Abstract
White thread blight is one of the most common diseases affecting several tree species that grow in warm and humid regions. The typical symptoms of this disease include blighted leaves hanging by a white thread of fungal hyphae. Leaf samples exhibiting white thread blight symptoms were collected from neem (Azadirachta indica A. Juss) and Brazilian cherry pitanga (Eugenia uniflora L.) plants in Northeastern Brazil, and from Indian green-tea (Camellia sinensis (L.) O. Kuntze), coffee (Coffea arabica L.), and persimmon (Diospyros kaki L.) in agricultural areas neighboring the Atlantic forest in Southeastern Brazil. Fungal isolates were obtained indirectly from leaf fragments or directly by transferring mycelia and sclerotia to culture medium. Bright field and scanning electron microscopy images revealed the association of Rhizoctonia-like hyphae and basidiospores with the infected leaves. In pathogenicity tests, Rhizoctonia-like fungal isolates induced leaf necrosis on their hosts, and the pathogens were re-isolated from inoculated plants. Phylogenetic analyses based on sequences of the ITS rRNA region indicated the occurrence of Ceratobasidium lineages distinct from previously reported Ceratobasidium species. Our study leads to the description of two new species of Ceratobasidium: the fungal isolates from A. indica, C. sinensis, and E. uniflora were classified as Ceratobasidium niltonsouzanum sp. nov., and those obtained from C. arabica and D. kaki as Ceratobasidium chavesanum sp. nov.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
White thread blight was first described on coffee plants in Northern India. The fungal pathogen was initially described as Pellicularia koleroga Cooke (Cooke 1876). Typical signs of the disease include white mycelial cords visible on the infected branches and leaves. Diseased leaves dry up but remain attached to the plant hanging by the fungal hyphae. Often, necrotic lesions are evident on the affected leaves. In severely colonized leaves, hymenia can be observed on the leaf surface. The fungus can form small black sclerotia on the branches and leaves of some host plants (Wolf and Bach 1927; Furtado 1997; Lourd and Alves 1997).
The etiology of white thread blight has been taxonomically revised. The previously described Pellicularia koleroga (Cooke 1876) was reclassified as Corticium koleroga (Von Hoehnel 1910). Later on, other names were also assigned such as Botryobasidium koleroga (Cooke) Venkatarayan, Hyphocnus koleroga Stevens & Hall, Koleroga noxia Donk and Ceratobasidium noxium (Donk) P. Roberts (Venkatarayan 1949). White thread blight was also associated with Corticium stevensii Burt, Ceratobasidium stevensii (Burt) Venkatar. and Ceratobasidium anceps (Bres. & Syd.) H.S. Jacks. (Mendes et al. 1998). Ceratobasidium and Thanatephorus are Rhizoctonia-like fungi and share common morphological traits. Ceratobasidium has two nuclei per cell (i.e., binucleate Rhizoctonia) whereas Thanatephorus has multinucleated cells (González Garcia et al. 2006). Ceratobasidium species produce coriaceous fruiting bodies containing globose basidiospores, which vary from 2 to 4 in number per basidium (Rogers 1935).
Species of Ceratobasidium can infect several members of Annonaceae, Rosaceae, Rubiaceae, Rutaceae, and Teaceae families, and are found mostly in tropical agroecosystems in Africa, Asia, and South America (Wolf and Bach 1927). In Brazil, white thread blight commonly occurs on fruit trees and ornamentals in the Amazon (Benchimol et al. 2001; Benchimol and Bastos 2004; Costa et al. 2013). High temperature and humidity favor the occurrence and spread of these pathogens (Furtado 1997; Souza et al. 2009).
The association of Ceratobasidium species with the white thread blight disease on several hosts in Brazil were mostly derived from the fungal morphological traits solely. Therefore, these identifications are likely to be less accurate, as these Rhizoctonia-like fungi exhibit extensive morphological variations, which could lead to misidentification of the pathogen (González Garcia et al. 2006). In Brazil, only a single study has included phylogenetic information based on the rDNA ITS-5.8S molecular marker to differentiate between Ceratobasidium spp. from C. sinensis and D. kaki (Ceresini et al. 2012). This study reported the occurrence of two distinct lineages of Ceratobasidium spp. infecting each one of the hosts. However, the authors did not propose new species designation to accommodate the two new phylogenetic groups (Ceresini et al. 2012). A new species Ceratobasidium tradescantiae D. M. Macedo, associated with Tradescantia fluminensis Vell. (Commelinaceae), was described recently in Brazil. However, the authors did not perform phylogenetic identification and only used morphological markers (Macedo et al. 2016).
