Abstract
Objectives
Hyperlipidemia, excessive amounts of fat and fatty substances in the blood, is a major risk factor for cardiovascular disease. The measuring of fat or fatty substance in blood is usually obtained through a blood lipid test which includes total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL). In the Medical Device industry, Point-of-care (POC) methods provide fast, portable and easy to use devices suitable for rapid determination of Lipid levels. Here, we evaluated the analytical performance of PixoTest POCT Lipid System (iXensor Co., Ltd.), a newly developed POC lipid panel analyzer.
Materials and Methods
PixoTest POCT Analyzer accuracy evaluation with the Cobas c111 Analyzer (Roche Diagnostics Ltd.) as a reference method was carried out according to CLSI EP 09-A3 guidelines. This study enrolled one hundred and twenty (120) subjects and conducted over three (3) clinical sites (40 subjects each), administered through nine (9) trained operators (total) performed under manufacturer’s protocol using three (3) reagent lots per blood sample (capillary & venous). Linear regression, bias plot and other statistical analyses were extrapolated from the test data in this study.
Results
Passing-Bablok regression analysis of the data between PixoTest POCT System and Roche Cobas c111 Analyzer with the venous blood showed a slope of 1.03, an intercept of − 0.1 and correlation coefficient of 0.99. The results for capillary blood showed a slope of 1.01, an intercept of − 0.04 and correlation coefficient of 0.99. Passing-Bablok regression analysis of the data (n = 120) from the comparison between the PixoTest POCT System and Roche Cobas c111 Analyzer with the TC venous blood of total 3 lots at 3 sites showed a slope of 0.99, an intercept of − 0.337 and correlation coefficient of 0.995 and the TC capillary blood showed a slope of 0.99, an intercept of − 0.105 and correlation coefficient of 0.984. TG venous blood of total 3 lots at 3 sites showed a slope of 0.99, an intercept of 0.0246 and correlation coefficient of 0.998 and TG capillary blood showed a slope of 0.99, an intercept of − 0.329 and correlation coefficient of 0.998. HDL venous blood showed a slope of 0.99, an intercept of − 0.0297 and correlation coefficient of 0.987 and the HDL capillary blood showed a slope of 0.99, an intercept of − 0.0403 and correlation coefficient of 0.989.
Conclusions
The evaluation results of PixoTest POCT system’s accuracy, linearity, and correlation with Roche Cobas c111 Analyzer, the PixoTest POCT System displayed great performance results for all evaluated data points. Thus, the PixoTest POCT System is capable of providing accurate and reliable lipid measurements in the POC setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cardiovascular disease (CVD) is the leading cause of death, about one-third of all death, in the world [1]. Globally, CVD and diabetes mellitus are major contributing factors to public health problems including hypertension, stroke, ischemic heart disease, and peripheral arterial occlusive disease [1, 2]. Blood lipids, which includes total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL) and high-density lipoprotein (HDL), is a risk factor index for cardiovascular disease. When certain lipids levels (TC, TG, and LDL) in the blood are high, the possibility or increased risk of atherosclerosis (artery plaque build-up) narrowing the arteries restricting or blocking blood flow throughout the body [3,4,5], will increasing the risk of developing heart diseases. There are many factors that can influence a person’s blood lipid profile, such as diet, age, weight, BMI, gender, genetics, diseases, and lifestyle [6].
The importance and effectiveness of point-of-care (POC) methods is the capability to quickly and easily provide diagnostic results to assist in the control or monitoring of blood lipid levels [7,8,9]. Despite the convenience and lowered costs, the performance of some POC methods are weaker in comparison to laboratory examinations, such as large bias and weak linearity. For instance, CardioChek PA (PTS Diagnostic Inc.) appeared to have the correlation coefficient of 0.86, a regression line to reference with slope of 0.72 and an intercept of 1.76 for their total cholesterol measurement of 106 patients [10].
