Abstract
Sesame plants with yellows symptoms and shortened internodes were observed in the surveyed sesame fields of a non-citrus-growing region in southeastern Iran. Leaf samples were collected from both symptomatic and asymptomatic plants. Concurrently, insects (Circulifer haematoceps) were also captured from the plants using a sweep net. Then, DNA was extracted from the symptomatic and non-symptomatic samples, likewise from the collected C. haematoceps insects. In the PCR assays, a DNA fragment with the expected size of 336 bp was amplified from the symptomatic plant’s DNA using Spiroplasma citri–specific primers. Besides, in the PCR assays, approximately 72.4% of the tested leafhopper samples were positive for S. citri, and in the experimental transmission assays, 26.6% of the periwinkle plants that were fed on by the field-collected leafhoppers showed symptoms. S. citri was also cultured from the symptomatic plants as well as the insects. Finally, the spiralin gene of an S. citri strain (KSC) isolated from one of the periwinkle plants was cloned and partially sequenced. BLAST and phylogenetic analysis of the obtained sequence revealed 100% and 87% homology of the KSC strain with the Iranian S. citri Marvdasht strain and the R8A2 reference strain, respectively. The present findings contribute to the knowledge on the sesame yellows disease caused by S. citri, as well as the high-frequency infectivity of the leafhopper C. haematoceps to S. citri, in the surveyed region. Furthermore, the finding of unique spiralins within Iranian populations of S. citri, including the KSC strain, may indicate that these strains are endemic in Iran. Due to the principal role of the leafhopper vector C. haematoceps in spreading the pathogen, controlling the vector insects is the key strategy for the management of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Three species of the Spiroplasma genus have been described as plant pathogens, i.e., Spiroplasma citri, S. kunkelii, and S. phoeniceum. S. citri is the first cultured and characterized plant pathogenic mollicute that was isolated from citrus stubborn–affected trees in Morocco and the USA (Saglio et al. 1971, 1973; Fudl-Allah et al. 1972). Researchers showed that the disease occurs in many Mediterranean and Near East countries, including Iran (Bové et al. 1988; Rahimian 1983). The pathogen has a wide host range and infects non-rutaceous hosts among woody and herbaceous plants (Nejat et al. 2011). For instance, the carrot purple leaf and safflower necrotic yellows diseases caused by S. citri have been reported from the USA and Mexico (Lee et al. 2006; Swisher et al. 2016), and Iran (Khanchezar et al. 2012), respectively. Depending on the type of host plant, the infected plants show symptoms such as reduced leaf size (little leaf), deformed fruits, yellow discoloration of foliage, stunting, and wilting (Cacciola et al. 2017). The other species, S. kunkelii, and S. phoeniceum, cause corn stunt and periwinkle yellows diseases, respectively (Whitcomb et al. 1986; Saillard et al. 1987; Chang 1998). Meanwhile, the full genome of the three species has been sequenced to clarify their evolutionary biology (Davis et al. 2015, 2017, 2019; Rattner et al. 2021).
S. citri is a phloem-limited pathogen and is transmitted under natural conditions either by grafting or by phloem-sap feeding leafhopper insects (Hemiptera: Cicadellidae), primarily Circulifer tenellus and C. haematoceps, in the USA and Old World, respectively (Weintraub and Beanland 2006; Bové et al. 2003). Since the two leafhopper species are polyphagous and capable of long-distance migration, they play a significant role in the epidemiology of diseases caused by the pathogen (Fletcher 1983; Bové et al. 1988).
Many attempts have been made to consider the molecular interaction of S. citri with insect vectors (Perilla-Henao and Casteel 2016). In this interest, it has been shown that several proteins are involved in this interaction such as spiralin, which is the main membrane protein of S. citri with an abundance of more than 22% of total membrane proteins (Wróblewski et al. 1977). Spiralin was found as a lectin capable of binding to insect glycoproteins, and it is required for S. citri adhesion and penetration into insect cells (Killiny et al. 2005; Duret et al. 2014). Accordingly, it has been concluded that this lipoprotein is overexpressed in leafhopper vectors during S. citri transmission to plant hosts, allowing spiroplasma cells to adhere and internalize in vector midgut and salivary glands cells (Dubrana et al. 2016). Besides, some proteins encoded by genes on S. citri plasmids contribute to the pathogen transmission by C. haematoceps (Berho et al. 2006; Breton et al. 2010).