Despite the fact that white thread blight is particularly relevant for important tree crop species of the Brazilian tropical agroecosystem, information regarding the etiology of the Ceratobasidium species associated with the disease is limited. The present study determined the etiology of white thread blight associated with A. indica, C. sinensis, C. arabica, D. kaki, and E. uniflora using both morphological and phylogenetic approaches, which resulted in the description of the new species: C. niltonsouzanum and C. chavesanum.
Material and methods
Fungal isolation
Plant leaf samples exhibiting white thread blight symptoms were collected from Indian green-tea (Fig. 1a), coffee (Fig. 2a, b and f), and persimmon (Fig. 2d and e) in agricultural areas surrounding the remnants of Atlantic forest in Southeastern Brazil in 2005 and 2010, and from Brazilian cherry pitanga (Fig. 1b and d) and neem (Fig. 1e–g) plants in Northeastern Brazil during the rainy season of 2015 and 2017. Direct isolation was performed by transferring fungal mycelia and sclerotia to potato-dextrose-agar (PDA) medium and the plates incubated at 25 °C. For indirect isolation, small, colonized leaf fragments from diseased leaves were used. The leaf fragments were disinfested in 2% sodium hypochlorite for 2 min, washed with sterile distilled water, then transferred to PDA medium and the plates incubated at 25 °C. Pure cultures were obtained by transferring fungal hyphal tips to fresh PDA medium. Isolates were deposited at the Mycological Collection (CML) of the Federal University of Lavras (Table 1).
Ceratobasidium niltonsouzanum sp. nov. a White thread blight symptoms on Indian green-tea (arrow indicates mycelial cord). b White thread blight symptoms on Brazilian cherry pitanga leaves. c Pathogenicity tests. d Fungal microsclerotia on Brazilian cherry pitanga leaf surface. e Symptom of white thread blight on neem. f Hymenium on the abaxial surface of neem leaves. g Mycelial cord (arrow) on neem branch. h–i Cultures of C. niltonsouzanum CML 3598 grown on PDA for 30 (I) days at 25 °C in the dark. j Microsclerotia on the surface of a colony after 60 days at 25 ° C in the dark. k Scanning electron micrograph of basidiospores. l-m Basidia and basidiospores. n Binucleate hyphae stained with DAPI. o Hyphae with 90° branching (indicated by arrow). p Hyphopodia. Scale bars: j: 200 μm; k: 10 μm; l-p: 40 μm
Ceratobasidium chavesanum sp. nov. a Symptoms of white thread blight on coffee. b Mycelial cord (indicated by arrow) on a coffee leaf. c Symptom of black rot on a coffee leaf inoculated with C. chavesanum CML 3474. d Symptoms of fruit rot on persimmon. e. Mycelial cord (indicated by arrow) on persimmon branches. f Appearance of leathery mycelia on coffee fruits. g Symptoms of white thread on persimmon leaves. h-i Culture of C. chavesanum CML 3474 grown on PDA for 30 days at 25 °C in darkness. j Hyphae with 90° branching (indicated by arrow). k Binucleated hyphae stained with DAPI. Scale bars: J–K: 10 μm
Phylogenetic analysis
Fungal isolates were identified based on phylogenetic analysis of the Internal Transcribed Spacer ITS-5.8S region of rDNA. DNA from fungal mycelia was extracted using the specific Axy Prep Multisource Genomic DNA Miniprep kit (Axygen Biosciences,) following the manufacturer’s instructions.
The PCR reactions were done in a final volume of 25.0 μL containing 12.5 μL Taq 2× Max Mix PCR, 2 μL of each primer and 9 μL ultra-pure water. The ITS-5.8S primers used were ITS1 (5’-TCC GTA GGT GAA CCT GCG G-3 ‘) and ITS 4 (5’-TCC TCC GCT TAT TGA TAT GC-3′) (White et al. 1999). The PCR conditions were as follows: denaturation at 94 °C for 30 s, annealing at 54 °C for 1 min, extension at 72 °C for 1 min, for 35 cycles, and final extension at 72 °C for 10 min. Analysis of the PCR products was done on 2% agarose gel stained with GelRed (Biotium Inc.) in 1× TAE buffer (Sambrook et al. 1989). PCR products were purified before sequencing using the Wizard SV Gel and PCR Clean-Up System (Promega). Sequencing of PCR amplicons was performed at the facilities of the Biological Institute of São Paulo using a DNA automated sequencer ABI 3700 Prism (Applied Biosystems).