The source of blood sample for blood lipid analysis is also an important factor, but many lipid POC analyzers are only capable of using capillary blood (Accutrend Plus (Roche) and BeneCheck PLUS (General Life Biotechnology Co., Ltd) [11, 12]), causing inconvenience and potential applicability at certain clinical sites. Furthermore, the evaluation of TC, TG, and HDL are also important factors to obtaining an informative lipid panel report. For example, products like BeneCheck PLUS only provides TC per specimen sample while Accutrend Plus is able to test TC and TG individually, per specimen, but lacks HDL or LDL. As a result, patients are required to find alternative methods of measuring missing biomarkers to complete a full lipid panel. These inconveniences reduce efficiency and efficacy diminishing the advantages of POC devices. Consequently, the difficulty of developing POC devices for lipid panel is that, the system should integrate many different biomarkers while keeping each analytical performance reliable, while simultaneously retaining operational flexibility. Hence, improvement of POC methods with new diagnostic techniques is an unmet clinical need.
We analyzed the performance of a newly developed POC blood lipid analyzer incorporating three key biomarkers TC, TG, and HDL, PixoTest POCT System (iXensor Co., Ltd., Taipei, Taiwan), based on the analysis of accuracy, linearity, and correlation with the reference method, Roche Cobas c111 Analyzer (Roche Diagnostics Ltd.).
2 Materials and Methods
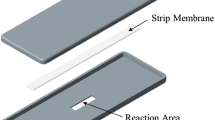
The specification of PixoTest POCT Lipid System as shown in Table 1, utilizes an enzymatic chromatography assay that is completed within 3 min. Figure 1 shows the structure of PixoTest Lipid Test Strip that is used for the quantitative measurement of TC, TG, and HDL cholesterol in whole blood. Estimated values of LDL cholesterol are calculated.
The PixoTest Lipid Test Strip utilizes enzymatic reactions to produce a color change when blood sample is applied, the enzymatic reactions that occurs are listed below:
In TC measurements, Cholesterol esters are transferred to colored-dyed substrate through cholesterol esterase, cholesterol oxidase, and peroxidase to produces color change.
Total cholesterol (TC) reactions
In TG measurements, Triglycerides are transferred to colored-dyed substrate through Lipoprotein Lipase, Glycerol kinase, and Glycerolphosphate oxidase to produce color change.
Triglycerides (TG) reactions
In HDL measurements, Total Cholesterol specimen will first delete very low-density lipoprotein (VLDL) and LDL, then Cholesterol esters are transferred to colored-dyed substrate through cholesterol esterase, cholesterol oxidase, and peroxidase to produces color change.
High-density lipoprotein cholesterol (HDL) Reactions
In our study, both capillary blood (CB) and venous whole blood (VB) were collected for testing samples. When whole blood sample is applied to PixoTest Lipid Test Strip, the color density of the reaction area is read by reflectance photometry as shown in Fig. 2. The level of color density is proportional to the concentration of biomarkers, the higher the concentration the darker color
In order to evaluate the accuracy of the PixoTest POCT System, we performed a method comparison study with Roche Cobas c111 Analyzer as reference method. We assessed one hundred and twenty (120) subjects from three (3) clinical sites through nine (9) operators as shown in Table 2. At each site, three (3) operators conducted tests on forty (40) different subjects using fresh capillary and venous blood. Furthermore, each subject used three (3) PixoTest Lipid Test Strips per blood sample, each test strip was taken from a different reagent lot, while following Clinical and Laboratory Standards Institute (CLSI) EP09-A3 guideline. Samples collected from one hundred and twenty (120) subjects were placed in anti-coagulate tubes (Heparin) prior to testing, using both the PixoTest POCT System and the Roche Cobas c111 Analyzer, with all tests completed within a period of three weeks.
The inclusion criteria of subject recruitment are:
- 1.
20 years or older.
- 2.
Willing to complete all study procedures.
- 3.
For TC and TG, HCT 30–55%; for HDL, HCT 30–52%.
- 4.
Subjects includes healthy subjects, outpatients, and inpatients.
The exclusion criteria of subject recruitment are:
- 1.
Taking prescription anticoagulants (such as Warfarin or Heparin) or having clotting problems that may prolong bleeding. Taking Plavix or aspirin daily is not excluded.
- 2.
Hemophilia or any other bleeding disorder.
- 3.
Reports having an infection with a blood borne pathogen (e.g. HIV, hepatitis).
- 4.
Working for a competitive medical device company.