Sesame (Sesamum indicum L.) is cultivated in arid and semiarid regions of the world and is the second oldest oilseed crop after coconut to humans (Budowski and Markley 1951). Due to the beneficial effect of sesame oil on human health and its use in the food industry, the amount of acreage devoted to sesame crop is increasing. However, throughout the world, including Iran, diseases such as phyllody, which is caused by phytoplasmas, have a major impact on sesame production (Salehi and Izadpanah 1992; Asghari Tazehkand et al. 2017; Devanna et al. 2020). Moreover, sesame yellows disease caused by S. citri has been reported as a threat in Turkey (Kersting et al. 1993) and the southwest part of Iran (Salehi and Izadpanah 2002).
The southwest part of the Kerman province (Sirjan region), located in southeastern Iran, is a non-citrus-growing and sesame production region. Sesame fields in the region were surveyed to identify plant pathogenic mollicutes infecting sesame. Thus, sesame plants with foliar yellowing were observed, which led us to study the etiology of the syndrome.
This paper presents studies that confirm sesame yellows syndrome in southeastern Iran is caused by S. citri. Conventional and molecular methods were used to verify S. citri infection of the symptomatic sesame plants and its detection in the sesame field–collected C. haematoceps insects. Furthermore, the spiralin gene of an isolated strain from an experimentally infected periwinkle plant fed on by naturally infected C. haematoceps insects was partially sequenced.
Materials and methods
Symptomatology and field sampling
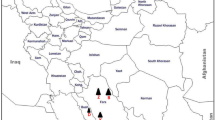
Sesame fields located in the Kerman province of Iran (Sirjan region, Fig. 1) were surveyed at the flowering stage. Some plants showed yellows, stunting, epinasty, and rosette leaves (Fig. 2). Leaf samples were collected from both symptomatic and symptomless plants in three fields (A–C), ranging from 8 to 10 ha, and approximately 500 m apart from each other. Besides, five batches of the leafhopper insects (C. haematoceps, Fig. 2e) were randomly captured from the plants at five locations within each field using a sweep net. Lastly, the leaf and insect samples were transferred to a laboratory, where they were processed for polymerase chain reactions (PCR), pathogen isolation, molecular cloning, and bioassay experiments.
DNA extraction from field samples
The field-collected leaf samples from the three fields, as well as those from the experimentally insect-inoculated periwinkle plants, were washed with sterile distilled water. The leaf midribs were cut with sterile razor blades, and 0.3 g of the midribs of each sample was macerated in 2 ml of the CTAB buffer (Maixner et al. 1995) and was incubated at 60 °C for 30 min. One milliliter of the homogenate was then centrifuged at 3,200 × g for 1 min. The supernatant was transferred to a 1.5-ml microtube and mixed with an equal volume of chloroform-isoamyl alcohol (24:1), and was centrifuged at 15,000 × g for 15 min. Then, the supernatant was collected, and 0.6 volume of isopropanol was added and centrifuged at 15,000 × g for 15 min. Finally, the pellet was washed three times with 70% ethanol, air-dried, and dissolved in 100-µl sterile distilled water, and stored at − 20 °C until used. For DNA extraction from insects, a single insect or two of them from each insect batch were randomly selected and homogenized in 400 μl of the CTAB buffer, and DNA was extracted as the plant samples.
PCR detection of S. citri
The primer pair of Sc1 (5′-ATTTTCAATTTGATGTTTATCAAGACAAC-3′) and Sc2 (5′-CAAAATCACTTGCTCCTGCATTTGG-3′), which amplify a part of the spiralin gene of S. citri (Supplemental Fig. S1), was used for detection of S. citri from the extracted DNA of the infected plants and insects. The sequence of the primers was generously provided by Dr. Colette Saillard and Dr. Xavier Foissac (UMR1090, Génomique, Diversité et Pouvoir Pathogéne, INRA, Université Victor Ségalen Bordeaux 2, BP 81, F-33883 Villenave d’Ornon, France). PCRs were carried out in reaction volumes of 25 µl containing 1.5 units of Taq DNA polymerase, 1X PCR buffer, 0.2 mM dNTPs (Cinagen, Iran), 10 pM each primer, and 1 µl of the extracted DNA. PCR was performed in a thermocycler (Biometra) with initial denaturing at 93 °C for 3 min, followed by 35 repeated cycles of 95 °C for 1 min, 62 °C for 1 min, and 72 °C for 2 min and a final extension at 72 °C for 10 min. Subsequently, the PCR products were electrophoresed on a 1% agarose gel containing 0.5 µg/ml ethidium bromide and photographed by a gel document (Biometra). Additionally, DNA extracted from plant samples was subjected to nested PCR using phytoplasma universal primers according to the previously described procedures (Deng and Hiruki 1991; Schneider et al. 1995; Gundersen and Lee 1996).