Nucleotide sequences were analyzed and edited using SeqAssem software (Hepperle 2004). All the sequences were deposited in GenBank (Table 1). Additional ITS-5.8S rDNA sequences from Ceratobasidium spp. and Thanatephorus cucumeris (asexual state = Rhizoctonia solani) reference isolates, including most of the anastomosis groups (AGs) and known species described so far associated with diverse hosts, were obtained from GenBank and added to the global phylogeny (Tables 1 and 2). Sequences were aligned using the ClustalW program (Thompson et al. 1994) in MEGA v.6 software (Tamura et al. 2013). Prior to phylogenetic analyses, MrModeltest 2.3 (Posada and Buckley 2004) was used to ascertain the models of nucleotide evolution that best fit the data for ITS-5.8S DNA. The best model for Ceratobasidium AGs and T. cucumeris (R. solani) data was the GRT + G model and for Ceratobasidium species was the GTR + I + G model. Bayesian phylogenetics inference analyses were performed using Metropolis-coupled Markov chain Monte Carlo (MCMCMC) algorithm in MrBayes v.3.2 (Ronquist et al. 2011). Phylogenetic analysis was conducted in the CIPRES web portal (Miller et al. 2010). Markov chains were run simultaneously from random trees up to 10,000,000 generations. Trees were sampled at every 1000 generations up to 10,000 trees in total. After discarding the first 2500 trees from each analysis as ‘burn-ins’, only the remaining 7500 trees were utilized to calculate the posterior probabilities of the branches, which were determined by the majority consensus of the trees sampled. FigTree (Rambaut 2009) was used to visualize and export trees to graphic programs.
Cultural and morphological characteristics
The cultural and morphological analyses were performed at the Electron Microscopy Lab at UFLA using the ex-type strains of the two new species described in this study, C. niltonsouzanum CML 3598 and C. chavesanum CML 3474. Fungal cultures were characterized after 10, 30 and 60 days of growth on PDA medium at 25 °C in the dark. The cultures were evaluated for color, appearance of the colony, presence of mycelia tufts and sclerotia. The hyphae dimensions were determined from 10-day-old cultures using the Zeiss Observer Z1 inverted microscope and the Zen 2012 software. Sclerotia images and measurements were acquired from 60-day-old cultures using a Nikon SMZ1500 stereoscope microscope and the NIS Elements D3.2 software. Thirty hyphae and sclerotia were sampled and measured.
Number of nuclei per cell was determined on 10-day-old colonies. Fragments with 0.5 cm diameter were fixed in Karnovsky solution, pH 7.2. These fragments were subjected to fluorescent staining of nuclei with DAPI (4′6-Diamidine-2′-phenylindole dihydrochloride) (VectaShield H-1200 Vector) mounting medium, and septa with 0.1 mg/mL Calcofluor White (Sigma) in potassium phosphate buffer pH 7.2. The fixed fragments were placed in drops of DAPI solution on coverslips and observed for stained nuclei after 5 min. Then, the same fragments were treated with drops of Calcofluor White solution on the coverslips and observed after 5 min. A Scanning Laser Confocal Zeiss Observer Z1 LSM780 with the Zen 2012 software was used to acquire the images, with 40×/1.3 oil objective, 4.4 zoom, 512 × 512 resolution, Ch1 detector, MBS 405 nm beam splitter, pinhole 68.5, argon laser 405 ηm at 10%, master gain 470, digital gain 1, and emission filter range at 420 to 480 ηm.
Fungal structures from infected leaf samples from the field were characterized using scanning electron microscope (SEM). Leaf fragments with white thread blight symptoms were fixed in Karnovsky solution pH 7.2 for a minimum of 24 h, transferred to 0.05 M cacodylate buffer solution and washed three times for 10 min. Fragments were soaked for 1 h in 1.0% osmium tetroxide in water and washed three times with distilled water. Then, the samples were dehydrated in an acetone series (25, 50, 75 and 90% acetone for 10 min each and three times in 100% acetone for 10 min). When the dehydration was completed, the samples were placed in a Balzers CPD 030 critical point and the acetone was replaced with CO2 (Bozzola and Russell 1999). The specimens obtained were covered with gold on the Balzers SCD 050 evaporator. Observation was done using a Zeiss LEO EVO 40 Scanning Electron Microscope and the Smart SEM software. The acquired images were edited and diagrammed using Corel Draw software. Drawings of morphological structures were made based on these images.