The data exclusion criteria
- 1.
The PixoTest POCT System user recognizes that an error was made and documents the details;
- 2.
The PixoTest POCT System error or failure requires the user to retest; if displayed, the error (e.g. error number or failure type) shall be documented;
- 3.
The PixoTest POCT System QC result are out of tolerance or not obtained;
- 4.
The sample was tested when QC results from the reference measurement procedure was out of acceptable limits or were not obtained;
- 5.
The blood sample was outside the manufacturer’s specifications for an influence quantity, such as packed cell volume;
- 6.
Information required to determine that the sample met the inclusion criteria is missing (e.g. no value for packed cell volume).
Passing-Bablok regression analysis was then carried out. All statistical analyses were performed with Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA) and MATLAB 2013.
3 Results
3.1 Total Cholesterol (TC)
According to the Passing-Bablok regression analysis of the data shown in Fig. 3, the comparison of the VB (n = 360) between the PixoTest POCT System and Roche Cobas c111 showed a slope of 0.99, an intercept of − 0.337 and correlation coefficient of 0.995. Similarly, the CB (n = 360) also showed high accuracy of the PixoTest POCT System with a slope of 0.99, an intercept of − 0.105 and correlation coefficient of 0.994 as shown in Fig. 4.
3.2 Triglycerides (TG)
As shown in Fig. 5, the Passing-Bablok regression analysis of the data from the comparison of VB (n = 360) between the PixoTest POCT System and Roche Cobas c111, the performance showed a slope of 0.99, an intercept of 0.0246 and correlation coefficient of 0.998; and the CB (n = 360) results showed a slope of 0.99 with intercept of − 0.329 and correlation coefficient of 0.998 as shown in Fig. 6.
3.3 High-Density Lipoprotein (HDL)
By comparing the PixoTest POCT System and Roche Cobas c111, the Passing-Bablok regression analysis of the VB data (n = 360) indicated in Fig. 7, shows a slope of 0.99, an intercept of − 0.0297 and correlation coefficient of 0.987. On the other hand, the CB (n = 360) showed similar results with a slope of 0.99, an intercept of − 0.0403 and correlation coefficient of 0.989 as shown in Fig. 8.
4 Discussion and Conclusion
Monitoring blood lipid levels is critical in the prevention and treatment of a broad range of diseases, such as cardiovascular diseases, obesity and other physiological disorders. Nowadays, point-of-care testing devices play an important role in the biomedical device industry. POC devices can provide a convenient method for quick screening to monitor patients’ lipid levels. POC devices do not require a clinical laboratory to analyze the sample, which is costly, time consuming and requires vein puncture for venous blood specimen samples. The National Cholesterol Education Program (NCEP) stated that POC tests can be used as long as the test results are standardized in accordance to clinical laboratories standards [13]. In order to provide informative results, a blood lipid measuring system needs be able to analyze many different biomarkers simultaneously. Hence, iXensor Co., Ltd. has developed PixoTest POCT System, a comprehensive solution for measuring lipid levels. The PixoTest POCT System, measures TC, TG, and HDL with one sample specimen in a single test, drastically simplifying and facilitating in the evaluation of patient blood lipid levels.
The accuracy of PixoTest POCT System is comparable to laboratory methods. The clinical trial results, from three (3) different sites of one hundred and twenty (120) independent participants (total), showed that TC, TG, and HDL evaluations, for both VB and CB specimen samples had 100% accuracy within 15% absolute bias in comparison to laboratory method (Roche Cobas c111). These results further indicate that PixoTest System has accuracy consistent not only to laboratory method in all three biomarkers, but also to different blood sample sources (VB or CB), resolving the single sample restriction previously known to some other POC devices. Moreover, the testing range of the clinical trial is comprehensive for all three biomarkers, covering a broad range from healthy individuals to those with high levels of TC, TG, and HDL. We have collected samples from 120 subjects with TC concentration from 107 to 442 mg/dL, TG concentration from 45 to 648 mg/dL, and HDL concentration from 25 to 94 mg/dL, respectively. Under these testing conditions, comparisons of PixoTest POCT System to Roche Cobas c111 Analyzer have correlation coefficients (R) higher than 0.99 (all three biomarkers), indicating a great linearity between laboratory method and PixoTest POCT System. Based on our clinical test results of both VB and CB specimen samples, the analytical data from different sites shows great accuracy and high correlation to the reference method (Roche Cobas c111 Analyzer) with linearity and accuracy performances between biomarkers to be almost identical. Most measurement systems often find it hard to consider all biomarkers accurately at the same time. CardioChek PA is examined to have a correlation coefficient of 0.86 for TC while only 0.77 for HDL from 106 patients [9]. Whereas PixoTest POCT test results of TC, TG, and HDL, all show great linearity with correlation coefficients larger than 0.98 and regression lines of slope approximately equal to 1.0 with absolute intercept no larger than 0.33. This evidence further indicates the consistency and versatility of PixoTest POCT System under various conditions for comprehensive lipid panel biomarkers.