S. citri isolation from field samples
Collected leaf samples from the symptomatic sesame plants were washed with sterile distilled water, and their midribs were cut with sterile razor blades. Then, 0.3 g of the midribs of each sample was minced in 2 ml of the LD10 medium (Lee and Davis 1984). After incubation for 30 min at room temperature, the macerate was passed through a sterile filter membrane (0.45-µm pore diameter). Tenfold serial dilutions of the filtrate were then prepared using the medium and incubated at 32 °C. To isolate S. citri from the insects, three individuals of the leafhopper C. haematoceps collected from each field were crushed in 2 ml of the medium, and the pathogen was isolated as described for plant samples.
Insect transmission assays
Periwinkle (Catharanthus roseus (L.) G. Don) plants were grown from seeds in pots under greenhouse conditions. Then, to examine the leafhopper C. haematoceps harboring S. citri, the collected insects from a batch of each field, in groups of 6 to 10, were caged and fed on five young periwinkle plants (in the stage of 6–8 leaves) in an insect-proof greenhouse at 32 ± 2 °C. The PCR detection of S. citri and its in vitro isolation from symptomatic periwinkle plants were carried out as described above.
Spiralin gene amplification, cloning, and sequencing
A DNA fragment containing spiralin gene of an isolated S. citri strain from an experimentally infected periwinkle plant fed on by naturally infected C. haematoceps was amplified using the primer pair of D (5′-GTATAAAGTAGGGTTAGAAGC-3′) and D′ (5′-CCCTTGTGAATCACCACC-3′) (Supplemental Fig. S1) (Foissac et al. 1996). The PCR conditions and electrophoresis were as described above, except that the annealing temperature was increased to 57 °C and 1 µl of S. citri culture (108 cells/ml) was directly used as the DNA template. The amplified products (DD′-fragment) were ligated into the TA cloning vector (pTZ57R/T, InsTA Clone PCR Cloning Kit; Fermentas, USA) and transferred into competent cells of Escherichia coli strain DH5α. Then, recombinants were selected on a lysogeny broth agar plate plate with X-gal and 100 μg/mL ampicillin. A white recombinant colony was sub-cultured, and the plasmids were extracted using a plasmid purification kit (Fermentas, Lithuania). Following PstI digestion of the extracted plasmids and verifying the insert by electrophoresis (data not shown), the fragment was partially sequenced by Macrogen Inc., South Korea (http://www.macrogen.com).
Phylogenetic analysis
The resulting sequence containing a part of the spiralin gene was trimmed, and BLAST-searched in the NCBI database (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to find its homology with those available in GenBank (Supplemental Table S1, Carle et al. 2010; Khanchezar et al. 2014). All the spiralin gene-related sequences and their deduced amino acids were then retrieved from GenBank and imported into the MEGA 6.0 (Tamura et al. 2013) and aligned using the ClustalW algorithm with default parameters. Subsequently, the phylogenetic tree was constructed using the maximum likelihood method with 1000 bootstrap replications (Fig. 4). The aligned deduced amino acid sequences were processed by the Jalview program (Waterhouse et al. 2009) (Supplemental Fig. S5). The identity matrix (Supplemental Fig. S4) of the 3′ part of the spiralin gene of S. citri strains was also generated using the MatGat software (Campanella et al. 2003).
Results
Disease incidence and PCR detection of S. citri in field samples
Following confirmation of the sesame yellows syndrome caused by S. citri, the disease incidence in the surveyed fields was visually estimated as 50%. Thus, the Sc1/Sc2 primer pair successfully amplified products with the expected 336 bp from the extracted DNA of the symptomatic sesame plants, but not from an asymptomatic sample (Supplemental Fig. S2). In the insect DNA samples obtained by maceration of two or individual insects, the 336 bp DNA fragment was also amplified from 21 out of 29 DNA samples (Supplemental Fig. S3, Table 1). Furthermore, in nested-PCR assays using universal primers for the detection of phytoplasmas, no amplicons were obtained from the symptomatic plant samples.
Isolation of S. citri from field samples
Within a week, the tubes with the LD10 medium, inoculated by the filtrates from the plant and insect samples, showed a color change from red to yellow at the dilution of 10−4 or 10−5 without bacterial contaminations. The presence of helical-shaped spiroplasma cells was confirmed by the dark-field microscopy of the cultures. S. citri was cultured from all the symptomatic leaf samples collected from the surveyed sesame fields. It was also isolated from all the sampled insect batches except for two of them (Table 1).