Pathogenicity tests
Three-month-old seedlings of E. uniflora, A. indica and C. arabica were used for the pathogenicity tests. Prior to inoculation, the plants were covered with plastic bags and placed in a humid chamber for 10 days. The Ceratobasidium spp. isolates CML 3598, CML 3596 and CML 3474, were used for inoculations on E. uniflora, A. indica, and C. arabica cv. Mundo Novo, respectively. These isolates were cultured at 25 °C in the dark for seven days on PDA medium containing sterilized tooth-picks on top. After incubation, the colonized tooth-picks were gently inserted in the leaves at the plants apex. To maintain the plants under high humidity, a cotton wool ball moistened with sterilized water was placed inside the plastic bags covering the plants (Souza et al. 2009). The inoculated plants were incubated at 25 °C and 12 h photoperiod and observed daily. Pathogenicity tests were done with six replicates per treatment and per plant species, plus a control using sterilized tooth-picks. The experiment was replicated once.
Results
Phylogenetic analysis
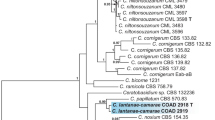
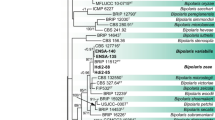
For phylogenetic analyses based on the ITS-5.8S rRNA region, sequences of isolates from this study were compared with sequences of reference Ceratobasidium spp. (binucleate Rhizoctonia AGs) isolates (Fig. 3), all the known Ceratobasidium species (Fig. 4), and Thanatephorus cucumeris (multinucleate R. solani AGs) (Fig. 5) deposited in the GenBank. The dataset from representative strains of this study and sequences of reference isolates of Ceratobasidium spp. (binucleate Rhizoctonia AGs) consisted of 784 aligned nucleotide positions with 404 variable sites, 233 of which were phylogenetically informative. A similar dataset comparing our sequences with the reference isolates of the known Ceratobasidium species consisted of 672 aligned nucleotide positions with 301 variable sites, 218 of which were phylogenetically informative. The dataset from strains of this study and sequences of reference isolates of R. solani AGs consisted of 839 aligned nucleotide positions with 360 variable sites, 197 of which were phylogenetically informative. The phylogenetic tree inferred from ITS-5.8S rRNA region clearly distinguished the isolates from this study from both binucleate Ceratobasidim (AGs and known species) (Figs. 3 and 4) and multinucleate R. solani AGs described (Fig. 5).
Bayesian phylogenetic tree based on the nucleotide sequences of the ITS-5.8S rRNA regions showing the relationships among Ceratobasidium species (binucleate Rhizoctonia). Posterior probability values >0.6 are indicated above the nodes. Anastomosis Groups (AG-A - AG-U and CAG-1 - CAG-7), haplotype (H), and sequences from ex-type (T) strains are indicated. The tree is rooted with Waitea circinata (GenBank accession number AJ000195). CML: Abbreviation of Mycological Collection at the Federal University of Lavras - UFLA
Bayesian phylogenetic tree based on the nucleotide sequences of the ITS-5.8S rRNA region showing the relationships among known Ceratobasidium species. Posterior probability values >0.6 are indicated above the nodes. Sequences from ex-type (T) strains are indicated. The tree is rooted with C. albasitensis and C. ramicola. CML: Abbreviation of Mycological Collection at the Federal University of Lavras - UFLA
Bayesian phylogenetic tree based on the nucleotide sequences of the ITS-5.8S rRNA region showing the relationships among Thanatephorus cucumeris (multinucleate R. solani AGs) and two new Ceratobasidium species causing white thread blight on tropical plants in Brazil. Posterior probability values >0.6 are indicated above the nodes. Anastomosis Groups (AG) and subgroups, haplotype (H), and sequences from ex-type (T) strains are indicated. The tree is rooted with Waitea circinata (GenBank accession number AJ000195). CML: Abbreviation of Mycological Collection at the Federal University of Lavras - UFLA
The sequences from our isolates grouped into two distinct monophyletic clades with high support (posterior probability = 1) in all the phylogenetic analyzes (Figs. 3, 4 and 5) and were distinct from those of the multinucleate R. solani clade (posterior probability = 1) (Fig. 5). The first clade included 10 isolates from C. arabica and D. kaki, while the second clade comprised nine isolates from A. indica, C. sinensis, and E. uniflora. We propose to accommodate isolates representing the first clade within the new species Ceratobasidium niltonsouzanum sp. nov., and those grouping in the second sister clade within another species named Ceratobasidium chavesanum sp. nov. Both species are described in this study.