Under the evaluation of accuracy, linearity and correlation between PixoTest POCT Lipid System and Roche Cobas c111 Analyzer, the PixoTest POCT Lipid System displayed great performance results for all evaluated biomarkers. Thus, the PixoTest POCT Lipid System is a perfect solution for lipid measurements in the POC settings, as a portable, easy-to-use, and convenient way to control and monitor blood lipid levels.
References
World Health Organization. Health topics: Cardiovascular diseases. http://www.who.int/topics/cardiovascular_diseases/en/.
Scarborough, P., Wickramasinghe, K., Bhatnagar, P., et al. (2011). Trends in coronary heart disease 1961–2011. London: British Heart Foundation.
New, M. I., Roberts, T. N., Bierman, E. L., & Reader, G. G. (1963). The significance of blood lipid alterations in diabetes mellitus. Diabetes,12, 208–212.
Frantz, I. D., Dawson, E. A., Ashman, P. L., Gatewood, L. C., Bartsch, G. E., Kuba, K., et al. (1989). Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arteriosclerosis, Thrombosis, and Vascular Biology,9, 129–135.
Ravelli, A., van der Meulen, J. H. P., Osmond, C., Barker, D., & Bleker, O. (2000). Infant feeding and adult glucose tolerance, lipid profile, blood pressure, and obesity. Archives of Disease in Childhood,82, 248–252.
Department of Health. (2009). Putting prevention first. NHS Health Check: Vascular risk assessment and management. Best practice guidance. London: Department of Health.
Pai, N. P., Vadnais, C., Denkinger, C., Engel, N., & Pai, M. (2012). Point-of-care testing for infectious diseases: Diversity, complexity, and barriers in low- and middle-income countries. PLOS Medicine,9, e1001306.
Rapi, S., Bazzini, C., Tozzetti, C., Sbolci, V., & Modesti, P. A. (2009). Point-of-care testing of cholesterol and triglycerides for epidemiologic studies: Evaluation of multicare-in system. Translational Research,153(2), 71–76.
Whitehead, S. J., Ford, C., & Gama, R. (2014). A combined laboratory and field evaluation of the Cholestech LDX and CardioChek PA point-of-care testing lipid and glucose analysers. Annals of Clinical Biochemistry,51(Pt 1), 54–67.
Panz, V. R., Raal, F. J., Paiker, J., Immelman, R., & Miles, H. (2005). Performance of the CardioChek PA and Cholestech LDX point-of-care analysers compared to clinical diagnostic laboratory methods for the measurement of lipids. Cardiovascular Journal South Africa,16(2), 112–117.
Batki, A. D., Holder, R., Thomason, H. L., & Thorpe, G. H. G. Boehringer Mannheim Accutrend GC meter for the determination of blood glucose and cholesterol. Medical Devices Agency evaluation report 1995 MDA/95/11.
NHS Purchasing and Supply Agency. (2009). Buyer’s guide. Point-of-care testing for cholesterol measurement.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. (2001). Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA,285(19), 2486–2497. https://doi.org/10.1001/jama.285.19.2486.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, SY., Tsai, TM., Chen, YY. et al. Performance Evaluation of a New Mobile POC Lipids Analyzer. J. Med. Biol. Eng. 40, 143–148 (2020). https://doi.org/10.1007/s40846-019-00495-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-019-00495-6