Insect transmission assays
Four out of 15 periwinkle plants fed on by the collected insects from the fields showed marginal and interveinal leaf chlorosis after 3 weeks, followed by wilting after 1 month (Fig. 3). The Sc1/Sc2 primer pair also amplified the 336 bp DNA fragments from the symptomatic periwinkle plants. Moreover, S. citri was cultured from the diseased plants and confirmed the cultures by dark-field microscopy.
Spiroplasma citri symptoms on periwinkle plants fed on by Circulifer haematoceps insects collected from sesame fields in the Kerman province of Iran. The insects, in groups of 6 to 10, were caged and fed on young periwinkle plants. After 3 weeks, four of the 15 treated plants showed symptoms. A healthy plant is represented by (a); (b) mild chlorosis; (c) marginal chlorosis in expanded leaves; (d) marginal and interveinal chlorosis in both young and old leaves; and (e) the onset of wilting after 1 month. S. citri was also isolated from the symptomatic plants
Spiralin gene amplification, sequencing, and phylogenetic analysis
PCR amplified a 1053-bp DNA fragment (data not shown) from the S. citri KSC strain (KSC stands for Kerman S. citri) that was isolated from a symptomatic periwinkle plant (Fig. 3) fed on by the field-collected leafhopper C. haematoceps. The spiralin gene of the KSC strain was partially sequenced, and following the BLAST analysis, the related S. citri strains (78, Alcanar 254, GII3-3X, Qualubia, Fasa II, Firuzabad II, Fewa, 1SP, Shiraz I, Corse, 24.86, R8-A2, 14.76, Palmyre, Darab II, Fasa I, and Marvdasht) were found.
The 3′ part of the KSC strain spiralin gene showed 100% and 87.2% identity with the Iranian S. citri strains, Marvdasht and Firuzabad II, respectively, and 89% identity with the reference R8A2 strain (Supplemental Fig. S4). The KSC and Marvdasht strains formed a single cluster based on the phylogenetic tree (Fig. 4). Furthermore, the multiple alignments of the spiralin partial sequence of the S. citri strains illustrated the insertion of alanine and serine into the positions 130 and 131 of the KSC strain spiralin similar to the other Iranian strains, i.e., Marvdasht, Firuzabad II and Darab II (Supplemental Fig. S5).
Maximum likelihood phylogenetic tree of the 3′ part of the spiralin gene of Spiroplasma citri KSC strain (isolated from an experimentally infected periwinkle plant by the sesame field–collected Circulifer haematoceps), including spiralin gene sequences of S. citri strains and the other two phytopathogenic spiroplasmas, S. phoeniceum, and S. kunkelii, obtained from GenBank. The reference strain of S. citri, the KSC strain, and other Iranian S. citri strains are shown on the tree as red, green, and purple, respectively. S. melliferum was used as an outgroup taxon. Numbers on the tree branches represent the bootstrap values (> 50%). See Supplemental Table S1 for more information on strains
Discussion
Phytopathogenic mollicutes (spiroplasmas and phytoplasmas) cause notable economic losses in agricultural products. The damage is mainly due to the systemic inhabitant of these organisms in the sieve tube elements of plants, as well as their wide host range and insect transmissibility. As opposed to phytoplasmas, spiroplasmas can be grown in axenic culture (Saglio et al. 1971, 1973; Seemüller et al. 2002).
In the present study, PCR and in vitro culture results confirmed that S. citri is the causative agent of sesame yellows syndrome in the Kerman province’s southwestern region. Furthermore, the infectivity of the field-collected leafhopper C. haematoceps to S. citri was verified by the experiments. The isolation of S. citri from 26.6% of the periwinkle plants fed on by field-collected leafhoppers, and the detection of the pathogen in approximately 72.4% of the leafhopper samples tested in PCR assays, indicates the principal role of the vector in the spread of S. citri to the sesame fields. Results of the research conducted in a citrus-growing area of Turkey showed that 87% of the sesame field–collected C. haematoceps insects were infected by S. citri, and 25% of them successfully transferred the pathogen to periwinkle plants. Moreover, 50% of the insects from maize were positive for S. citri, but those from potato and cotton were negative (Kersting and Sengonca 1992). Therefore, our findings are consistent with the evidence in Turkey, showing that the C. haematoceps vector and sesame are the two preferred hosts for increasing the inoculum of S. citri.