Ceratobasidium chavesanum was distinguished from C. niltonsouzanum by nine fixed nucleotide polymorphisms in the ITS-5.8S rDNA sequence. These fixed nucleotide differences were at the following positions (C. chavesanum/C. niltonsouzanum): 205–206 (TT/GC), 235 (A/G), 438 (A/T), 445 (C/T), 511 (A/G), 621 (G/A), 630 (T/C), and 657 (G/A for most of the isolates; C for CML 3598 from Eugenia uniflora).
Cultural and morphological characteristics
The fungal cultures of C. niltonsouzanum CML 3598 and C. chavesanum CML 3474 initially (10 days old) had white-colored aerial mycelia and reverse with concentric zones. The colonies reverse turned to brown color with aging and the aerial mycelia produced tufts (up to 30 days old) (Fig. 1h–i and Fig. 2h–i). Black and irregular-shaped sclerotia were produced by the C. niltonsouzanum ex-type strain CML 3598 after 60 days of incubation (Fig. 1j). Ceratobasidium chavesanum did not produce sclerotia. The isolate CML 3598 of C. niltonsouzanum was obtained from E. uniflora leaves on which hymenia and basidia with four unicellular, oblong to elliptical and hyaline basidiospores were present (Fig. 1k–m). Hyphopodia (Fig. 1p) and black, irregular-shaped sclerotia were also observed in these leaves (Fig. 1d). The C. niltonsouzanum isolate CML 3598 produced basidiospores on PDA medium after 45 days of incubation (not shown). However, it was not possible to observe basidiospores development in subsequent cultures. No basidiospores were observed for C. chavesanum. Isolates from both species were binucleate (Fig. 1n and Fig. 2k).
Microscopic analyses of C. niltonsouzanum and C. chavesanum mycelium revealed the association of hyphae with typical characteristics of Rhizoctonia-like fungi. The hyphae presented branching with 90° angulation (Fig. 1n and 2 j, k). There were mycelial chords in both isolates, with larger cells. The morphological markers for C. niltonsouzanum and C. chavesanum observed in vitro and/or in vivo were illustrated with drawings derived from digital images (Fig. 6).
Pathogenicity test
Ten days after inoculation, the first symptoms of disease were observed on C. arabica inoculated with C. chavesanum isolate CML 3474 (Fig. 2c) and on E. uniflora inoculated with C. niltonsouzanum isolate CML 3598 (Fig. 1c). Initially, a small patch of necrosis was visible, which later developed into leaf blight. Re-isolations of the pathogens validated Koch’s postulates. Leaves were suspended by fungal mycelium but fungal mycelial cord was absent in the plant branches. However, inoculation with C. niltonsouzanum CML 3596 did not produce symptoms in A. indica.
Taxonomy
On the basis of the phylogenetic analysis and morphological traits, the Ceratobasidium isolates obtained from A. indica, C. arabica, C. sinensis, D. kaki, and E. uniflora were split into two new species:
Ceratobasidium niltonsouzanum M.P. Melo, S.I. Moreira, P.C. Ceresini, sp. nov. MycoBank 823,294.
Etymology: In honor of Prof. Dr. Nilton Luiz de Souza, for his vast research contribution on the genus Rhizoctonia in Brazil.
Specimen examined: Brazil, State of Piauí, Teresina, Centro de Ciências Agrárias, Campus da Universidade Federal do Piauí (42 ° 46 ‘57’ ‘W; 05 ° 02’ 45 “S), in Eugenia uniflora, April 2015, Maruzanete Pereira de Melo (Holotype VIC 44275, Ex-type CML 3598).
Ceratobasidium niltonsouzanum: fixed nucleotide differences in ITS-5.8S rDNA specific to C. niltonsouzanum: 205–206 (GC), 235 (G), 438 (T), 445 (T), 511 (G), 621 (A), 630 (C), and 657 (A for most of the isolates; C for CML 3598 from Eugenia uniflora). The leaves of the host species Eugenia uniflora show the characteristic presence of hymenia, and branched hyphae with clear septa. The hyphae are branched with 90° angulation and have two nuclei per cell. Hyphopodia are globose to subglobose, with 3.5 to 5.0 μm diameter. On PDA, two kinds of aerial mycelia are present in 10-day-old cultures. One type with 1.5 (1.0 to 2.0) μm width and another, thick-walled, with 2.0 (1.5 to 2.5) μm width, which compose mycelial chords.
