tóth B, Kótai C, Bartók T, Véha A, Ács K, Vágvölgyi C, Lehoczki-Krsjak S (2018) Reduction of deoxynivalenol (DON) contamination by improved fungicide use in wheat. Part 1. Dependence on epidemic severity and resistance level in small plot tests with artificial inoculation. European Journal of Plant Pathology 151:39–55
Nicolli CP, Machado FJ, Spolti P, Del Ponte EM (2018) Fitness traits of deoxynivalenol and nivalenol-producing Fusarium graminearum species complex strains from wheat. Plant Disease 102:1341–1347
Nicolli CP, Spolti P, Tibola CS, Fernandes JMC, Del Ponte EM (2015) Fusarium head blight and trichothecene production in wheat by Fusarium graminearum and F. meridionale applied alone or in mixture at post-flowering. Tropical Plant Pathology 40:134–140
Paul PA, Bradley CA, Madden LV, Lana FD, Bergstrom GC, Dill-Macky R, Esker PD, Wise KA, McMullen M, Grybauskas A, Kirk WW, Milus E, Ruden K (2018) Meta-Analysis of the effects of QoI and DMI fungicide combinations on Fusarium head blight and deoxynivalenol in wheat. Plant Disease 102:2602–2615
Paul PA, Lipps PE, Hershman DE, McMullen MP, Draper MA, Madden LV (2008) Efficacy of Triazole-Based fungicides for Fusarium head blight and deoxynivalenol control in wheat: A multivariate meta-analysis. Phytopathology 98:999–1011
Pirgozliev SR, Edwards SG, Hare MC, Jenkinson P (2003) Strategies for the control of Fusarium head blight in cereals. In: Xu X, Bailey JA, Cooke BM (eds) Epidemiology of Mycotoxin Producing Fungi. Springer Netherlands, Dordrecht, 731–742
Qiu J, Shi J (2014) Genetic relationships, carbendazim sensitivity and mycotoxin production of the Fusarium graminearum populations from maize, wheat and rice in Eastern China. Toxins 6:2291–2309
Qiu J, Xu J, Shi J (2014) Molecular characterization of the Fusarium graminearum species complex in Eastern China. European Journal of Plant Pathology 139:811–823
R Core Team (2021) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Russell PE (2004) Sensitivity baselines in fungicide resistance research and management. FRAC Monograph, 3. CropLife International, Brussels 3:60
Schmale DG, Wood-Jones AK, Cowger C, Bergstrom GC, Arellano C (2011) Trichothecene genotypes of Gibberella zeae from winter wheat fields in the eastern USA. Plant Pathology 60:909–917
Spolti P, Barros NC, Gomes LB, dos Santos J, Del Ponte EM (2012a) Phenotypic and pathogenic traits of two species of the Fusarium graminearum complex possessing either 15-ADON or NIV genotype. European Journal of Plant Pathology 133:621–629
Spolti P, Del Ponte EM, Dong Y, Cummings JA, Bergstrom GC (2014) Triazole sensitivity in a contemporary population of Fusarium graminearum from New York wheat and competitiveness of a tebuconazole-resistant isolate. Plant Disease 98:607–613
Spolti P, de Jorge BC, Del Ponte EM (2012b) Sensitivity of Fusarium graminearum causing head blight of wheat in Brazil to tebuconazole and metconazole fungicides. Tropical Plant Pathology 37:419–423
Spolti P, Del Ponte EM (2013) Agressividade diferencial de espécies do complexo Fusarium graminearum em interação com o fungicida tebuconazole na redução do rendimento de trigo. Ciência Rural 43:1569–1575
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–9
Umpiérrez-Failache M, Garmendia G, Pereyra S, Rodríguez-Haralambides A, Ward TJ, Vero S (2013) Regional differences in species composition and toxigenic potential among Fusarium head blight isolates from Uruguay indicate a risk of nivalenol contamination in new wheat production areas. International Journal of Food Microbiology 166:135–140
Ward TJ, Bielawski JP, Kistler HC, Sullivan E, O’Donnell K (2002) Ancestral polymorphism and adaptive evolution in the trichothecene mycotoxin gene cluster of phytopathogenic Fusarium. Proceedings of the National Academy of Sciences 99:9278–9283
Ward TJ, Clear RM, Rooney AP, O’Donnell K, Gaba D, Patrick S, Starkey DE, Gilbert J, Geiser DM, Nowicki TW (2008) An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genetics and Biology 45:473–484
Wegulo SN, Baenziger PS, Hernandez Nopsa J, Bockus WW, Hallen-Adams H (2015) Management of Fusarium head blight of wheat and barley. Crop Protection 73:100–107
Wegulo SN, Bockus WW, Nopsa JH, De Wolf ED, Eskridge KM, Peiris KHS, Dowell FE (2011) Effects of integrating cultivar resistance and fungicide application on Fusarium head blight and deoxynivalenol in winter wheat. Plant Disease 95:554–560
Willyerd KT, Li C, Madden LV, Bradley CA, Bergstrom GC, Sweets LE, McMullen M, Ransom JK, Grybauskas A, Osborne L, Wegulo SN, Hershman DE, Wise K, Bockus WW, Groth D, Dill-Macky R, Milus E, Esker PD, Waxman KD, Adee EA, Ebelhar SE, Young BG, Paul PA (2012) Efficacy and stability of integrating fungicide and cultivar resistance to manage Fusarium head blight and deoxynivalenol in wheat. Plant Disease 96:957–967
Yin Y, Liu X, Li B, Ma Z (2009) Characterization of sterol demethylation inhibitor-resistant isolates of Fusarium asiaticum and F. graminearum collected from wheat in China. Phytopathology 99:487–497
Acknowledgements
The authors thank the Programa de Pós-graduação em Fitopatologia (UFV) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for providing a graduate scholarship to F. J. Machado and for a research fellowship for Dauri J. Tessmann, Eduardo S. G. Mizubuti and E. M. Del Ponte. We thank FAPEMIG (Fundação de Pesquisa do Estado de Minas Gerais) for the financial support (APQ—03945-16).
Author information
Authors and Affiliations
Contributions
Conceptualization: F.J.M, E.M.D, and D.J.T.; methodology: F.J.M., E.S.G.M. and E.M.P.; investigation: F.J.M., C.N.S., G.F.P., D.J.T., E.S.G.M. and E.M.P.; statistical analysis: F.J.M., C.N.S., G.F.P., D.J.T., E.S.G.M. and E.M.P.; writing—original draft preparation: F.J.M. and E.M.P.; writing—review and editing: F.J.M., C.N.S., G.F.P., D.J.T., E.S.G.M. and E.M.P.; supervision: E.M.P. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Machado, F.J., Silva, C.N., Paiva, G.F. et al. Sensitivity to tebuconazole and carbendazim in Fusarium graminearum species complex populations causing wheat head blight in southern Brazil. Trop. plant pathol. 49, 157–167 (2024). https://doi.org/10.1007/s40858-023-00616-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-023-00616-2