The leafhopper C. haematoceps has also been reported as a vector of S. citri from the southern part of the Kerman province (Jiroft region), which is one of the main territories of citrus production in Iran where the stubborn disease is also widespread (Omidi et al. 2011). In the present study, however, the pathogen was found in sesame fields located in a non-citrus-growing region of the province. On the other hand, S. citri is a non-seed-borne pathogen, and therefore sesame plants are naturally infected by the leafhopper carrying the pathogen. Additionally, S. citri has no transovarial transmission, and the vector insects habitually acquire the pathogen from permanent reservoir plants around the fields. Sesame is also a preferred host plant for leafhoppers, particularly for C. haematoceps. Therefore, sesame is a distinguished field test plant for monitoring phytoplasmas (Asghari Tazehkand et al. 2017) and spiroplasmas diseases (Omidi et al. 2011) in agricultural regions as well as virus diseases that are transmitted by leafhoppers (Hasanvand et al. 2018).
The C. haematoceps’ primary host plants, Salsola kali and Alhagi camelorum, are well known and prevalent across all arid and semi-arid areas of Iran. Consequently, there appears to be a close relationship between the leafhopper vector, the ubiquity of the leafhopper host plants, and the prevalence of S. citri in the country (Bové et al. 1988). It is worth noting that the stubborn disease is prevalent in all citrus-growing regions in southern and northern Iran. However, due to adverse conditions for the vector activity, particularly the high humidity and lack of the vector host plants, the disease incidence on the northern coast of the country is mainly due to using infected budwoods (Bové et al. 1988).
Zarei et al. (2017) have detected S. citri in experimental periwinkle plants fed on by the leafhopper C. haematoceps collected from sesame fields in southwestern Iran (Fars province) using PCR. In the same study, based on spiralin gene sequence analysis, the C. haematocps-associated strains of S. citri have been grouped along with the previously defined groups of I and VI. In the present study, the spiralin partial sequence of the S. citri KSC strain was also found identical to the identified Iranian S. citri Marvdasht strain (Zarei et al. 2017) and was classified in the group VI. In the alignment of the partial putative spiralin of the worldwide S. citri strains, only a few of the Iranian strains, including the KSC strain, have a pair of amino acids (alanine and serine) insertion into the protein in the palaces 131 and 132 (Supplemental Fig. S5). Consequently, such strains appear to be insightful candidates for assessing their interactions with the leafhopper vector C. haematoceps, towards a smarter perception of spiralin performance against insect transmission efficiency.
Duret et al. (2014) showed that spiralin is essential for the S. citri GII3 strain to bind to and invade insect cells, which appears to be a fundamental requirement for the pathogen internalization. Moreover, strong adhesion (recognition) of S. citri to the insect cells has the potential to enhance the efficacy of insect transmission (Duret et al. 2014). Hence, the sequences of amino acids in spiralin may have varying impacts on this recognition. However, for a more accurate evaluation of the influence of different spiralins on the vector-pathogen interaction, insect transmissibility screening of such strains is recommended. Recent studies have also revealed that S. citri strains that contain plasmids (pSci1 to -6) encoding membrane-related proteins (pSci1 to -5) and the hydrophilic protein P32 (pSci6) are implicated in transmission by C. haematoceps, since they have not been found in non-insect transmissible strains (Breton et al. 2010). Although several potential proteins have been identified in S. citri-vector interactions (Fletcher et al. 1998; Berho et al. 2006), the essential insect receptors which mediate the S. citri movement within the vector are still unknown.
In conclusion, the findings of this study showed that S. citri is involved in the etiology of sesame yellows in the studied region, and C. haematoceps plays a key role in its spreading. Considering the pathogen was detected in a non-citrus-growing region and has no vertical transmission in plants, in addition to vector control, eradicating weeds (particularly those of perennial) help to manage the disease. Moreover, finding unique spiralins within Iranian populations of S. citri, including the KSC strain, may be an evidence of the endemic nature of these strains in Iran.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Asghari Tazehkand S, Hosseinipour A, Heydarnejad J, Rahimian H, Massumi H (2017) Identification of phytoplasmas associated with sesame phyllody disease in southeastern Iran. Archives of Phytopathology and Plant Protection 50:761–775