Basidiospores are produced in hymenium on A. indica and E. uniflora leaves, above epidermis. Typical basidia contain four unicellular, oblong to elliptical and hyaline basidiospores (6–) 8 (−11) × (2–) 3 (−4) μm in size. Mycelial chords ranging from white to brown in color are evident on leaves of Camelia sinensis (Fig. 1a) and branches of Azadirachata indica (Fig. 1g). Irregularly shaped microsclerotia, black in color, around 200 × 500 μm in size, were visible on leaves and branches of E. uniflora and A. indica (Fig. 1d). The strains isolated from E. uniflora leaves produced black sclerotia with irregular shape, measuring 130 × 105 μm on average after 60 days of incubation on PDA. The culture on PDA grows forming concentric rings with white aerial mycelia, tufts development and reverse browning color with age (Fig. 1h-i).
Ceratobasidium chavesanum M.P. Melo, J.A. Ventura, H. Costa, P.C. Ceresini, sp. nov. MycoBank 823,295.
Etymology: In honor of Prof. Dr. Geraldo Martins Chaves, an eminent Brazilian scientist and coffee plant pathologist.
Specimen examined: Brazil, State of Espírito Santo, Conceição do Castelo (41° 17 ‘45 “W, 20° 22’ 08” S), in Coffea arabica, May 2010, Hélcio Costa and José Aires Ventura (Holotype VIC 44207, Ex-type CML 3474).
Ceratobasidium chavesanum: fixed nucleotide differences in ITS-5.8S rDNA specific to C. chavesanum: 205–206 (TT), 235 (A), 438 (A), 445 (C), 511 (A), 621 (G), 630 (T), and 657 (G). Mycelial chords are produced on branches and leaves of coffee plants (Fig. 2b), besides rich mycelial development on leaves, branches and fruits (Fig. 2a, b and f). No production of basidiospores was observed. The hyphae are branched with 90° angulation. Ten-day-old PDA cultures presented aerial mycelia with hyphae 1.5 (1.0 to 2.0) μm width and thick-walled mycelial chords hyphae 2.0 (1.5 to 2.5) μm width, and presence of two nuclei per cell. The initially white fungal cultures become brown to dark brown with aging and show compact tufts (Fig. 2h-i).
Morphological structures of the C. niltonsouzanum observed in vivo (as basidia and basidiospores, hyphopodia and sclerotia) and in vitro (as hyphae from aerial mycelia and from mycelial chords) were illustrated in drawings derived from digital images (Fig. 6a). Similarly, C. chavesanum morphological structures were drawn based on structures observed in vitro, such as aerial mycelia and mycelial chords (Fig. 6b).
Discussion
Using both morphological and phylogenetic characters, our study described two new binucleate Ceratobasidium species: C. niltonsouzanum sp. nov. and C. chavesanum, sp. nov, affecting important crop species in the Brazilian agroecosystem. Ceratobasium niltonsouzanum was morphologically distinct from C. chavesanum based on vegetative structures. Not all morphological structures used to distinguish between these two species were easily produced in culture, and the use of the ITS-rDNA sequences was a robust marker to infer their distinction.
Ceratobasidium niltonsouzanum was obtained from pitanga, neem and green-tea trees. In the phylogenetic analysis, the isolates formed three different haplotypes (Figs. 3 and 4). The first clade was composed by pitanga isolates, the second by green-tea isolates, and the last clade by isolates from green-tea and neem tree. Previous studies reported that the fungus associated with green-tea plants was Pellicularia koleroga but subsequently the pathogen was reclassified to Ceratobasidium anceps (Furtado 1997, 2005). Our study provides evidence that, in fact, the new species C. niltonsouzazum (but not C. anceps) is associated with white thread blight on green-tea plants.
Meanwhile, Ceratobasidium isolates obtained from C. arabica and D. kaki were described here as Ceratobasidium chavesanum. The clade corresponding to C. chavesanum showed three haplotypes (Figs. 3 and 4). In Brazil, coffee white thread blight was identified in Acre and Rondônia plantations. Similarly, as cited above, P. koleroga (or C. anceps) was also associated with coffee white thread blight (Garcia and Veneziano 1998; Cavalcante and Sales 2001). Because this former identification was based only on morphological characters and no fungal cultures or ITS-rDNA data is available for comparison, we cannot assert that the coffee associated pathogen described in Acre and Rondônia is indeed C. chavesanum. However, it is plausible that those isolates were misidentified and that C. chavesanum was, in fact, the pathogen associated with white thread blight of coffee in those localities.