Berho N, Duret S, Danet JL, Renaudin J (2006) Plasmid pSci6 from Spiroplasma citri GII-3 confers insect transmissibility to the non-transmissible strain S. citri 44. Microbiology 152:2703–2716
Bové JM, Renaudin J, Saillard C, Foissac X, Garnier M (2003) Spiroplasma citri, a plant pathogenic mollicute: relationships with its two hosts, the plant and the leafhopper vector. Annual Review of Phytopathology 41:483–500
Bové, JM, Fos, A, Lallemand J, Raie A, Ali, Y, Ahmed N, Saillard C, Vignault JC (1988) Epidemiology of Spiroplasma citri in the Old World. In: Proceedings, 10th International Organization of Citrus Virologists Conference, pp. 295–299. Riverside, CA
Breton M, Duret S, Danet JL, Dubrana MP, Renaudin J (2010) Sequences essential for transmission of Spiroplasma citri by its leafhopper vector, Circulifer haematoceps, revealed by plasmid curing and replacement based on incompatibility. Applied and Environmental Microbiology 76:3198–3205
Budowski P, Markley KS (1951) The chemical and physiological properties of sesame oil. Chemical Reviews 48:125–151
Cacciola SO, Bertaccini A, Pane A, Furneri PM (2017) Spiroplasma spp.: a plant, arthropod, animal and human pathogen. In: Harsimran G, Garg H (eds) Citrus Pathology. InTech press, pp. 31–51
Campanella JJ, Bitincka L, Smalley J (2003) MatGAT: an application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinformatics 4:29
Carle P, Saillard C, Carrère N, Carrère S, Duret S, Eveillard S, Gaurivaud P, Gourgues G, Gouzy J, Salar P, Verdin E (2010) Partial chromosome sequence of Spiroplasma citri reveals extensive viral invasion and important gene decay. Applied and Environmental Microbiology 76:3420–3426
Chang CJ (1998) Pathogenicity of aster yellows phytoplasma and Spiroplasma citri on periwinkle. Phytopathology 88:1347–1350
Davis RE, Shao J, Dally EL, Zhao Y, Gasparich GE, Gaynor BJ, Athey JC, Harrison NA, Donofrio N (2015) Complete genome sequence of Spiroplasma kunkelii strain CR2-3x, causal agent of corn stunt disease in Zea mays L. Genome Announcements 3:e01216-e1315
Davis RE, Shao J, Zhao Y, Gasparich GE, Gaynor BJ, Donofrio N (2017) Complete genome sequence of Spiroplasma citri strain R8–A2T, causal agent of stubborn disease in citrus species. Genome Announcements 5:e00206-e217
Davis RE, Shao J, Zhao Y, Wei W, Bottner-Parker K, Silver A, Stump Z, Gasparich GE, Donofrio N (2019) Complete genome sequence of Spiroplasma phoeniceum strain P40T, a plant pathogen isolated from diseased plants of Madagascar periwinkle [Catharanthus roseus (L.) G. Don]. Microbiology Resource Announcements 8:e01612-e1618
Deng S, Hiruki D (1991) Amplification of 16S rRNA genes from culturable and nonculturable mollicutes. Journal of Microbiological Methods 14:53–61
Devanna P, Naik MK, Bharath R, Bhat KV, Madupriya P (2020) Characterization of 16SrII group phytoplasma associated with sesame phyllody disease in different cropping seasons. Indian Phytopathology 73:563–568
Dubrana MP, Béven L, Arricau-Bouvery N, Duret S, Claverol S, Renaudin RJ, Saillard C (2016) Differential expression of Spiroplasma citri surface protein genes in the plant and insect hosts. BMC Microbiology 16:1–14
Duret S, Batailler B, Dubrana MP, Saillard C, Renaudin J, Béven L, Arricau-Bouvery N (2014) Invasion of insect cells by Spiroplasma citri involves spiralin relocalization and lectin/glycoconjugate-type interactions. Cellular Microbiology 16:1119–1132
Fletcher J (1983) Brittle root of horseradish in Illinois and the distribution of Spiroplasma citri in the United States. Phytopathology 73:354–357
Fletcher J, Wayadande A, Melcher U, Ye F (1998) The phytopathogenic mollicute-insect vector interface: a closer look. Phytopathology 88:1351–1358
Foissac X, Saillard C, Gandar J, Zreik L, Bové JM (1996) Spiralin polymorphism in strains of Spiroplasma citri is not due to differences in posttranslational palmitoylation. Journal of Bacteriology 178:2934–2940
Fudl-Allah AE-SA, Calavan EC, Igwegbe ECKA (1972) Culture of a mycoplasmalike organism associated with stubborn disease of citrus. Phytopathology 62:729–731
Gundersen DE, Lee IM (1996) Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathologia Mediterranea 35:144–151
Hasanvand V, Kamali M, Heydarnejad J, Massumi H, Kvarnheden A, Varsani A (2018) Identification of a new turncurtovirus in the leafhopper Circulifer haematoceps and the host plant species Sesamum indicum. Virus Genes 54:840–845
Kersting U, Baspinar UH, Cinar A, Sengonea, C, Uygun N (1993) New findings on the epidemiology of Spiroplasma citri in the Eastern Mediterranean region of Turkey. In: Proceedings, 12th International Organization of Citrus Virologists Conference, pp. 336–341. Riverside, CA
Kersting U, Sengonca C (1992) Detection of insect vectors of the citrus stubborn disease pathogen, Spiroplasma citri Saglio et al., in the citrus growing area of South Turkey. Journal of Applied Entomology 113:356–364
Khanchezar A, Izadpanah K, Salehi M (2012) Partial characterization of Spiroplasma citri isolates associated with necrotic yellows disease of safflower in Iran. Journal of Phytopathology 160:331–336
Khanchezar A, Béven L, Izadpanah K, Salehi M, Saillard C (2014) Spiralin diversity within Iranian strains of Spiroplasma citri. Current Microbiology 68:96–104
Killiny N, Castroviejo M, Saillard C (2005) Spiroplasma citri spiralin acts in vitro as a lectin binding to glycoproteins from its insect vector Circulifer haematoceps. Phytopathology 95:541–548
Lee IM, Davis RE (1984) New media for rapid growth of Spiroplasma citri and corn stunt spiroplasma. Phytopathology 74:84–89
Lee IM, Bottner KD, Munyaneza JE, Davis RE, Crosslin JM, du Toit LJ, Crosby T (2006) Carrot purple leaf: a new spiroplasmal disease associated with carrots in Washington State. Plant Disease 90:989–993
Maixner M, Arhens U, Seemüller E (1995) Detection of the German grapevine yellows (Vergilbungskrankheit) MLO in grapevine, alternative wild-type hosts and a vector by a specific PCR procedure. European Journal of Plant Pathology 101:241–250
Nejat N, Vadamalai G, Dickinson M (2011) Spiroplasma citri: a wide host range phytopathogen. Plant Pathology Journal 10:46–56
Omidi M, Hosseini-Pour A, Rahimian H, Massumi H, Saillard C (2011) Identification of Circulifer haematoceps (Hemiptera: Cicadellidae) as vector of Spiroplasma citri in the Kerman province of Iran. Journal of Plant Pathology 93:167–172
Perilla-Henao LM, Casteel CL (2016) Vector-borne bacterial plant pathogens: interactions with hemipteran insects and plants. Frontiers in Plant Science 7:1163
Rahimian H (1983) Distribution and symptoms of citrus stubborn disease in South East of Iran. In: Proceedings, 7th Plant Protection Conference of Iran, p. 74. College of Agriculture, Univ. Teheran, Karaj, Iran
Rattner R, Thapa SP, Dang T, Osman F, Selvaraj V, Maheshwari Y, Pagliaccia D, Espindola AS, Hajeri S, Chen J, Coaker G (2021) Genome analysis of Spiroplasma citri strains from different host plants and its leafhopper vectors. BMC Genomics 22:1–14
Saglio P, Lafléche D, Bonissol C, Bové JM (1971) Culture in vitro des mycoplasmes associés au stubborn des agrumes et leur observation au microscope électronique. Comptes rendus de l'Académie des Sciences 272:1387–1390
Saglio P, Lhospital M, Lafléche D, Dupont G, Bové JM, Tully JG, Freundt EA (1973) Spiroplasma citri gen. and sp. n.: a mycoplasma-like organism associated with “stubborn” disease of citrus. International Journal of Systematic and Evolutionary Microbiology 23:191–204
Saillard C, Vignault JC, Bové JM, Raie A, Tully JG, Williamson DL, Fos A, Garnier M, Gadeau A, Carle P, Whitcomb RF (1987) Spiroplasma phoeniceum sp. nov., a new plant-pathogenic species from Syria. International Journal of Systematic and Evolutionary Microbiology 37:106–115
Salehi M, Izadpanah K (2002) A disease of sesame in Iran caused by Spiroplasma citri. In Proceedings, 15th International Organization of Citrus Virologists Conference, pp. 401–402. Riverside, CA
Salehi M, Izadpanah K (1992) Etiology and transmission of sesame phyllody in Iran. Journal of Phytopatholology 135:37–47
Schneider B, Seemüller E, Smart CD, Kirkpatrick BC (1995) Phylogenetic classification of plant pathogenic mycoplasma-like organisms or phytoplasmas. In: Razin S, Tully JG (eds) Molecular and diagnostic procedures in mycoplasmology, vol 1. Academic Press, San Diego, pp 369–380
Seemüller E, Garnier M, Schneider B (2002) Mycoplasmas of plants and insects. In: Razin S, Herrmann R (eds) Molecular Biology and Pathogenicity of Mycoplasmas. Kluwer Academic/Plenum Publishers, New York, pp 91–115
Swisher KD, Velásquez-Valle R, Mena-Covarrubias J, Munyaneza JE (2016) Occurrence and molecular detection of Spiroplasma citri in carrots and its insect vector, Circulifer tenellus, in Mexico. Journal of Plant Pathology 98:355–360
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30:2725–2729
Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ (2009) Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191
Weintraub PG, Beanland L (2006) Insect vectors of phytoplasmas. Annual Review of Entomology 51:91–111
Whitcomb RF, Chen TA, Williamson DL, Liao C, Tully JG, Bové JM, Mouches C, Rose DL, Coan ME, Clark TB (1986) Spiroplasma kunkelii sp. nov.: characterization of the etiological agent of corn stunt disease. International Journal of Systematic and Evolutionary Microbiology 36:170–178
Wróblewski H, Johansson KE, Hjérten S (1977) Purification and characterization of spiralin, the main protein of the Spiroplasma citri membrane. Biochimica et Biophysica Acta - Biomembranes 465:275–289
Zarei Z, Salehi M, Azami Z, Salari K, Béven L (2017) Stubborn disease in Iran: diversity of Spiroplasma citri strains in Circulifer haematoceps leafhoppers collected in sesame fields in Fars province. Current Microbiology 74:239–324
Funding
The office of vice-chancellor for research, Shahid Bahonar University of Kerman, Iran, funded this work.