From a taxonomic point of view, the morphological features of the basidiospores are considered as significant characters for identification of species from the genus Ceratobasidium (Oberwinkler et al. 2013). Ceratobasidium niltonsouzanum isolates produced basidiospores both in nature and in culture medium. These fungal cultures were obtained from hyphal tips. Therefore, C. niltonsouzanum is likely a homothallic fungus. No basidiospores were detected on infected leaves of Camelia sinensis sampled from the Ribeira Valley in São Paulo State. In contrast, abundant sclerotia were present on leaves from E. uniflora and A. indica,. It is likely that temperature and humidity conditions did not favor sexual phase formation. While environmental factors may influence the Thanatephorus teleomorph formation, these conditions are yet to be clearly understood. Conditions favorable for sporulation seem to differ between isolates or species (Sneh et al. 1991; González Garcia et al. 2006). Despite the absence of the sexual phase of C. chavesanum, leathery mycelia in the leaves and fruit of coffee trees in the field may indicate basidiospores production. Hymenium producing basidia are likely to exist in leathery mycelia.
Ceratobasidium niltonsouzanum can be differentiated from certain species based on morphological markers. The production of four basidiospores per basidium separate this species from C. tradescantia, which forms two basidiospores per basidia (Macedo et al. 2016). Ceratobasidium tradescantia, related to T. fluminensis in Brazil, also presents hymenium on host leaves. Ceratobasidium lantana-camarae Barreto was the second species described in Brazil, morphologically different from C. niltonsouzanum with smaller and apiculate basidiospores (Barreto et al. 1995). Basidiospores of C. niltonsouzanum and C. pseudocornigerum Christiansen are similar in form but the basidiospores of C. cornigerum are shorter and usually widest at the basal end (Kotiranta and Saarenoksa 2005; Oberwinkler et al. 2013).
Presence of black microsclerodes attached to the leaves and branches, and the production of basidiospores were confirmed in E. uniflora and A. indica diseased samples. These fungal structures are crucial for the long-term survival of the pathogen. They were also observed on apple and citrus leaf surfaces (Wolf and Bach 1927; Lourd and Alves 1987). The sclerotia dimensions formed on plant tissues were larger than those produced in vitro. Differences in the sclerotia dimensions may occur due to environmental conditions, such as nutrition, temperature, and light.
In conclusion, the present work proposes the description of two new species of Ceratobasidium, Ceratobasidium chavesanum in coffee and persimmon, and Ceratobasidium niltonsouzanum in green-tea, pitanga and neem. These two species are causal agents of white thread blight. White thread blight has been gradually recognized as an emerging disease with a great potential to impact the production of coffee, persimmon, Indian green-tea, pitanga and neem. Knowledge of the etiology of this disease is crucial for employing suitable management measures. Possibly, diverse host plants may serve as inoculum sources. These two fungal species are believed to be associated with other hosts, specifically in the northern parts of Brazil, which have favorable climatic conditions for disease development.
References
Barreto RW, Evans HC, Ellison CA (1995) The mycobiota of the weed Lantana camara in Brazil, with particular reference to biological control. Mycological Research 99:769–782
Benchimol RL, Bastos CN (2004) Queima-do-fio em três espécies de plantas ornamentais. Comunicado Técnico, Embrapa, p 1–3
Benchimol RL, Poltronieri LS, Trindade DR, Albuquerque FC (2001) White-thread-blight: five new hosts in the state of Pará, Brazil. Fitopatologia Brasileira 26:778
Bozzola JJ, Russell LD (eds) (1999) Electron microscopy: principles and techniques for biologists, 2nd edn. Jones and Bartlett Publishers, Sudbury
Burt EA (1918) Corticiums causing Pellicularia koleroga disease of the coffee plant, Hypochnose of pomaceous fruits and Rhizoctonia disease. Annals of the Missouri Botanical Garden 5:5119–5132
Cavalcante MJB, Sales F (2001) Ocorrência da Queima-do-fio (Pellicularia koleroga) em cafezais em Rio Branco. Comunicado Técnico, Embrapa, p 1-2
Ceresini PC, Costa-Souza E, Zala M, Furtado EL, Souza NL (2012) Evidence that the Ceratobasidium-like white-thread blight and black rot fungal pathogens from persimmon and green-tea crops in the Brazilian Atlantic Forest agroecosystem are two distinct phylospecies. Genetics and Molecular Biology 35:480–497