Author information
Authors and Affiliations
Contributions
Study conception and design: Akbar Hosseinipour. Data curation: Mojtaba Rezaee Ahmadabady. Analysis and interpretation of data: Mojtaba Rezaee Ahmadabady and Akbar Hosseinipour. Writing—original draft: Akbar Hosseinipour. Review, editing, and validation: Hossain Massumi.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
40858_2021_488_MOESM1_ESM.jpg
Supplementary file1 Fig. S1 Schematic diagram representing positions of the D/D' and Sc1/Sc2 primer pairs on a part of the Spiroplasma citri genome R8A2 strain (GenBank, CP013197), which were used for the detection of S. citri in plants and insects, and spiralin gene amplification, respectively. The coding region of the spiralin gene was shown with the cyan arrow (JPG 1407 KB)
40858_2021_488_MOESM2_ESM.jpg
Supplementary file2 Fig. S2 Agarose gel electrophoresis of a PCR-amplified 336 bp DNA fragment (red arrow) from Spiroplasma citri-infected sesame plants in the Kerman province of Iran. PCR DNA templates were extracted from asymptomatic (lane 1) and symptomatic sesame plants from three fields (lanes 2 to 4). M, DNA size marker (1Kb DNA ladder, Fermentas) (JPG 426 KB)
40858_2021_488_MOESM3_ESM.jpg
Supplementary file3 Fig. S3 PCR amplification of Spiroplasma citri-specific DNA fragment (336 bp, red arrows) from five batches of insects (Cirulifer haematoceps) collected from sesame fields (A-C) in the Kerman province of Iran. The PCR DNA templates were extracted from a pair of insects (panel a) or a single insect from each batch (panel b). In the latter, four out of the five insect batches of the field C were tested. M, DNA size marker (1Kb DNA Ladder, Fermentas) (JPG 746 KB)
40858_2021_488_MOESM4_ESM.jpg
Supplementary file4 Fig. S4 The identity matrix compares the 3′ part of the spiralin gene of the Spiroplasma citri KSC strain (isolated from an experimentally infected periwinkle plant by the sesame field-collected Circulifer haematoceps) to that of other S. citri strains, S. phoeniceum, S. kunkelii, and S. melliferum. The reference strain of S. citri, the KSC strain, and other Iranian S. citri strains are shown on the tree as red, green, and purple, respectively. See Supplemental Table S1 for more information on strains (JPG 2024 KB)
40858_2021_488_MOESM5_ESM.jpg
Supplementary file5 Fig. S5 Multiple sequence alignments of the partial spiralin sequence of Spiroplasma citri strains. The alignment spiralin sequence of the isolated S. citri KSC strain in the present work is shown in the green row. Different amino acids of the spiralin of the KSC strain, compared with the reference S. citri strain (red sequence arrow), are indicated with black letters and with a purple box for the insertion of the alanine and serine amino acids. The other Iranian S. citri strains retrieved from GenBank are shown with green filled circles. Amino acid identity in the alignments is denoted by black dots. See Supplemental Table S1 for more information on strains (JPG 1742 KB)
Rights and permissions
About this article
Cite this article
Ahmadabady, M.R., Hosseinipour, A. & Massumi, H. Molecular detection and isolation of Spiroplasma citri causing yellows in sesame and its insect transmission by Circulifer haematoceps in a non-citrus-growing region of Iran. Trop. plant pathol. 47, 402–410 (2022). https://doi.org/10.1007/s40858-021-00488-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-021-00488-4