Cooke MC (1876) Some Indian fungi. Grevillea 4:116–118
Costa RC, Verzignassi JR, Poltronieri TPS, Poltronieri LS, Monteiro LC (2013) Novos hospedeiros de Ceratobasidium ochroleucum, agente causal da queima-do-fio, no Pará. Summa Phytopathologica 39:62
Furtado E (1997) Doenças do chá. In: Kimati H, Amorim L, Bergamim Filho A, Camargo L and Rezende J (eds) Manual de Fitopatologia. 3rd edition. Agronômica Ceres, São Paulo, pp 257-260
Furtado EL (2005) Doenças do chá. In: Kimati H, Amorim L, Bergamim Filho A, Camargo L, Rezende J (eds) Manual de Fitopatologia, vol 2, 4th edn. Agronômica Ceres, São Paulo, pp 257–260
Garcia A, Veneziano W (1998) Queima do fio, mal de koleroga ou mal de hilachas (sinonimia: Pillicularia koleroga: Koleroga noxia donk= Corticium koleroga): Uma doença em expansão nos cafeeiros de Rondônia. Comunicado técnico, Embrapa, p 1-11
González Garcia V, Portal Onco MAP, Rubio Susan VR (2006) Review, biology and systematics of the form genus Rhizoctonia. Spanish Journal of Agricultural Research 4:55–79
Hepperle D (2004) SeqAssem Win32-version. A sequence analysis tool contig assembler and trace data visualization tool for molecular sequences. Available at http://www.sequentix.de
Kotiranta H, Saarenoksa R (2005) Ceratobasidium and Oliveonia (Basidiomycota, Aphyllophorales) in Finland. Annales Botanici Fennicci 42:237–245
Lourd M, Alves B (1987) Lista de hospedeiro e etiologia da queima-do-fio das plantas frutíferas na região Amazônica. Fitopatologia Brasileira 12:88–89
Lourd M, Alves MLB (1997) Lista de hospedeiros e etiologia da queima do fio das plantas frutíferas da região Amazônica. Fitopatologia Brasileira 12:88–89
Macedo DM, Pereira OL, Hora Junior BT, Weir BS, Barreto RW (2016) Mycobiota of the weed Tradescantia fluminensis in its native range in Brazil with particular references to classical biological control. Australasian Plant Pathology 45:45–56
Mendes MAS, da Silva VL, Dianese JC (1998) Fungos em Plantas no Brasil. Embrapa-SPI, Embrapa Cenargem. 555p
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. 1–8, in: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA
Oberwinkler F, Riess K, Bauer R, Kirschner R, Garnica S (2013) Taxonomic re-evaluation of the Ceratobasidium-Rhizoctonia complex and Rhizoctonia butinii, a new species attacking sprume. Mycological Progress 12:763–776
Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology 53:793–808
Rambaut A (2009) FigTree 1.2.2. Available at: http://tree.bio.ed.ac.uk/software/figtree/. Accessed on October 20, 2015
Rogers DP (1935) Notes on lower basidiomycetes. University of Iowa Studies in Natural History 17:1–43
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard AM, Huelsenbeck JP (2011) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61:539–542
Sambrook J, Fritschi EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Sneh B, Burpee L, Ogosh A (1991) Identification of Rhizoctonia species. APS Press. St. Paul. 133p
Souza EC, Basseto MA, Boliani AC, Takada HM, Ceresini PC (2009) Patogenicidade cruzada de Ceratobasidium spp. do caquizeiro (Diospyros kaki) e do chá (Camellia sinensis) e reação de cultivares de caqui ao patógeno. Summa Phytopathologica 35:9–14
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30:2725–2729
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22:4673–4680
Venkatarayan S (1949) The validity of the name Pellicularia koleroga Cooke. Indian Phytopathology 2:186–189
Von Hoehnel, F (1910) Fragments Zur Mykologie. X. Sitzungber. K. Akad. Wiss. Wien, Math-Natur. Wiss. Klass. 199. 395, 1910
White TJ, BrunsT, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. New York, USA. Academic Press, pp 315–322
Wolf F, Bach WJ (1927) The thread blight disease caused by Corticium koleroga (Cooke) Hohn., on citrus and pomaceus plants. Phytopathology 17:689–707
Acknowledgements
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Programa Nacional de Pós-Doutorado (PNPD) for granting post-doctoral fellowship to MPM, KSM and SIM. This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG) and Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP, grant number 04/1980-0 to PCC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Simon Shamoun
Rights and permissions
About this article
Cite this article
de Melo, M.P., Matos, K.S., Moreira, S.I. et al. Two new Ceratobasidium species causing white thread blight on tropical plants in Brazil. Trop. plant pathol. 43, 559–571 (2018). https://doi.org/10.1007/s40858-018-0237-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-018-0237-x